Abstract
Objectives
To design microneedles that minimize pain, this study tested the hypothesis that microneedles cause significantly less pain than a 26-gage hypodermic needle, and that decreasing microneedle length and the number of microneedles reduces pain in normal human subjects.
Methods
Single microneedles with lengths ranging from 480 to 1450 μm, widths from 160 to 465 μm, thicknesses from 30 to 100 μm and tip angles from 20° to 90°; and arrays containing 5 or 50 microneedles were inserted into the volar forearms of ten healthy, human subjects in a double-blinded, randomized study. Visual analog scale pain scores were recorded and compared to each other and to the pain from a 26-gage hypodermic needle.
Results
All microneedles investigated were significantly less painful than the hypodermic needle with microneedle pain scores varying from 5 to 40% of the hypodermic needle. Microneedle length had the strongest effect on pain, where a three-fold increase in length increased the pain score by seven fold. The number of microneedles also affected the pain score, where a 10-fold increase in the number of microneedles increased pain just over two-fold. Microneedle tip angle, thickness and width did not significantly influence pain.
Discussion
Microneedles are significantly less painful than a 26-gage hypodermic needle over the range of dimensions investigated. Decreasing microneedle length and number of microneedles reduces pain.
Keywords: Hypodermic needle, microneedle dimensions, microneedle length, transdermal drug delivery, visual analog pain scale
INTRODUCTION
Micron-scaled needles, i.e., microneedles, have been developed as a hybrid approach between transdermal patches and hypodermic needles to overcome the individual limitations of injections and patches, and to create a minimally invasive and less painful method of transdermal drug and vaccine delivery 1. A major limitation of hypodermic needles is the pain and risk of infection from bloodborne pathogens. Pain from needle insertion leads to distress and poor patient compliance 2, and in extreme cases can produce needle phobia, which is characterized by fear, anxiety and vasovagal reaction that can lead to fainting or sometimes even death 3–5. Furthermore, the hazardous practice of needle reuse found predominantly in developing countries puts millions of people at risk. In the year 2000, an estimated 40% of the 16 billion injections administered worldwide were from reused needles, which led to an estimated 21 million, 2 million and 260,000 new cases of hepatitis B, hepatitis C and HIV infections, respectively 6.
To eliminate the pain and risk associated with hypodermic needles, transdermal drug patches have been developed 7. However, transdermal delivery is currently limited in scope because of the formidable transport barrier provided by the stratum corneum 7. Because of this barrier, fewer than 20 drugs exist as transdermal patches, which are all small and lipophilic molecules with low dose requirements 7.
In a synergistic approach, microneedles assembled on a patch have been successfully used to deliver a variety of large and hydrophilic compounds into the skin. In vitro delivery of small molecules like calcein and large compounds like proteins, DNA and nanoparticles has been shown 8–10. In vivo delivery has been shown for insulin 11 and vaccines, for example, against influenza and hepatitis B 12,13.
Excitement about microneedles is based in part on the expectation that they cause less pain than hypodermic needles. However, this expectation has not been fully validated. Only one study has formally measured the pain caused by microneedles in human subjects. It found that insertion of 400 microneedles measuring 150μm in length was painless as compared to a 2-mm deep insertion of a 26-gage hypodermic needle 14. In a related study, scraping the skin with microneedle-like structures measuring 50–200 μm in length was similarly found to be painless 12. However, no study has examined the pain caused by microneedles in detail or determined how microneedle geometry influences pain. Addressing this issue is important because larger microneedles are stronger, can deliver more drug, and are generally easier to handle, but are expected to cause more pain. Design of an effective microneedle drug delivery system that minimizes pain requires a quantitative understanding of the dependence of pain on microneedle geometry.
Therefore, as the first study to examine the relationship between pain and micron-scale trauma to the skin, this study investigated the influence of microneedle design on pain by fabricating microneedles over a broad range of dimensions by varying microneedle length, width, thickness, tip angle and number of microneedles in an array; and comparing the pain they stimulated to a 26-gage hypodermic needle in healthy human subjects.
MATERIALS AND METHODS
Fabrication and assembly of stainless steel microneedles
Using methods described in detail previously 15, microneedle geometries were first drafted in AutoCAD software (Autodesk, Cupertino, CA, USA) and then cut into 50, 75 or 125 μm thick stainless steel sheets (Trinity Brand Industries, SS 304; McMaster-Carr, Atlanta, GA, USA) using an infrared laser (Resonetics Maestro, Nashua, NH, USA). Single, in-plane microneedles (microneedle shafts oriented parallel to the base substrate) were fabricated in different geometries and thicknesses. ‘Out-of-plane’ microneedles (shafts bent at 90° to the base substrate) were fabricated as two-dimensional arrays after manually bending microneedles perpendicularly out of the plane of their base substrate. To deburr and clean microneedle edges and to make the tips sharp, microneedles were electropolished, washed under running water, dried using compressed air, and stored in air-tight containers until further use.
For insertion into skin, single microneedles were firmly held in slots axially cut through the flat ends of teflon rods (3 mm diameter, McMaster-Carr) and clamped using a one-piece shaft collar (McMaster-Carr). Out-of-plane microneedles were assembled into adhesive patches using methods described in detail previously 15. Briefly, out-of-plane microneedle arrays were attached to a single-sided medical foam tape (TM9942, MACtac, Stow, OH, USA) and a perforated, double-sided, poly-ethylene-terephthalate carrier tape (63.5 μm thick; T04314A, MACtac) was then attached on top of the array with the microneedles passing through the perforations. The final patch had an adhesive layer surrounding the microneedles to hold the microneedles firmly against the skin after insertion. All the microneedles were assembled in a laminar flow hood for cleanliness. The final microneedle-teflon rod assemblies were autoclaved and the microneedle adhesive patches were ethylene oxide sterilized (AN 74j, Andersen Sterilizers, Haw River, NC, USA) before use.
Pain study design
Range of microneedle dimensions
Microneedle length, width, thickness, tip angle (Fig. 1) and the number of microneedles were independently varied over a wide range to determine their influence on pain in human subjects. The following microneedle dimensions were investigated. Microneedle length: 480, 700, 960 and 1450μm; microneedle tip angle: 20°, 55° and 90°; microneedle width: 160, 245 and 465μm; microneedle thickness: 30, 45 and 100μm; and the number of microneedles: 5 and 50. A 5-mm deep insertion of a 26-gage (outer diameter: 460μm) hypodermic needle was used as a positive control for comparison with all the different microneedle insertions. The flat tip of a teflon rod pressed against the skin served as a negative control to account for possible pain associated with the teflon rod of the insertion device.
Figure 1.
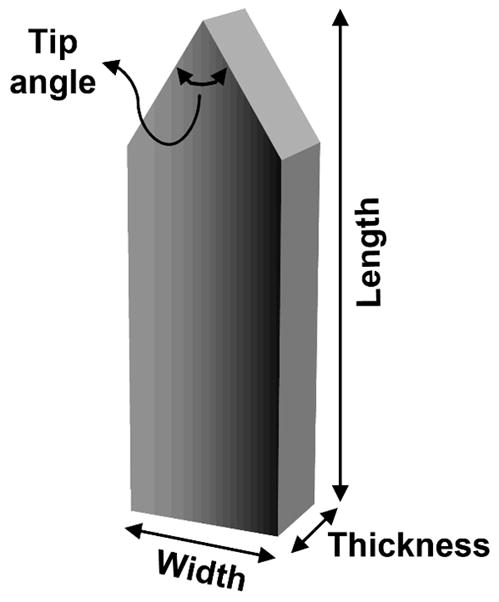
Schematic of a microneedle illustrating its typical geometry and characteristic dimensions of length, width, thickness and tip angle investigated during the pain study.
Because microneedle dimensions decrease from their AutoCAD design values during the laser cutting and electropolishing steps of the fabrication process, the final dimensions of the microneedles after fabrication were visually measured (n=5 for each dimension configuration) using a stereomicroscope (SZX12, Olympus America, Melville, NY, USA) and a precision micropositioning stage that was equipped with a digital readout display (Boeckeler Instruments, Tucson, AZ, USA). On average, the variability in the dimensions of the fabricated microneedles was low with a mean standard deviation of about 5% (range: 1 to 15%) for all the microneedle geometric parameters.
Statistical design
The study was carried out using the within-subject repeated measures design. In this design, all the subjects in a study received identical treatments, which allows for comparisons within the subjects, such that every subject acts as his or her own control 16. To avoid carryover effects (i.e. influence of previous insertions) caused by too many insertions on a subject during a single session, the study was conducted in two stages. Each stage consisted of 10 subjects. The different microneedle dimensions to be studied were divided between the two stages. In stage I, the effects of microneedle length and microneedle tip angle were investigated (Table 1); and in stage II, the effects of microneedle numbers, thickness and width were investigated (Table 1). All insertions were performed in triplicate for every subject.
Table 1.
Geometry of microneedles used in the pain study
| Length (μm) | Width (μm) | Thickness (μm) | Tip angle | Number of micronedles | ||
|---|---|---|---|---|---|---|
| Stage I | Length study | 480 ± 10 700 ± 20 960 ± 20 1450 ± 10 |
160 ± 10 | 45 ± 5 | 55° | 1 |
| Tip angle study | 480 ± 10 | 160 ± 10 | 45 ± 5 | 20° 55° 90° |
1 | |
| 960 ± 20 | 160 ± 10 | 45 ± 5 | 20° 55° 90° |
1 | ||
| Stage II | Number study | 620 ± 20 | 160 ± 10 | 45 ± 5 | 55° | 5 50 |
| Thickness study | 700 ± 20 | 160 ± 10 | 30 ± 10 45 ± 5 100 ± 5 |
55° | 1 | |
| Width study | 700 ± 20 | 160 ± 10 245 ± 10 465 ± 10 |
45 ± 5 | 55° | 1 | |
| Positive control* | 5 mm deep insertion of a 26 gauge hypodermic needle | |||||
| Negative control* | Flat end of a 3 mm diameter teflon rod | |||||
Positive and negative controls used in both stages of the pain study.
Randomization for bias reduction
To reduce investigator bias and subject preconceived bias to hypodermic needle pain, the subjects and the two investigators performing the study were blinded to the type of microneedle being inserted. Further, the location of insertions on the forearms was also randomized. This was done by stamping rectangular grids of dots onto the subjects’ volar forearms using a custom-designed rubber stamp (Dixie Seals and Stamps, Atlanta, GA, USA) to demarcate 40 treatment sites, and then randomly distributing the insertions amongst the treatments sites, with a single insertion per site.
Volunteer recruitment
Normal human subjects were recruited from the student and staff population at the Georgia Institute of Technology (Atlanta, GA, USA). Allergy to stainless steel was used as the subject exclusion criteria. In addition, individuals conducting research with microneedles were excluded from the study to reduce subject bias. The use of human subjects in the pain study was approved by the Institutional Review Boards at the Georgia Institute of Technology and Emory University. There were three males and seven females (18 to 40 years of age) in each stage of the pain study, with four subjects common to both stages of the study.
Insertion protocol
Upon receiving written consent of the subjects, the forearms of the subjects were cleaned using isopropanol swabs (Becton and Dickinson, Franklin Lakes, NJ, USA). Next, the rubber stamp was stamped on the forearms of the subjects. Subjects were then introduced to the use of the visual analog scale (VAS). Throughout the study, the same investigator performed the insertions and the same second investigator performed the pain score measurement.
Insertions were performed manually on the volar forearms of the subjects with a gap of at least 30 s between insertions. All treatment sessions began with the insertion of a microneedle (55° tip angle, 700 μm long, 160 μm wide and 45 μm thick), a negative control teflon rod and a positive control hypodermic needle, in that order, to help the subjects understand the use of the VAS and calibrate their responses to the range of sensations to be encountered. The remaining insertions were then performed according to the randomized sequence for each subject, and pain scores were recorded. Insertion sites were visually examined to note signs of skin reaction. If bleeding was observed after hypodermic needle insertions, a cotton swab dipped in isopropanol was immediately applied to stop the blood flow.
Upon completion of the insertions, the dot-grid was removed using isopropanol swabs. The subjects were contacted after 24 h to check for any adverse sensations or reactions during that period.
Measurement of visual analogue pain scores
Each subject was presented with a ruler containing a 100-mm slot with “No Pain” written at the left end and “Worst Pain” at the right end. There were no other markings visible to the subject. Immediately after each insertion, the blinded subjects were asked to move the slider to the place along the slot that best described his or her pain. The blinded observer recorded the location of the slider along the slot in millimeters, which was visible on the back side of the ruler.
Staining of insertion sites
To validate penetration of microneedles into the skin after insertion, three randomly-selected microneedle insertion sites on each subject of stage II of the pain study were stained with gentian violet (2% solution, Humco, Texarkana, TX, USA), a violet-colored topical antifungal agent that preferentially stains sites of microneedle penetration into skin, even after cleaning the skin with isopropanol. To prevent bias during insertion for sites to be stained, the investigator performing the insertions was blinded to the random sites requiring staining. Upon completion of all insertions, images of the stained sites were collected using a digital camera (DMC-TZ3S, Panasonic, Secaucus, NJ, USA).
Statistical analysis
VAS pain scores were analyzed for statistical significance using parametric tests on the basis of a previous study, which found no significant differences in conclusions between parametric and non-parametric tests analyzing VAS pain scores, even when the data were not normally distributed 17.
For each subject, average raw VAS pain scores (based on triplicate measurements) for each microneedle geometry were calculated and then normalized to the average raw hypodermic needle pain score to account for the variability in a subject’s perception of hypodermic needle pain and thereby provide a common reference point. Because all the negative controls had a VAS score of zero (no pain), no adjustment to the raw VAS scores was necessary to account for pain caused by the microneedle insertion device. The percentage of subjects reporting painless insertions (i.e., VAS = 0) for each microneedle geometry was also reported. Box plots for raw and normalized VAS pain scores were plotted to show the range and variation of the pain scores across the subjects (Minitab, ver. 15, State College, PA, USA). To identify if the microneedles and the 26 gage hypodermic needle produce significantly different pain sensations, an omnibus F-test was conducted for each stage of the pain study using the raw (unaveraged) VAS pain scores of all the microneedle configurations and the 26 gage hypodermic needle (repeated measures ANOVA test, NCSS, Kaysville, Utah, USA), where p<0.05 was considered statistically significant. When Mauchly’s test indicated that the compound symmetry assumption used in this test was invalid, the lower bound F-statistic was calculated and the corresponding corrected p-value was reported. Finally, Tukey’s multiple comparison test was used to identify microneedle configurations significantly different in pain level from the hypodermic needle.
To compare pain levels amongst different microneedle designs, the normalized VAS pain scores for each microneedle geometry were statistically tested using the repeated measures ANOVA method (NCSS), where p<0.05 was considered statistically significant, followed by Tukey’s multiple comparison test.
RESULTS
Microneedle fabrication and insertion into skin
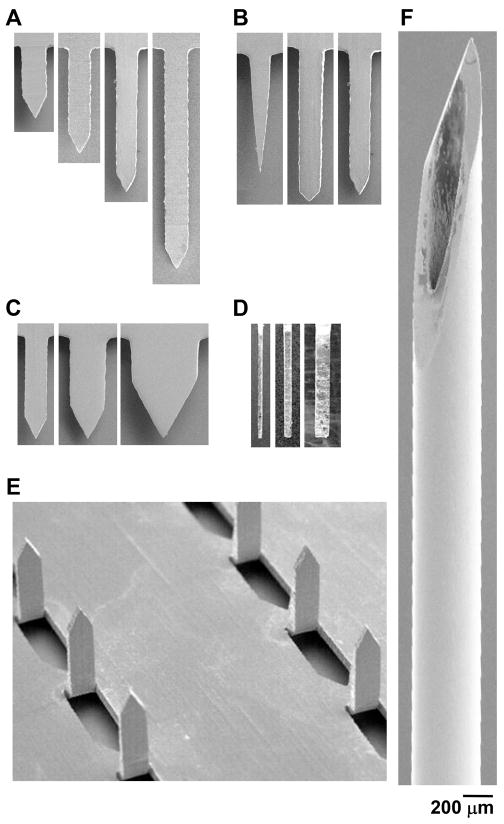
Microneedles were fabricated with a range of geometries in order to compare pain between microneedles and a hypodermic needle, and to quantify differences in pain resulting from different microneedle designs. To determine the effect of microneedle geometry, single microneedles of different lengths (Fig. 2A), widths (Fig. 2B), tip angles (Fig. 2C) and thicknesses (Fig. 2D) were fabricated. To study the influence of the number of microneedles, arrays of microneedles with 5 or 50 microneedles were also fabricated (Fig. 2E). To facilitate handling, single microneedles (Fig 3A) and arrays of microneedles (Fig. 3B and 3C) were mounted onto holders.
Figure 2.
Representative microneedles used for insertion. Scanning electron microscopy images of microneedles used in the length study (A), tip-angle study (B), width study (C), thickness study (side view) (D) and number of microneedles study (E). A 5 mm-long, 26-gage hypodermic needle was used as a positive control (F). All images are at the same magnification.
Figure 3.
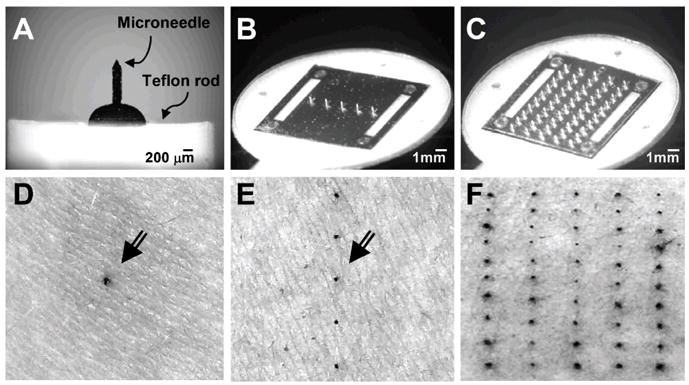
Microneedle devices and stained skin penetration sites. Brightfield microscopy images of microneedles assembled into devices: a single microneedle affixed to a teflon rod holder (A), a five-microneedle array assembled as an adhesive patch (B) and a 50-microneedle array assembled as an adhesive patch (C). Brightfield microscopy images of the skin surface of human forearms after inserting microneedles and applying gentian violet to stain the sites of microneedle insertion, which demonstrates microneedle penetration into the skin, using: a single microneedle (D), an array of five microneedles (E) and an array of 50 microneedles (F). Arrows in (D) and (E) point to the stained insertion sites.
To verify that microneedles penetrated into the skin, three randomly selected sites on each subject of stage II were stained with gentian violet as described in the Materials and Methods section. This protocol selectively stained sites of skin perforation, as shown in the representative images for a single microneedle (Fig. 3D), a microneedle array with 5 microneedles (Fig. 3E) and a microneedle array with 50 microneedles (Fig. 3F). Analysis of images from all of the stained sites on all subjects indicated successful microneedle penetration into the skin in all cases (data not shown).
Microneedles vs. 26-gage hypodermic needle
Our next objective was to determine if microneedles cause less pain than a 26-gage hypodermic needle. We compared the pain reported for a hypodermic needle to microneedles over a range of dimensions that varied length (480, 700, 960 and 1450μm), width (160, 245 and 465μm), thickness (30, 45 and 100μm), tip angle (20°, 55° and 90°) and the number of microneedles (5 and 50). A 5-mm deep insertion of a 26-gage hypodermic needle was used as a positive control. Although hypodermic needles are inserted clinically for example, 8 to 12 mm deep during vaccination 18 and insulin delivery 2,19, we restricted the insertion depth in this study to 5 mm in order to reduce the possibility of bleeding, which could complicate analysis.
Among stage I data, the raw VAS pain scores for microneedles ranged from a minimum of 2±2 mm (mean ± standard deviation) to a maximum of 15±17 mm, whereas the pain score for the hypodermic needle was 39±21 mm. In stage II, the microneedle pain scores ranged from a minimum of 2±2 mm to a maximum of 11±9 mm and the hypodermic needle pain score was 24±16 mm. Based on repeated measures ANOVA, each of the microneedles was found to have a pain score significantly smaller than the hypodermic needle positive control (Tukey’s pairwise comparison, p < 0.05). These results demonstrate that all the microneedles over a wide range of dimensions investigated in this study were less painful than the 26-gage hypodermic needle.
Effect of microneedle length
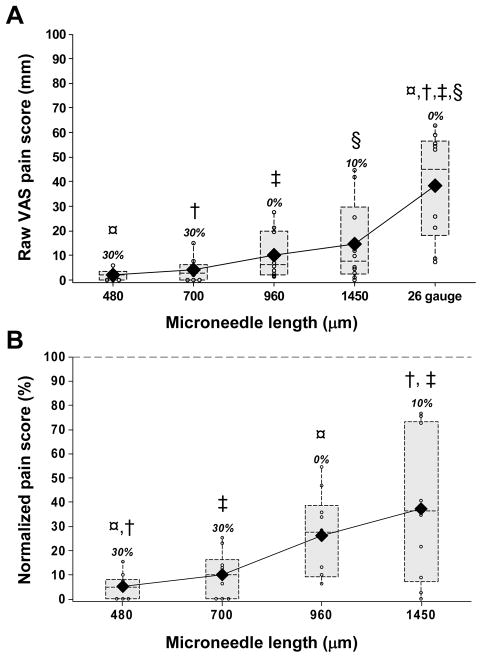
To study the dependence of pain on microneedle geometry, we hypothesized that increasing microneedle length should increase pain, because pain receptors innervate both the epidermis and the dermis, and longer penetrations should therefore excite more receptors 20. Consistent with this hypothesis, mean pain scores increased with increasing microneedle length, over a range of 2±2 mm for the shortest microneedles (480 μm) to 15±17 mm for the longest (1450 μm) (corrected lower bound p=0.03) (Fig. 4A). In addition to calculating mean pain scores, we also tabulated the fraction of subjects who reported each microneedle treatment as completely painless (i.e. VAS pain score = 0). This analysis showed that the frequency of painless insertions decreased with an increase in microneedle length: 30% of subjects reported both the 480 μm and the 700 μm long microneedles as completely painless, whereas just 0% and 10% reported the 960 μm and 1450 μm long microneedles painless, respectively.
Figure 4.
The effect of microneedle length. Box plots of pain scores after insertion of 480, 700, 960 and 1450 μm long single microneedles (160 μm wide, 45 μm thick and a tip angle of 55°): raw visual analog scale (VAS) pain scores (A) and the normalized pain scores (B), which were calculated as the ratio of the microneedle raw VAS score and the 26-gage hypodermic needle raw VAS pain score for the same subject. The normalized pain score of the hypodermic needle (i.e., 100%) is represented by the horizontal dotted line in (B). The small open circles represent individual data points. Each dotted rectangular box represents the interquartile range (i.e., 25 – 75%) of the pain score for a particular microneedle length, with a horizontal line at the median value. The vertical lines (whiskers) extend from the box boundary to the maximum and the minimum data points within one and a half times the interquartile range. The solid diamonds represent the mean pain scores for each insertion. The numbers above each box present the percentage of subjects who reported the insertions to be painless (i.e., VAS pain score of zero).
Although raw pain scores generally showed variability among subjects, hypodermic needle pain was especially variable, ranging from 7 mm to 63 mm, with a mean value of 39 mm. Because of this large inter-subject variability in pain perception, we re-analyzed the data by normalizing all the microneedle VAS scores from each subject to that subjects’s own hypodermic needle control. In this way, all data are presented relative to a common reference pain level that is familiar to most people and of direct relevance to medical applications.
Carrying out a more detailed analysis using these normalized pain scores indicated that the 480μm long microneedles produced just 5% of the pain of the 26-gage hypodermic needle (Fig. 4B). Increasing microneedle length sharply increased pain, such that a three-fold increase in the microneedle length from 480 μm to 1450 μm caused the pain to increase more than seven fold from 5% to 37% (corrected lower bound p = 0.013).
Comparing pairwise among the microneedles: (i) increasing microneedle length by at least 700μm (i.e., 480 μm vs. 1450 and 750 μm vs. 1450 μm) significantly increased pain levels (Tukey’s multiple comparison, p < 0.002); increasing microneedle length by 480 – 490 μm sometimes increased pain significantly (i.e., 480 μm vs. 960 μm, p = 0.02) and sometimes did not (960 μm vs. 1450 μm, p = 0.38); and (iii) increasing microneedle length by up to 260 μm (i.e., 480 μm vs. 700 μm and 700 μm vs. 960) did not significant increase pain (Tukey’s multiple comparison, p > 0.1). This analysis suggests that the threshold for distinguishing differences in pain due to increment in microneedle length was approximately 500μm.
Effect of the number of microneedles
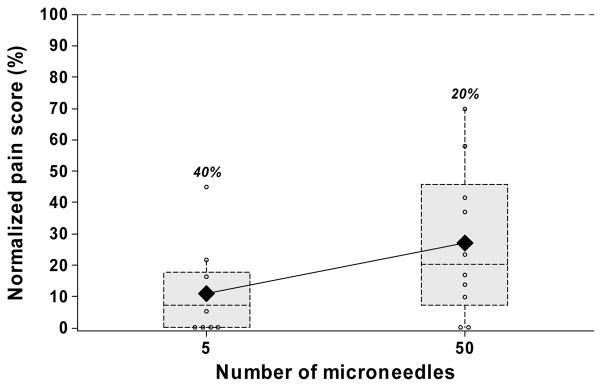
Drug and vaccine delivery applications will often require multiple microneedles. We hypothesized that increasing the number of microneedles should increase pain, because more sensory nerves would be excited using a larger number of microneedles. Microneedle arrays with 5 or 50 microneedles were used to test this hypothesis. Insertion of a 5-microneedle array caused pain at a low level, corresponding to 10% of the hypodermic needle (Fig. 5). A 10-fold increase in the number of microneedles to a 50-microneedle array produced a relatively small increase in the pain of just 2.5 fold (p=0.004), which corresponded to 25% of the hypodermic needle. The arrays with 5 and 50 microneedles were reported to be completely painless by 40% and 20% of the subjects, respectively.
Figure 5.
The effect of the number of microneedles. Box plots of normalized pain scores after insertion of microneedle arrays having 5 and 50 microneedles. All microneedles were 620 μm long, 160 μm wide, 45 μm thick and had a tip angle of 55°.
Effect of microneedle tip angle
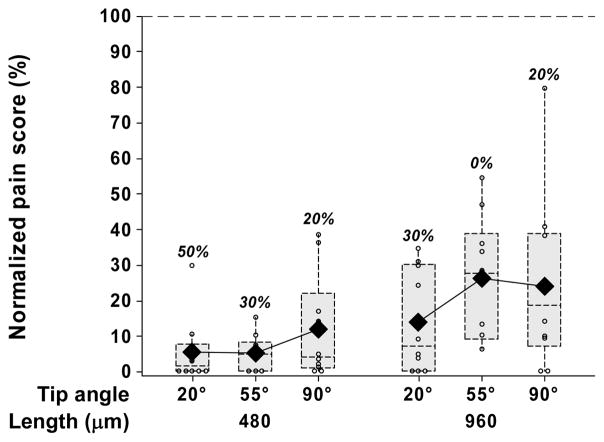
We next hypothesized that larger tip angles would cause more tissue deformation during microneedle insertion and thereby cause more pain than microneedles with smaller tip angles. To test this hypothesis, we examined microneedles with tip angles of 20°, 55° and 90°, each at two different lengths of 480 μm and 960 μm.
At both microneedle lengths tested, no consistent or statistically significant (p > 0.12) relationship was observed between mean pain scores and microneedle tip angle over the relatively large range of tip angles considered (20° to 90°) (Fig. 6). For the 480 μm long microneedles, the percentage of subjects reporting completely painless insertions decreased with increasing tip angles, but there was no such trend for the 960 μm long microneedles (Fig. 6). Additional experiments using 960 μm long microneedles at the same tip angles, but with an increased microneedle thickness from 40 μm to 100 μm similarly showed no dependence of pain on microneedle tip angle (data not shown).
Figure 6.
The effect of microneedle tip angle. Box plots of normalized pain scores after insertion of 480 and 960 μm long single microneedles each with a tip angle of 20°, 55° and 90°. All microneedles were 160 μm wide and 45 μm thick.
This finding was contrary to our original hypothesis. To understand this observation, we examined the microneedle tips using high-magnification scanning electron microscopy and found them to be extremely sharp, with a radius of curvature less than 1 μm independent of tip angle. This suggests that pain may correlate with sharpness at the very tip of the microneedle, rather than the overall angle of the tip. A previous study found that the force required for microneedle insertion into skin also scaled with microneedle tip sharpness 21, which indicates that the force of insertion may correlate with pain. This would be consistent with previous studies involving hypodermic needles, which found that the intensity of pain scales with mechanical workload (area under the force-displacement curve) of hypodermic needle insertion 22,23. Altogether, these observations suggest a new hypothesis that pain depends primarily on the force of microneedle tip insertion and the depth to which the microneedle tip penetrates into the skin.
Effect of microneedle thickness and width
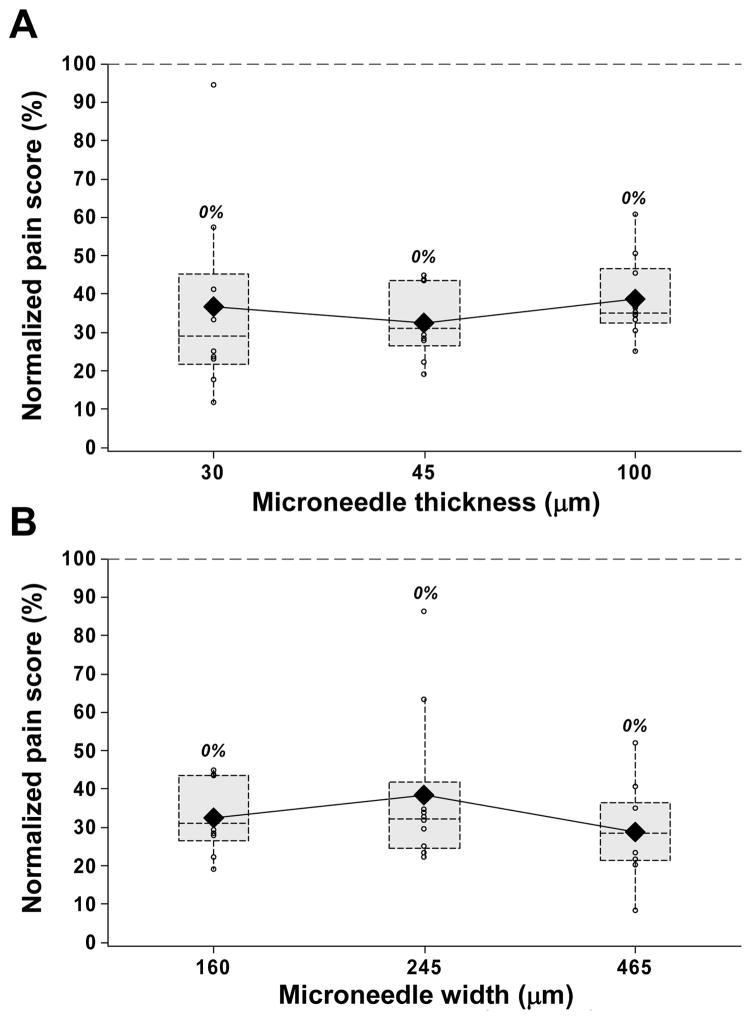
Our last hypothesis was that increased microneedle thickness and width would cause more pain by engaging more pain receptors during insertion. To test this hypothesis, microneedles with thicknesses of 30, 45 and 100 μm and widths of 160, 245, and 465 μm were examined. In contrast to this hypothesis, neither increasing microneedle thickness by more than three fold (p = 0.6, Fig. 7A) nor increasing microneedle width by almost three fold (p = 0.30, Fig. 7B) had a significant effect on pain scores.
Figure 7.
The effect of microneedle thickness and width. Box plots of normalized pain scores after insertion of 30, 45 and 100 μm thick single microneedles each 700 μm long, 160 μm wide and with a tip angle of 55° (A); and 160, 245 and 465 μm wide single microneedles each 700 μm long, 45 μm thick and with a tip angle of 55° (B).
These observations are, however, consistent with previous reports that pain is independent of needle diameter for lancets with diameters of 800, 400 and 300 μm inserted to a depth of 900 μm 24. They are also consistent with the new hypothesis that pain depends primarily on the force of microneedle tip insertion and not on other features of the microneedle shaft geometry other than length. In contrast, a study of hypodermic needles found that the likelihood of an insertion being painful increased with increasing needle diameter, but also noted that the intensity of those insertions rated as painful was insensitive to needle diameter 25.
Skin reaction to insertions
In addition to pain, microneedle insertions into the skin could cause skin irritation. Visual observation of the skin immediately after microneedle insertion revealed highly localized, mild erythema in the form of a light pink dot less than 1 mm across, which was observed at all microneedle insertion sites independent of microneedle geometry. Although this redness could be observed upon direct examination, essentially no cosmetic effect was evident when subjects were viewed from a distance. The erythema decreased somewhat during the 2 h study period and was self-reported by the subjects to be mostly resolved when they were contacted after 24 h. There were no signs of edema after any microneedle insertions.
The appearance of a tiny droplet of blood (e.g., 1 μl) was observed at the insertion site after some microneedle insertions, especially those involving 1450 μm long microneedles. The shorter microneedles (i.e. 480 and 700 μm) did not result in bleeding.
DISCUSSION
Degree of pain reduction
This study shows that microneedles caused significantly less pain than a 26-gage hypodermic needle. Using the shortest microneedles, pain scores were reduced by a factor of 20 compared to the hypodermic needle. Overall, pain scores from the diversity of microneedle geometries considered ranged from 5 to 40%, which was highly significant by statistical analysis. This level of pain reduction could also be significant to reduce needle anxiety and phobia.
Even though the pain reduction reported in this study is considerable, the degree of pain reduction from microneedle-based drug delivery is expected to be still greater. First of all, hypodermic needles are typically inserted 8 to 12 mm deep into the skin 2,18,19. In this study, we limited the insertion depth to just 5 mm to minimize bleeding. Because pain is known to depend strongly on device insertion depth, as shown in this study and in the literature 26, the actual pain caused by hypodermic needles in clinical practice is expected to be greater than in this study and thus the relative reduction in pain by using microneedles should also be greater.
In addition, the act of injecting fluid into the skin can itself cause pain, which means that the positive control hypodermic needle insertion used in this study is an even greater underestimate of the pain caused by hypodermic injection. Because solid microneedles do not involve fluid injection for drug delivery, the pain associated with fluid injection would also be eliminated. For these reasons, the relative decrease in pain enabled by the use of microneedles reported in this study is based on a conservative study design. Even greater pain reduction should be expected from drug delivery using solid microneedles as compared to actual hypodermic injections.
It should be noted that this analysis does not take into account pain that could be caused by local irritation by the drug formulation. Solid microneedles deliver a solid drug formulation into the skin, whereas hypodermic injection delivers a liquid drug formulation typically into deeper tissues. These differences may further influence pain in some cases.
Microneedle device optimization
Optimization of a microneedle device requires selecting microneedle dimensions and overall design that meets a number of constraints, including minimizing pain, providing sufficient mechanical strength for insertion into the skin, and delivering the required dose in a manner suitable for the target population. To guide optimization that minimizes pain, this study supported the hypothesis that decreasing microneedle length and the number of microneedles reduces pain. It also showed that microneedle tip angle, thickness and width did not have a significant effect on pain score. Altogether, these observations suggested an additional hypothesis that pain depends primarily on the force of microneedle tip insertion and the depth to which the microneedle tip penetrates into the skin, but not on other features of the microneedle shaft geometry besides length. Validation of this new hypothesis requires additional study.
These findings suggest that an optimal microneedle design should involve a small number of short microneedles. Due to the much steeper dependence of pain on microneedle length compared to the number of microneedles, minimizing length should be especially important. For ease of insertion 21 and possible reduction in pain, microneedle tips should also be sharp. Although microneedle thickness, width and tip angle do not appear to affect pain, they do affect microneedle mechanical strength 21,27 and therefore need to be considered.
Reduction of anxiety and needle phobia
The microscopic dimensions of microneedles may further decrease the perception of pain due to their patient-friendly appearance. It is well known that pain from the use of hypodermic needles can produce poor patient compliance and that needle phobia produces stress, anxiety and vasovagal reaction, which can interfere with treatments that use needles 2,3,5. Recent research using stress-reducing medical devices involving decorative and aesthetically pleasing syringes has shown significant reduction in needle phobia 28. Microneedles should offer similar advantages, due to their small size, inconspicuous profile and ability to be incorporated into Band Aid-like patches that can be aesthetically pleasing and easy to use. Although this blinded study did not assess this aspect of microneedles, we expect microneedle patches to be patient friendly and thereby further reduce anxiety and the perception of pain.
In conclusion, this study demonstrates that microneedles over a wide range of dimensions are significantly less painful than a 26-gage hypodermic needle and that decreasing microneedle length and number of microneedles reduces pain. These findings give insight into the thresholds and parameters that control pain due to micron-scale trauma to the skin and provide a rational basis to optimize microneedle geometry for drug delivery applications that minimize pain.
Acknowledgments
Funding source: This work was supported in part by the National Institutes of Health.
We would like to thank Dr. Mark Allen for use of the lasers in his lab and Richard Shafer, Dr. Shawn Davis, and Ed Birdsell for helpful discussions regarding laser operation. MRP is the Emerson-Lewis Faculty Fellow. This work was supported in part by the National Institutes of Health and took place in the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology.
References
- 1.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–7. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Hanas R. Reducing injection pain in children and adolescents with diabetes: a review of indwelling catheters. Pediatr Diabetes. 2004;5(2):102–11. doi: 10.1111/j.1399-543X.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract. 1995;41(2):169–75. [PubMed] [Google Scholar]
- 4.Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord. 2006;20(7):946–60. doi: 10.1016/j.janxdis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg. 2003;68(3):341–4. [PubMed] [Google Scholar]
- 6.Hauri A, Armstrong G, Hutin Y. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15(1):7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 7.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–24. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 8.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100(24):13755–60. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabri F, Bouris K, Jones T, Barrow D, Hann A, Allender C, Brain K, Birchall J. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br J Dermatol. 2004;150(5):869–77. doi: 10.1111/j.1365-2133.2004.05921.x. [DOI] [PubMed] [Google Scholar]
- 10.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–80. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 11.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–52. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 12.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8(4):415–9. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14(4):375–81. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92(2):502–4. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 15.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–37. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Homewood, IL: McGraw Hill/Irwin; 1996. [Google Scholar]
- 17.Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology. 1995;82(4):896–902. doi: 10.1097/00000542-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 18.CDC. Centers for Disease Control and Prevention. General recommendations on immunization. MMWR Recommendations and Reports. 2006;55:RR-15. [Google Scholar]
- 19.Hanas R, Lytzen L, Ludvigsson J. Thinner needles do not influence injection pain, insulin leakage or bleeding in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2000;1(3):142–9. doi: 10.1034/j.1399-5448.2000.010305.x. [DOI] [PubMed] [Google Scholar]
- 20.Oaklander AL, Siegel SM. Cutaneous innervation: Form and function. J Am Acad Dermatol. 2005;53(6):1027–37. doi: 10.1016/j.jaad.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 21.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37(8):1155–63. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3(1):41–49. doi: 10.1053/eujp.1998.0099. [DOI] [PubMed] [Google Scholar]
- 23.Egekvist H, Bjerring P, Arendt-Nielsen L. Regional variations in pain to controlled mechanical skin traumas from automatic needle insertions and relations to ultrasonography. Skin Res Technol. 1999;5(4):247–54. [Google Scholar]
- 24.Fruhstorfer H, Schmelzeisen-Redeker G, Weiss T. Capillary blood sampling: relation between lancet diameter, lancing pain and blood volume. Eur J Pain. 1999;3(3):283–86. doi: 10.1053/eujp.1999.0132. [DOI] [PubMed] [Google Scholar]
- 25.Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23(1–2):37–43. doi: 10.1080/08990220600700925. [DOI] [PubMed] [Google Scholar]
- 26.Fruhstorfer H, Müller T, Scheer E. Capillary blood sampling: How much pain is necessary?. Part 2: Relation between penetration depth and puncture pain. Pract Diabetes Int. 1995;12(4):184–85. [Google Scholar]
- 27.Park J-H, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Kettwich SC, Sibbitt WL, Jr, Brandt JR, Johnson CR, Wong CS, Bankhurst AD. Needle phobia and stress-reducing medical devices in pediatric and adult chemotherapy patients. J Pediatr Oncol Nurs. 2007;24(1):20–8. doi: 10.1177/1043454206296023. [DOI] [PubMed] [Google Scholar]