Abstract
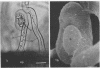
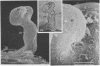
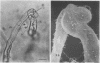
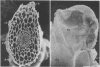
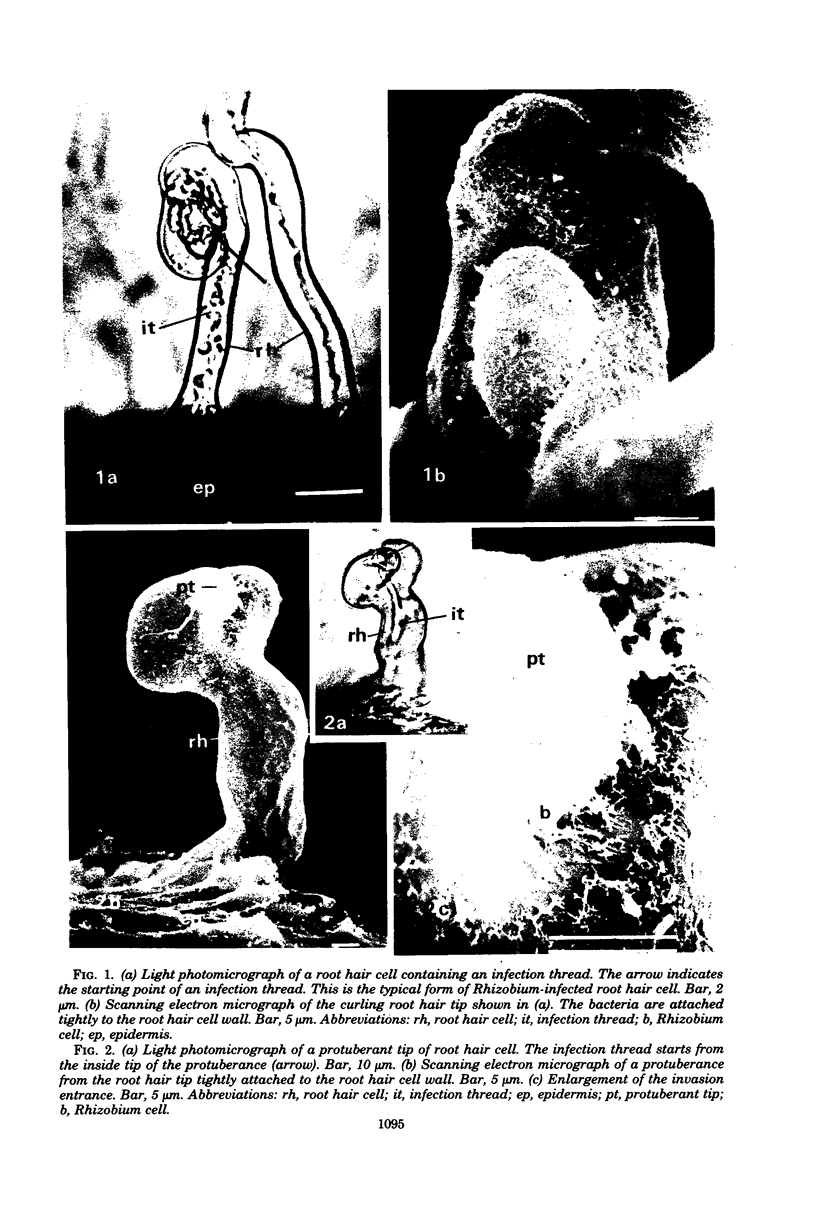
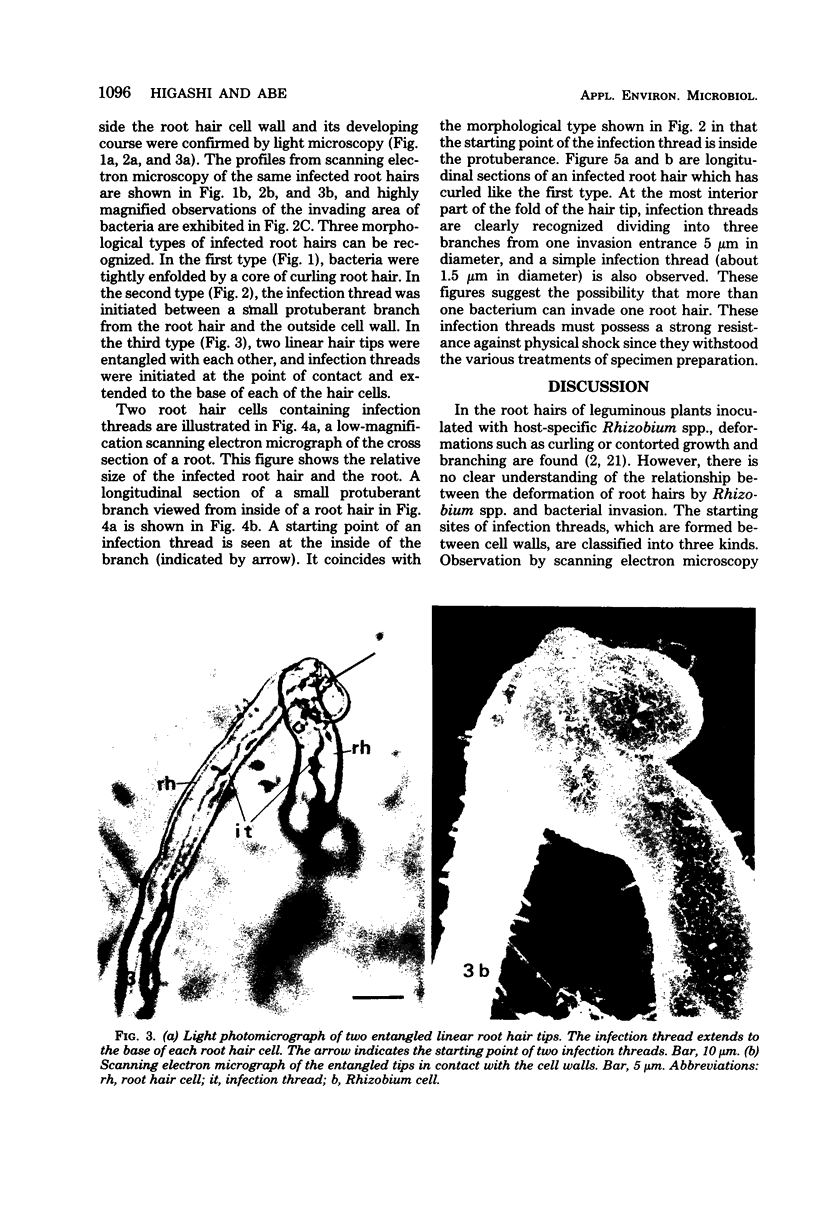
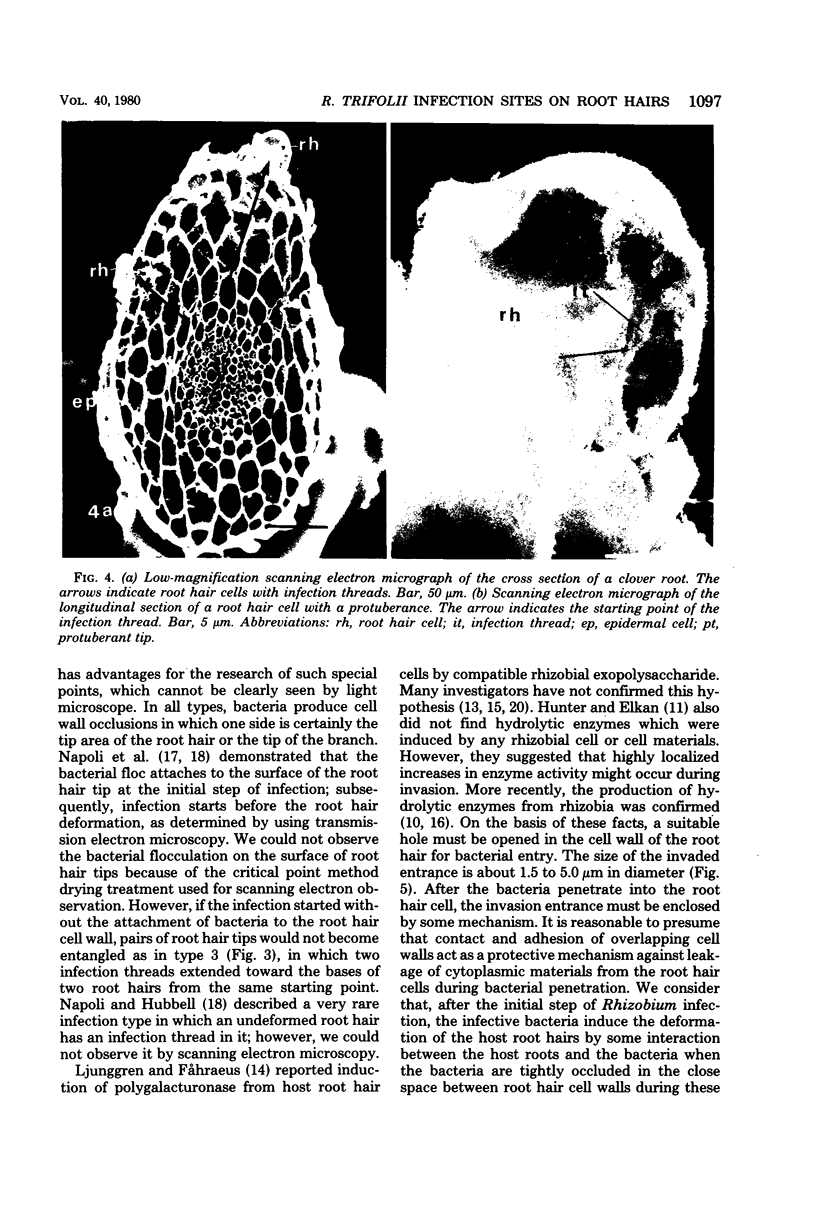
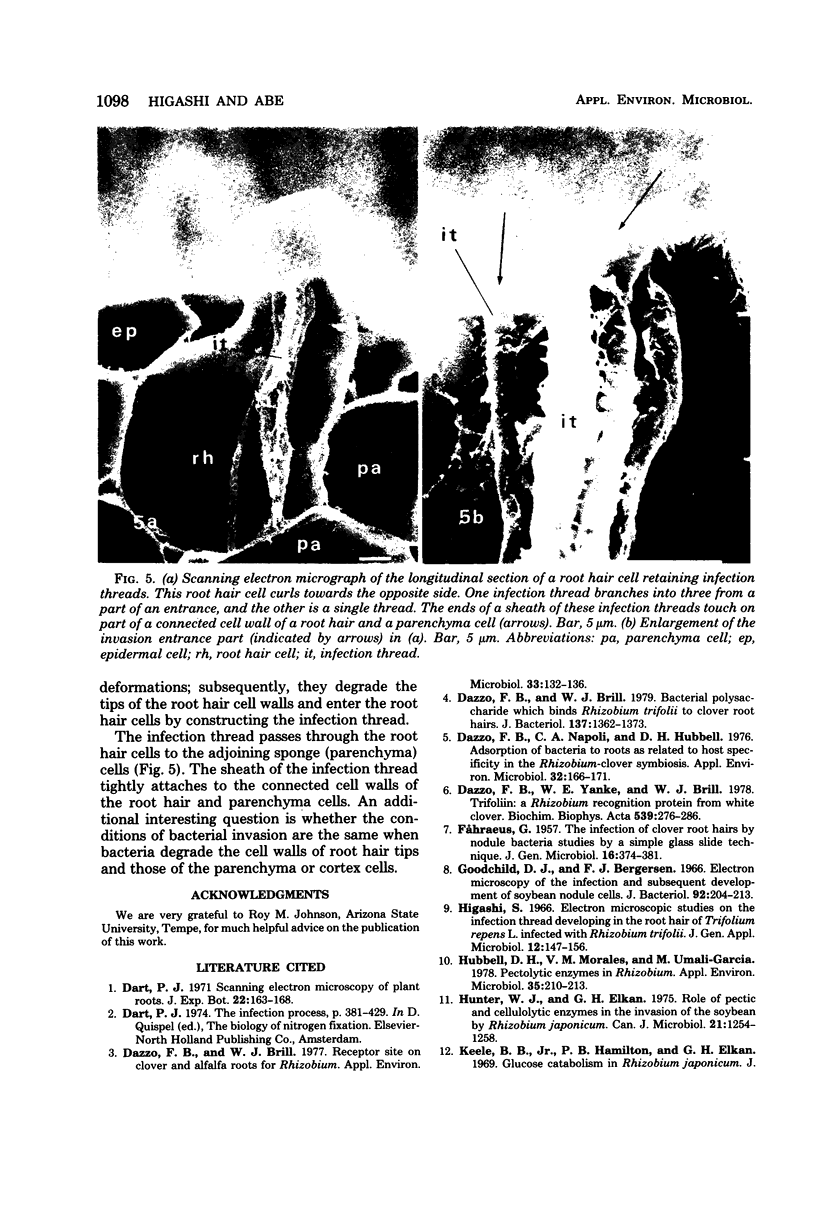
White clover root hairs which were inoculated with Rhizobium trifolii 4S (infectious strain) contained infection threads which were observed by light microscopy and scanning electron microscopy. Three morphological types of root hairs retaining infection threads were recognized. The bacteria were strongly attached between the surfaces of two plant cell walls as follows: between surfaces of a root hair tip curled back on itself, between a protuberance from a root hair and its cell surface, or between two root hair tips clinging together. An anatomical analysis documented the attachment site of the infection thread sheath from the inside of the root hair cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Receptor site on clover and alfalfa roots for Rhizobium. Appl Environ Microbiol. 1977 Jan;33(1):132–136. doi: 10.1128/aem.33.1.132-136.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Bergersen F. J. Electron microscopy of the infection and subsequent development of soybean nodule cells. J Bacteriol. 1966 Jul;92(1):204–213. doi: 10.1128/jb.92.1.204-213.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell D. H., Morales V. M., Umali-Garcia M. Pectolytic enzymes in Rhizobium. Appl Environ Microbiol. 1978 Jan;35(1):210–213. doi: 10.1128/aem.35.1.210-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. J., Elkan G. H. Role of pectic and cellulolytic enzymes in the invasion of the soybean by Rhizobium japonicum. Can J Microbiol. 1975 Aug;21(8):1254–1258. doi: 10.1139/m75-187. [DOI] [PubMed] [Google Scholar]
- LJUNGGREN H., FAHRAEUS G. The role of polygalacturonase in root-hair invasion by nodule bacteria. J Gen Microbiol. 1961 Nov;26:521–528. doi: 10.1099/00221287-26-3-521. [DOI] [PubMed] [Google Scholar]
- Lillich T. T., Elkan G. H. Evidence countering the role of polygalacturonase in invasion of root hairs of leguminous plants by Rhizobium spp. Can J Microbiol. 1968 Jun;14(6):617–625. doi: 10.1139/m68-104. [DOI] [PubMed] [Google Scholar]
- Macmillan J. D., Cooke R. C. Evidence agsinst involvement of pectic enzymes in the invasion of root hairs by Rhizobium trifolii. Can J Microbiol. 1969 Jun;15(6):643–645. doi: 10.1139/m69-111. [DOI] [PubMed] [Google Scholar]
- Martinez-Molina E., Morales V. M., Hubbell D. H. Hydrolytic enzyme production by Rhizobium. Appl Environ Microbiol. 1979 Dec;38(6):1186–1188. doi: 10.1128/aem.38.6.1186-1188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C. A., Hubbell D. H. Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl Microbiol. 1975 Dec;30(6):1003–1009. doi: 10.1128/am.30.6.1003-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]