Abstract
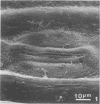
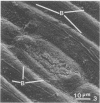
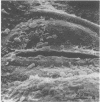
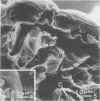
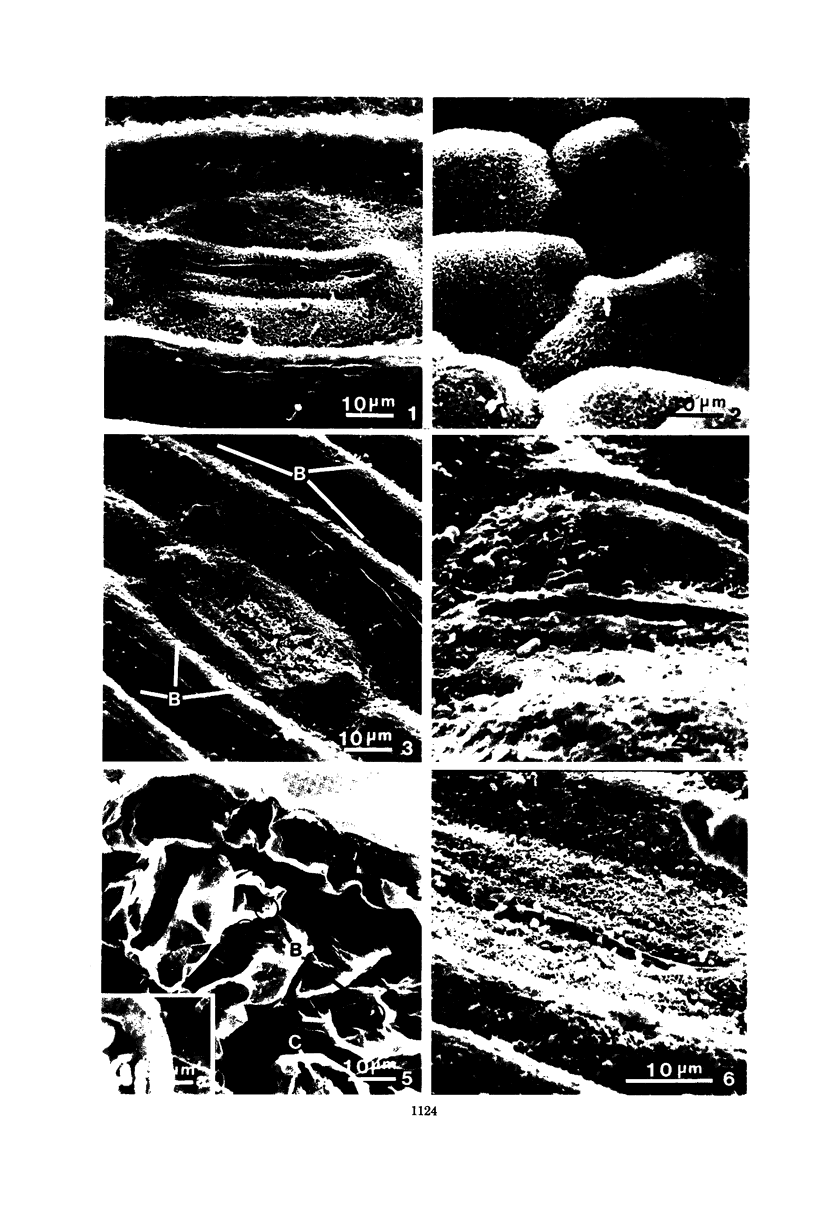
Wheat and alfalfa silages were examined by scanning electron microscopy and standard methods of microbial enumeration. Epiphytic microflora were present at levels of 106 to 108/g in the fresh-cut plants. This flora was initially observed microscopically primarily on the surfaces. After 4 days of fermentation, lactic acid bacteria were observed on the surface in high concentrations near open stomata and throughout the interior mesophyll air sac spaces. At 4 days, populations on interior surfaces were restricted to the exterior surfaces of the air sacs. After 8 days the mesophyllic cells showed marked deterioration, and bacteria were observed on their inner surfaces. At 32 days, the end of the fermentation, vascular bundles and epidermal cells remained intact whereas stomata and mesophyllic cells were collapsed and often contained microorganisms. It is concluded that the interior of the leaves offers substantial nutritional and environmental advantages to epiphytic flora and is an important if not major deterioration site in fermented products. Since little deterioration of exterior surfaces was observed, these sites may play a minor role in supplying nutrients for microbial growth.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl Environ Microbiol. 1976 Apr;31(4):562–568. doi: 10.1128/aem.31.4.562-568.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. H. Bacteria on Leaf Surfaces and in Intercellular Leaf Spaces. Science. 1965 Mar 5;147(3662):1151–1152. doi: 10.1126/science.147.3662.1151. [DOI] [PubMed] [Google Scholar]
- GIBSON T., STIRLING A. C., KEDDIE R. M., ROSENBERGER R. F. Bacteriological changes in silage made at controlled temperatures. J Gen Microbiol. 1958 Aug;19(1):112–129. doi: 10.1099/00221287-19-1-112. [DOI] [PubMed] [Google Scholar]
- Moon N. J., Hamann A. C., Reinbold G. W. Recovery of Lactobacillus bulgaricus and Streptococcus thermophilus on Nine Commonly Used Agar Media. Appl Microbiol. 1974 Dec;28(6):1076–1078. doi: 10.1128/am.28.6.1076-1078.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. V., Tukey H. B. Characterization of Leachate from Plant Foliage. Plant Physiol. 1964 Jul;39(4):590–593. doi: 10.1104/pp.39.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing V. O., Schroth M. N. Bacteria--plant cell surface interactions: active immobilization of saprophytic bacteria in plant leaves. Science. 1977 Aug 19;197(4305):759–761. doi: 10.1126/science.197.4305.759. [DOI] [PubMed] [Google Scholar]