Abstract
Background
Hyperbaric oxygen (HBO) decreases ischemia-reperfusion (IR) induced neutrophil-ICAM adhesion by blocking CD18 polarization. The purpose of this study was to evaluate whether this HBO effect is nitric oxide (NO) dependent and to determine if NO synthase (NOS) is required.
Methods
A gracilis muscle flap was raised in 9 groups of male Wistar rats. Global ischemic injury was induced by clamping the gracilis muscle pedicle artery and vein for 4 hours. The HBO treatment consisted of 100% O2 at 2.5 ATA during the last 90 minutes of ischemia. Groups were repeated with and without various NOS inhibitors and C-PTIO, a NO scavenger. Normal neutrophils (PMNs) were exposed to activated plasma on ICAM coated coverslips (% adherent) and labeled with FITC-anti-rat-CD11b for confocal microscopy (% polarized). The percent of adherent and polarized cells was reported as mean ± SEM. Statistical Analysis was by ANOVA. A p ≤ 0.05 was considered significant.
Results
C-PTIO treated IR-HBO plasma showed a significant increase in the percent polarization of CD18 compared to IR-HBO untreated plasma from 4.1±2.5 to 33.7±7.7 (p ≤ 0.05). The NO scavenger, C-PTIO, also increased the percent of adherent cells from 1.6±0.4 to 20.3±5.9 (p ≤ 0.05). Administration of LNAME and other NOS inhibitors prior to HBO treatment restored neutrophil adhesion and CD18 polarization to IR control values, significantly greater than IR-HBO alone.
Conclusions
These results suggest that the HBO reduction of IR induced neutrophil polarization of CD18 and adherence to ICAM is mediated through a nitric oxide mechanism that requires NOS.
Ischemia reperfusion (IR) injury can be a serious consequence of diseases and procedures where blood flow is interrupted, including myocardial infarction, cerebral ischemic stroke, transplantation, replantation, and free tissue transfer. Restoration of blood flow to ischemic tissue initiates the inflammatory cascade which increases the injury. The prevention, diagnosis and treatment of IR injury are important in order to improve survival and function of affected tissues. Hyperbaric oxygen (HBO) treatment in myocardial infarction,1,2 ischemic stroke 3 and skeletal muscle ischemia 4 have shown a positive effect on tissue survival, however, the absence of an elaborated mechanism of action has hindered wide acceptance of HBO treatment for IR injury.
Ischemia and reperfusion lead to injury of the microvasculature resulting in cell damage and death.5,6 Early efforts to explain this injury, based theories on the deleterious effects of oxygen free radicals produced by xanthine oxidase.7,8 Later experiments demonstrated the pathophysiologic role of free radicals produced from activated neutrophils.9,10
A morphologic analysis of skeletal muscle during IR showed an increase in the number of rolling and adherent neutrophils to the endothelium of the postcapillary venules compared with nonischemic controls.6 These adherent neutrophils release free radicals that damage the endothelium and provide a nidus for platelet aggregation.
Attachment of the neutrophil to venular endothelium is dependent upon the CD18 protein complex. This complex needs to be functionally present for neutrophil attachment to the intercellular adhesion molecule-1 (ICAM-1) found on endothelial cells.11 Larson, et. al.12 reported that neutrophils exposed to IR tissue show an up-regulation of CD18 protein on the neutrophil surface. 12 More importantly, IR induced CD18 molecule aggregates become polar by concentrating the surface protein to one area of the neutrophil making the cell more apt to attach to the venular endothelium.13 It has been reported that clustering of the adhesion molecules is closely associated with firm adhesion of leukocytes through the surface membrane CD18 integrin.14–16
Hyperbaric oxygen (HBO) treatment has been shown to decrease edema and necrosis in ischemic skeletal muscle.17–19 With microcirculation studies, researchers found a reduction in the number of leukocytes adherent to venular endothelium in groups treated with HBO during and immediately following the ischemic event.6 Further studies showed that HBO treatment prevented the neutrophil CD18 surface protein polarization and, therefore, the neutrophil attachment to the venular endothelium.20
Important molecules in cell signal transduction during IR include nitric oxide synthase (NOS), cGMP, and guanyl cyclase. Nitric oxide (NO) has been shown to decrease neutrophil-endothelial adhesion and improve survival rate when L-Arginine (L-Arg), a NO precursor, was infused into ischemic rectus femoris and gracilis muscles.21 Conversely, when ischemic rectus femoris was infused with L-nitro-aminomethylamine (L-NAME), a nitric oxide synthase inhibitor, the percent necrosis was unchanged from ischemic conditions.21,22 Studies by Elayan23 showed an increase in NO formation in the brain with HBO treatment. Hyperbaric oxygen has also been shown to decrease PMN cGMP24, and may interfere with guanylate cyclase interference of CD18 function.25 Nitric oxide, cGMP and guanylate cyclase have all been implicated in cytoskeleton rearrangement.24–26
Integrating the progression of the HBO and NO studies led to the purpose of this study which is to evaluate the role of nitric oxide in HBO mediated inhibition of CD18 surface polarity, and neutrophil adhesion, in vitro. The in vitro polarization and adhesion assays, with activated plasma from in vivo HBO treated animals, were used with and without carboxy-PTIO, a NO scavenger, to evaluate the role of nitric oxide in HBO mediated inhibition of CD18 surface polarity and neutrophil adhesion. Nitric Oxide synthase inhibitors (L-NAME, 7-NI, 1400W), were infused into HBO treated animals to assess the involvement of NOS isoforms in the HBO effect on CD18 polarization and adhesion.
Methods
This animal model and protocol were approved by the University Institutional Animal Care and Use Committee.
Rat Gracilis Muscle Model
Male Wistar rats weighing 250g ±17 were anesthetized with pentobarbital (50mg/kg, ip.) and remained anesthetized for the entire experiment, with pentobarbital supplementation (10mg/kg) as required. The right thigh musculature and femoral vasculature were exposed, and the gracilis muscle was dissected free on its vascular pedicle, using standard microsurgical technique. To raise this axial pattern flap, the epigastric and popliteal vessels and several gracilis muscle penetrators, as well as the obturator nerve, were divided.
Global ischemic injury was induced by clamping the pedicle (femoral artery and vein) for 4 hours. To simulate reperfusion the clamp was removed. The animals were randomly assigned to one of nine plasma groups: 1) non-ischemic control, 2) IR (4 hrs of ischemia, 15 min of reperfusion), 3) IR-HBO (100% O2 at 2.5 ATA during the last 90 min of ischemia), 4) IR-LNAME, 5) IR-HBO-LNAME, 6) IR-1400W, 7) IR-HBO-1400W, 8) IR-7NI, 9) IR-HBO-7NI. Plasma groups 1 and 3 were analyzed with (2 × 10−4 M) and without C-PTIO, a nitric oxide (NO) scavenger,27–29 by FITC MAB CD11b labeling and confocal microscopy. The plasma groups with C-PTIO were compared to plasma groups without C-PTIO using ANOVA. Groups 4–9 were treated with NOS inhibitors during the last 45 min of ischemia at the following doses; L-NAME and 1400W; 10mg/kg min, iv and 7-NI; 25mg/kg, ip. One cubic centimeter of blood was collected from each flap in a time-controlled manner, using a 25-gauge angiocatheter. The blood was centrifuged and the plasma was separated for the adhesion and polarization assays.
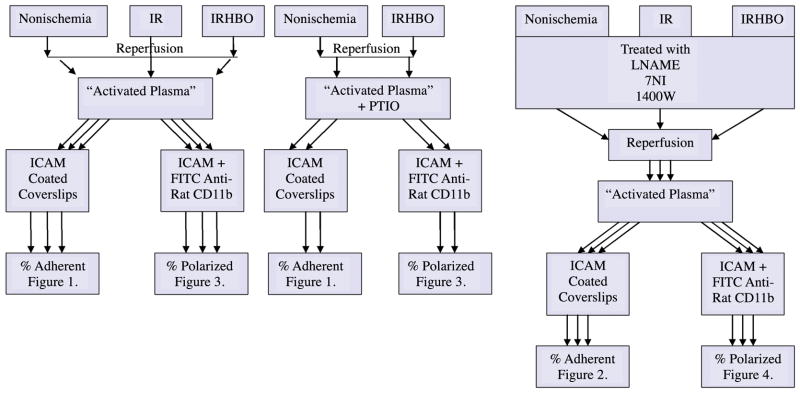
Figure 1 is a schematic diagram of the study design including methods and experimental groups.
Figure 1.
A schematic diagram of the study design including methods and experimental groups.
Hyperbaric Oxygen Treatment
Hyperbaric oxygen treatment consisted of 100% O2 at 2.5 ATA during the last 90 minutes of ischemia. (Model 1300, Sechrist Industries, Inc., Anaheim, CA)
Neutrophil separation13
Briefly, heparinized whole blood was withdrawn from normal Wistar rats (no surgical intervention). The neutrophils were separated by a Ficoll-hypaque density gradient (Sigma Chemical, Inc., St Louis, MO) and centrifugation. The neutrophil layer was centrifuged at 1500 rpm for 10 min to pellet the cells. The pellet was resuspended in 300 μl PBS with 2% goat serum (PBS+). These cells were counted by hemacytometer and used in the adhesion and polarization assays.
Adhesion Assay13
The adhesion assay was performed using normal neutrophils isolated from a neutrophil donor and plasma isolated from the gracilis muscle flap of the animals in each plasma group. The nonischemic control and the IR-HBO plasma were analyzed with and without C-PTIO.
The collected plasma from all the respective groups was placed with neutrophils from the neutrophil donor. This neutrophil/plasma mixture was incubated for 30 minutes at 37°C on ICAM-coated coverslips. The coverslips were gently washed with PBS+ to remove nonadherent cells. The coverslips were then placed under a light microscope and the adherent neutrophils were manually counted (400x). The number of adherent cells was divided by the total number of cells placed on the slide × 100 to get the percent adherence.
Polarization 13
The CD18 polarization assay was performed with the nonischemic control and the IR-HBO plasma groups with and without C-PTIO treatment and the six plasma groups with the NOS inhibitors. The neutrophils were incubated with the plasma in the presence of sICAM at 37°C for 30 minutes, centrifuged for 10 min at 1500 rpm, and washed with PBS+. The cells were labeled with FITC anti-rat CD11b protein for 30 minutes at 37°C. The neutrophils were washed 3 times with PBS+ and fixed with 1% paraformaldehyde in PBS. The neutrophils were then placed on a microscope slide and visualized under a Zeiss confocal microscope (Carl Zeiss, Inc, New York). Twenty consecutive isolated cells per assay were analyzed for mean fluorescent intensity.
Microscope analysis 13
The Z-stack analysis (LSM 510) was used to visualize 10 cross section views of the neutrophil. The outside surface of the neutrophil was divided into 3 equal areas and mean fluorescence intensity (I) of each area was recorded. Polarization of CD18 was calculated using the following formula: I Area 1/(I Area 2 + I Area 3) where Area 1 is the area with the highest intensity of fluorescence. Polarization is defined as two times the fluorescent intensity in one area compared to the sum of the remaining areas.13
Statistics
The results are reported as mean ± SEM of the raw data. Analysis of variance for repeated measures was used to evaluate the statistical difference between the groups and a Dunnett’s Post Hoc analysis was performed to determine between group differences. Arc sine transformations of the raw data were performed to meet the assumptions of ANOVA. A p ≤ 0.05 was considered significant.
Results
Polarization
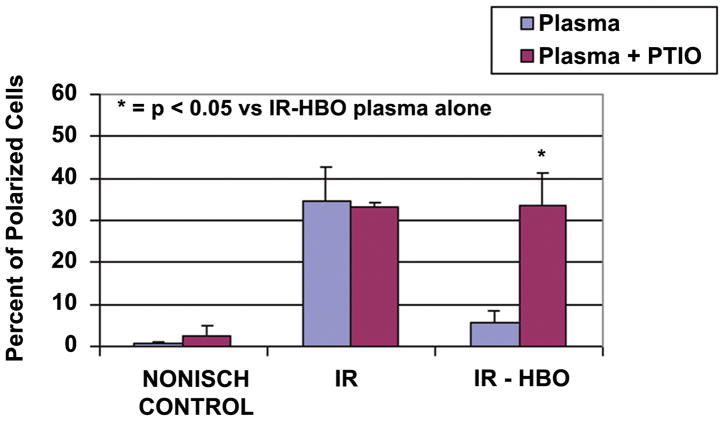
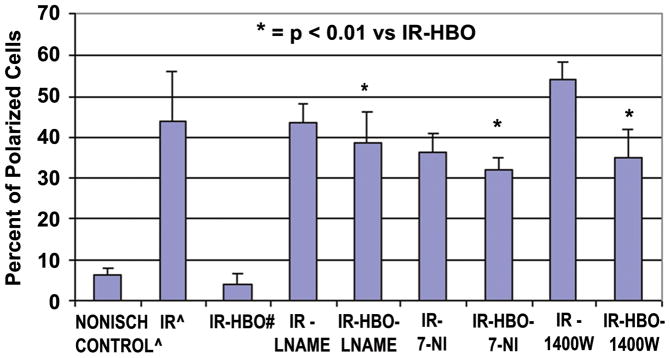
The pretreatment of plasma with the NO scavenger, C-PTIO, did not change the percent of polarized cells in the control plasma group. Conversely, C-PTIO treatment significantly reversed the effects of the HBO treatment in the IR-HBO treated group (33.7 ± 7.7 vs. 4.1 ± 2.5, p ≤0.05) (Figure 2). The isoform-selective inhibitors of NOS (Figure 3), L-NAME (ecNOS)38,39, 7-NI(nNOS),38,40,41 and 1400W(iNOS),38,42,43 used in conjunction with HBO treatment significantly increased (p < 0.01) polarization of CD18 compared to HBO treatment alone (5.5 ± 2.9 vs. 38.8 ± 7.5, 35.0 ± 8.2, 32 ± 6.6).
Figure 2.
Percent polarized cells from Nonischemic Control, IR, and IR-HBO plasma groups (blue bars) and plasma groups repeated with C-PTIO (red bars). N = 6 animals and 100 cells/group.
= p < 0.05 versus IR-HBO plasma alone.
Figure 3.
Percent polarized cells from Nonischemic Control13, IR13, and IR-HBO20 plasma groups, compared to IR and IR-HBO with L-NAME, with 7-NI and with 1400W. n = 6 animals and 100 cells/group. All the NOS inhibitors reversed the beneficial HBO effect.
* = p < 0.01 versus IR-HBO plasma alone.
^ The data for Nonischemic Control and IR plasma alone was published in PRS 114:1846–1850, 2004. # The data for IR-HBO plasma alone was published in J Surg Res 150:11–16, 2008. This Control Data is presented here for clarity and statistical comparison with the new experimental data.
Adhesion assay
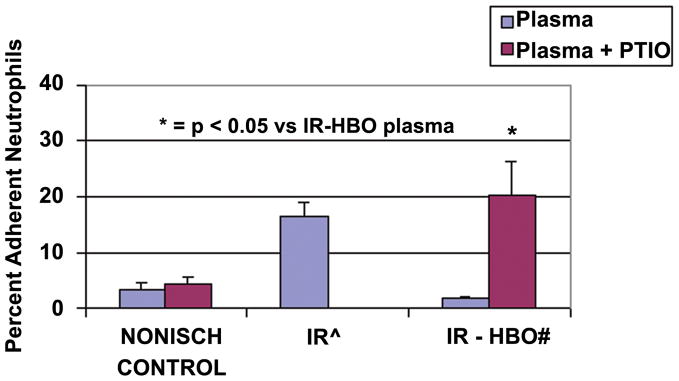
Ischemia reperfusion plasma caused a statistically significant increase in the percent of adherent neutrophils compared to non-ischemic control (16.7 ± 2.2 vs. 0.8 ± 0.09, p ≤ 0.05). The plasma from the IR-HBO flap showed a statistically similar percent of adherent neutrophils to the non-ischemic control (1.64 ± 0.35 vs. 0.8 ± 0.09). C-PTIO treatment of the plasma from the IR flap with HBO treatment reversed the effects of the relative nonadherence seen in the IR-HBO group (20.3 ± 5.9 vs. 1.64 ± 0.35, p ≤ 0.05) (Figure 4).
Figure 4.
Percent adherent neutrophils on coverslips coated with sICAM in 3 plasma groups: Nonischemic Control, IR13, IR-HBO20 (blue bars) and IR and IR-HBO repeated with C-PTIO (red bars). n =10/group. * = p < 0.05 versus IR-HBO plasma alone.
^ The data for IR plasma alone was published in PRS 114:1846–1850, 2004. # The data for IR-HBO plasma alone was published in J Surg Res 150:11–16, 2008. This Control Data is presented here for clarity and statistical comparison with the new experimental data.
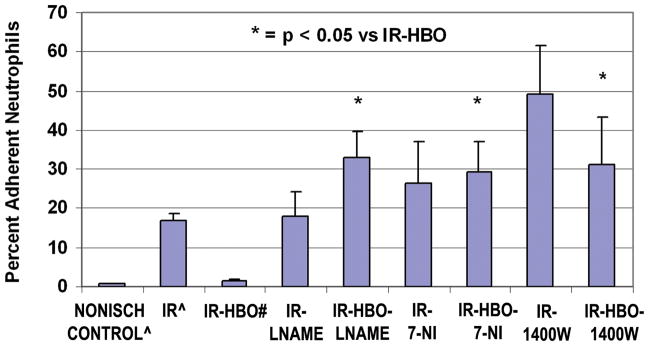
Isoform-selective inhibitors of NOS, LNAME (eNOS),38,39 7NI(nNOS) 38,40,41 and1400W(iNOS) 38,39, 42,43 used in conjunction with HBO treatment significantly (p <0.01) increased adhesion of PMNs to ICAM coated coverslips (Figure 5).
Figure 5.
Percent adherent neutrophils on coverslips coated with sICAM in Nonischemic Control13, IR13, and IR-HBO20 plasma groups, compared to IR-HBO with L-NAME, with 7-NI and with 1400W. n =10/group. The NOS inhibitors had no significant affect on the IR plasma alone, data not shown.
* = p < 0.05 versus IR-HBO plasma alone.
^ The data for Nonischemic Control and IR plasma alone was published in PRS 114:1846–1850, 2004. # The data for IR-HBO plasma alone was published in J Surg Res 150:11–16, 2008. This Control Data is presented here for clarity and statistical comparison with the new experimental data.
Discussion
The results from this study show that the beneficial HBO effect on neutrophil CD18 polarization and resultant endothelial adhesion is dependent on nitric oxide signaling and involves nitric oxide synthase.
The polarization and adhesion assays were designed to allow for treatment manipulation in vivo and in vitro. In vivo, the whole animal was exposed to IR from the gracilis skeletal muscle flap and HBO treatment. Plasma obtained from these subjects (activated plasma) was then exposed in vitro to normal neutrophils (from an animal with no intervention) and s-ICAM (either coated on coverslips or in solution). These assays allowed us to treat the animals in vivo and separate out the components of CD18 polarization and adhesion eliminating in vivo effects such as direct neutrophil exposure to HBO and differences in endothelial ICAM-1 expression.
To evaluate whether the HBO effect on neutrophil CD18 polarization and adhesion is NO dependent, carboxy-2-phenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl 3 oxide (C-PTIO),28 a NO scavenger, was used in the in vitro polarization and adhesion experiments.13 Activated plasma was scavenged free of NO with C-PTIO before reaction with ICAM and normal neutrophils. This stable, radical NO scavenger inhibits NO dependent events without affecting NO synthase.29,30 Figure 2 shows that when NO was scavenged from the IR-HBO plasma, the percent of polarized cells was equivalent to that in IR plasma. The beneficial HBO-induced decrease in neutrophil-ICAM adherence was also reversed in the absence of NO (Figure 4). Note that C-PTIO did not have an independent effect on neutrophils when administered to non-activated (nonischemic) plasma (Figure 2).
In order to evaluate if NOS is required for the HBO effect on neutrophils, isoform directed NOS inhibitors were used in the polarization and adhesion assays (Figures 3 and 5). Nitric oxide synthase inhibitors used were: NG-nitro-L-arginine methyl ester (L-NAME),31,32 7-nitroindazole (7-NI), 33,34 and N-(3-aminomethyl)benzyl)acetamidine (1400 W).35,36 Nitric oxide synthase inhibitors have been evaluated by investigators for their affinity for specific isoforms of NOS. The inhibitor L-NAME showed selectivity toward eNOS, 31,32 7-NI was shown as a potent inhibitor of nNOS, 31,33,34 and 1400W has been reported as a highly selective inhibitor of iNOS.35,36
Figures 3 and 5 show the results of isoform directed NOS inhibitors on in vitro polarization and adherence. The NOS inhibitors analyzed all reversed the beneficial HBO reduction in CD18 polarization and cell adhesion. It appears from this data that while the HBO-NO effect on polarization is NOS dependent, it is not isoform specific. It should be pointed out, however, that while some NOS inhibitors are preferentially selective for a particular NOS isoform, they are not completely specific, allowing for the possibility of crossover inhibition.
In this experiment we showed that removal of nitric oxide from the plasma using a nitric oxide scavenger reversed the effects of HBO treatment on neutrophil CD18 polarization and adhesion. The concentration of PTIO used here was reported by Janssen, et. al.28 showing cell cycle alterations with the depletion of nitric oxide. Certain structural rearrangements of cells are mediated by nitric oxide signal transduction. The Rho protein, as well as cGMP, has been implicated in nitric oxide involvement of pseudopodia and filopodia formation.24–26 Loitto, et al. reported a reduced amount of F-actin in PMNs exposed to NO.27 Though the exact mechanisms are not known, these studies recognize the nitric oxide dependent reorganization of the actin cytoskeleton.
The above reports suggest that HBO may increase NO concentration, but there is a mechanistic gap linking HBO and NO to neutrophil adhesion function. Polarization of expressed CD18 molecules is directly related to the actin cytoskeleton and actin binding proteins. Cytoskeletal linkages enable integrins (CD18) to mediate cell adhesion and regulate cell shape.37 Several reports implicate NO in the disruption of the actin cytoskeleton.38–40 Specifically, Saltzman, et al., have reported that NO may protect the GI mucosa from IR injury by maintaining perfusion, and inhibiting PMN adhesion.38 These authors also found that high NO concentrations disrupt the actin cytoskeleton.38
Because polarization of the CD18 molecules is required for firm adhesion to occur, nitric oxide could be interfering with CD18 function possibly through disruption of the actin cytoskeleton. The nitric oxide mediated inhibition of PMN c-GMP, inhibition of membrane guanylate cyclase, or a reduction in f-actin could all contribute to disruption of the actin cytoskeleton preventing CD18 polarization.24–27 Recently, Thom, et al., showed that neutrophils subjected to HBO treatment, release NO leading to an increase in nitrosylated actin that perturbs the intracellular F-actin assembly which regulates integrin function.42
This data corroborates other reports that the HBO inhibition of PMN-endothelial adhesion is dependent on NO and involves NOS.26, 43 The early upregulation of eNOS in IR 44 was blocked by the nonspecific NOS inhibitors, however, the late increase in iNOS was not tested in this protocol. 44 The increase in NO could be interfering with the cGMP, guanylate cyclase, or the cytoskeletal network (g-proteins and/or actin) to inhibit the polarization of CD18 surface protein molecules necessary for PMN-endothelial adhesion.
There is strong rationale for involvement of nitric oxide (NO) in IR and the effect of HBO on neutrophil CD18. Several investigators have shown interrelationships between IR injury, HBO and NO. We implicated nitric oxide as a step in the signaling pathway of hyperbaric oxygen inhibition of CD18 polarization and the resultant neutrophil-endothelial adhesion, however, a specific isoform was not identified. Further studies are needed to define the signaling complex initiated by hyperbaric oxygen.
Replants following long ischemia times, or replants or free tissue transfers complicated by thrombosis and a secondary ischemia time may benefit from hyperbaric oxygen therapy. Recently published data on the mechanism of IR injury suggest that increasing NO to the hypoxic tissue could greatly enhance the survival and function of a replanted tissue. Delivery of NO could be through hyperbaric oxygen treatment, infusion of a NO precursor or other method to facilitate the NO cell signaling cascade that improves outcomes following any ischemic event.
Acknowledgments
This work was completed at the Center for Biological Imaging, University of Nevada, Las Vegas. This center was built with a National Science Foundation Grant, DBI-9977509, and is supported by NIH Grant P20 RRO16464 from the INBRE Program of the National Center for Research Resources.
Footnotes
Financial Disclosure
There are no financial interests or commercial associations that may create a conflict of interest with information presented in this manuscript.
Products
| FITC anti-rat CD11b | BD Pharmingen, Torreyana, CA |
| rh-ICAM-1 | R&D Systems, Minneapolis, MN |
| Confocal Microscope & LSM 510 | Carl Zeiss, Inc., New York, NY |
| Hyperbaric Research Chamber-Model 1300 | Sechrist Industries, Inc., Anaheim, CA |
| Nembutal, sodium pentobarbital | Abbott Laboratories, North Chicago, IL |
| Histopaque 1077 & Histopaque 1119 | Sigma Chemical Co., St. Louis, MO |
| Carboxy-2-phenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl 3 oxide (C-PTIO) | Sigma Chemical Co., St. Louis, MO |
| L-nitro-aminomethylamine (L-NAME) | Sigma Chemical Co., St. Louis, MO |
| 7-nitro-indazole (7-NI) | Sigma Chemical Co., St. Louis, MO |
| N-(3-aminomethyl)benzyl)acetamidine (1400W) | Cayman Chemical Co., Ann Arbor, MI |
References
- 1.Sterling DL, Thornton JD, Swafford A, et al. Hyperbaric oxygen limits infarct size in ischemic rabbit myocardium in vivo. Circulation. 1993;88:1931–1936. doi: 10.1161/01.cir.88.4.1931. [DOI] [PubMed] [Google Scholar]
- 2.Cabigas BP, Su J, Hutchins W, et al. Hyperoxic and hyperbaric-induced cardioprotection: Role of nitric oxide synthase 3. Cardiovascular Research. 2006;72:143–151. doi: 10.1016/j.cardiores.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Helms AK, Whelan HT, Torbey MT. Hyperbaric oxygen therapy of cerebral ischemia. Cerebrovasc Dis. 2005;20:417–426. doi: 10.1159/000088979. [DOI] [PubMed] [Google Scholar]
- 4.Wong HP, Zamboni WA, Stephenson LL. Effect of Hyperbaric Oxygen on Skeletal Muscle Necrosis Following Primary and Secondary Ischemia in a Rat Model. Surgical Forum. 1996;XLVII:705–707. [Google Scholar]
- 5.Allen DM, Chen L, Seaber AV, Urbaniak JR. Pathophysiology and related studies of no reflow phenomenon in skeletal muscle. Clin Orthop. 1995;314:122. [PubMed] [Google Scholar]
- 6.Zamboni WA, Roth AC, Russell RC, Graham B, Suchy H, Kucan JO. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast Reconstr Surgery. 1998;91:1111–1121. doi: 10.1097/00006534-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Angel MF, Ramasastry SS, Swartz WM, et al. Free radicals: Basic concepts concerning their chemistry, pathophysiology, and relevance to plastic surgery. Plast Reconstr Surg. 1987;79:990. [PubMed] [Google Scholar]
- 8.Russell RC, Roth AC, Kucan JO, Zook EG. Reperfusion injury and oxygen free radicals: A review. J Reconstr Microsurg. 1989;5:79. doi: 10.1055/s-2007-1006855. [DOI] [PubMed] [Google Scholar]
- 9.Inauen W, Suzuki M, Granger DN. Mechanisms of cellular injury: Potential source of oxygen free radicals in ischemia/reperfusion. Microcirc Endothelium Lymphatics. 1989;5:143. [PubMed] [Google Scholar]
- 10.Pang CY. Ischemia-induced reperfusion injury in muscle flaps: Pathogenesis and major source of free radicals. J Reconstr Microsurg. 1990;6:77. doi: 10.1055/s-2007-1006807. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni WA, Stephenson LL, Roth AC, Suchy H, Russell RC. Ischemia-reperfusion injury in skeletal muscle: CD18-dependent neutrophil-endothelial adhesion and arteriolar vasoconstriction. Plast Reconstr Surg. 1997;99:2002–09. doi: 10.1097/00006534-199706000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Larson JL, Stephenson LL, Zamboni WA. Effect of hyperbaric oxygen on neutrophil CD18 expression. Plast Reconstr Surg. 2000;105:1375–81. doi: 10.1097/00006534-200004040-00017. [DOI] [PubMed] [Google Scholar]
- 13.Khiabani KT, Stephenson LL, Gabriel A, Nataraj C, Wang WZ, Zamboni WA. A Quantitative Method for Determining Polarization of Neutrophil Adhesion Molecules Associated with Ischemia Reperfusion. Plast Reconstr Surg. 2004;114:1846–1850. doi: 10.1097/01.prs.0000143580.45631.dd. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SI, Hotchin NA, Nash GB. Role of the cytoskeleton in rapid activation of CD11b/CD18 function and its subsequent down regulation in neutrophils. J Cell Science. 2000;113:2737–2745. doi: 10.1242/jcs.113.15.2737. [DOI] [PubMed] [Google Scholar]
- 15.Haverstick DM, Sakai H, Gray LS. Lymphocyte adhesion can be regulated by cytoskeleton-associated, PMN-induced capping of surface receptors. Am J Physiol. 1992;262:C916–C926. doi: 10.1152/ajpcell.1992.262.4.C916. [DOI] [PubMed] [Google Scholar]
- 16.Tohya K, Kimura M. Ultrastructural evidence of distinctive behavior of L-selectin and LFA-1 (αLβ2 integrin) on lymphocytes adhering to the endothelial surface of high endothelial venules in peripheral lymph nodes. Histochem Cell Biol. 1998;110:407–416. doi: 10.1007/s004180050301. [DOI] [PubMed] [Google Scholar]
- 17.Strauss MB, Hargens AR, Gershuni DH, et al. Reduction of skeletal muscle necrosis using intermittent hyperbaric oxygen in a model compartment syndrome. J Bone Joint Surg. 1983;65A:656. [PubMed] [Google Scholar]
- 18.Nylander G, Lewis D, Nordstrom H, Larson J. Reduction of postischemic edema with hyperbaric oxygen. Plast Reconstr Surg. 1985;76:596. doi: 10.1097/00006534-198510000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Skyhar MJ, Hargens AR, Strauss MB, et al. Hyperbaric oxygen reduces edema and necrosis of skeletal muscle in compartment syndromes associated with hemorrhagic hypotension. J Bone Joint Surg. 1986;68A:1218. [PubMed] [Google Scholar]
- 20.Khiabani KT, Bellister SR, Skaggs SS, Stephenson LL, Wang WZ, Zamboni WA. Reperfusion induced neutrophil CD18 polarization: Effect of hyperbaric oxygen. J Surg Res. 2008;150:11–16. doi: 10.1016/j.jss.2007.12.780. [DOI] [PubMed] [Google Scholar]
- 21.Meldrum DG, Stephenson LL, Zamboni WA. Effects of L-NAME and L-Arginine on ischemia-reperfusion injury in rat skeletal muscle. Plast Reconstr Surg. 1999;103(3):935–940. doi: 10.1097/00006534-199903000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel A, Stephenson LL, Zamboni WA. Effect of L-Arginine on Leukocyte Adhesion in Ischemia-Reperfusion Injury. Plastic Reconstructive Surgery. 2004;113:1698–1702. doi: 10.1097/01.prs.0000117364.53547.0e. [DOI] [PubMed] [Google Scholar]
- 23.Elayan IM. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J Neurophysiol. 2000;83(4):2022–9. doi: 10.1152/jn.2000.83.4.2022. [DOI] [PubMed] [Google Scholar]
- 24.Ke X. Nitric Oxide regulates actin reorganization through cGMP and Ca(2+)/calmodulin in RAW 264.7 cells. Biochim Biophys Acta. 2001;1539(1–2):101–13. doi: 10.1016/s0167-4889(01)00090-8. [DOI] [PubMed] [Google Scholar]
- 25.Ellis S. The novel Rho-family GTPase rif regulates coordinated actin-based membrane rearrangements. Curr Biol. 2000;10(21):1387–90. doi: 10.1016/s0960-9822(00)00777-6. [DOI] [PubMed] [Google Scholar]
- 26.Banick PD, Chen Q, Xu YA, Thom SR. Nitric oxide inhibits neutrophil beta 2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J Cell Physiol. 1997;172:12–24. doi: 10.1002/(SICI)1097-4652(199707)172:1<12::AID-JCP2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Loitto VM, Nilsson H, Sundqvist T, Magnusson KE. Nitric oxide induces dose-dependent CA(2+) transients and causes temporal morphological hyperpolarization in human neutrophils. J Cell Physiol. 2000;182:402–413. doi: 10.1002/(SICI)1097-4652(200003)182:3<402::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Janssen YMW, Soultanakis R, Steece K, et al. Depletion of nitric oxide causes cell cycle alterations, apoptosis, and oxidative stress on pulmonary cells. American Physio Society. 1998:L1100–07. doi: 10.1152/ajplung.1998.275.6.L1100. [DOI] [PubMed] [Google Scholar]
- 29.Akaike T, Yoshida M, Miyamoto Y, et al. Antagonistic Action of Imidazolineoxyl N-Oxides against Endothelium-Derived Relaxing Factor/NO through a Radical Reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 30.Maeda H, Akaike T, Yoshida M, Suga M. Multiple functions of nitric oxide in pathophysiology and microbiology: analysis by a new nitric oxide scavenger. J Leukoc Biol. 1994;56:588–592. doi: 10.1002/jlb.56.5.588. [DOI] [PubMed] [Google Scholar]
- 31.Southan GJ, Szabo C. Selective Pharmacological Inhibition of Distinct Nitric Oxide Synthase Isoforms. Biochemical Pharmacology. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 32.Boer R, Ulrich W-R, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of Arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Molecular Pharmacology. 2000;58:1026–1034. [PubMed] [Google Scholar]
- 33.Moore PK, Babbedge RC, Wallace P, Gaffen ZA, Hart SL. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br J Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babbedge RC, Blad-Ward PA, Hart SL, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substituted indazoles. Br J Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garvey EP, Oplinger JA, Furfine ES. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen LL, Scott JM, Topley P, Knowles RG, Keerie AJ, Frend AJ. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- 37.Calderwood DA, Shattil SJ, Ginsber MH. Integrins and Actin Filaments: Reciprocal Regulation of Cell Adhesion and Signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 38.Saltzman AL. Nitric oxide in the gut. New Horiz. 1995;3:33–45. [PubMed] [Google Scholar]
- 39.Frenkel SR, Clancy RM, Ricci JL, Di Cesare PE, Rediske JJ, Abramson SB. Effects of nitric oxide on chondrocytes migration, adhesion, and cytoskeletal assembly. Arthritis Rheum. 1996;39:1905–2012. doi: 10.1002/art.1780391118. [DOI] [PubMed] [Google Scholar]
- 40.Staykova MA, Berven LA, Cowden WB, Willenborg DO, Crouch MF. Nitric oxide induces polarization of actin in encephalitogenic T cells and inhibits their in vitro transendothelial migration in a p70S6 kinase-independent manner. FASEB J. 2003;17:1337–1339. doi: 10.1096/fj.02-0577fje. [DOI] [PubMed] [Google Scholar]
- 41.Ingram AJ, James L, Cai L, Thai K, Ly H, Scholey JW. NO Inhibits Stretch-induced MAPK Activity by Cytoskeletal Disruption. J Biol Chem. 2000;275:40301–40306. doi: 10.1074/jbc.M007018200. [DOI] [PubMed] [Google Scholar]
- 42.Thom SR, Bhopale VM, Mancini DJ, Milovanova TN. Actin S-nitrosylation inhibits neutrophil B2 integrin function. J Biol Chem. 2008;283:10822–10834. doi: 10.1074/jbc.M709200200. [DOI] [PubMed] [Google Scholar]
- 43.Thom SR. Effects of hyperoxia on neutrophil adhesion. Undersea Hyperb Med. 2004;31:123–131. [PubMed] [Google Scholar]
- 44.Baynosa RC, Naig AL, Murphy PS, Hansen BK, Fang XH, Stephenson LL, Khiabani KT, Wang WZ, Zamboni WA. The effect of hyperbaric oxygen on NOS activity and transcription in ischemia reperfusion injury. Journal of the American College of Surgeons. 2005;201(3):S57–S58. [Google Scholar]