Abstract
Purpose
Patients with acute leukemia refractory to induction or reinduction chemotherapy have poor prognoses if they do not undergo hematopoietic stem-cell transplantation (HSCT). However, HSCT when a patient is not in complete remission (CR) is of uncertain benefit. We hypothesized that pretransplantation variables may define subgroups that have a better prognosis.
Patients and Methods
Overall, 2,255 patients who underwent transplantation for acute leukemia in relapse or with primary induction failure after myeloablative conditioning regimen between 1995 and 2004 were reported to the Center for International Blood and Marrow Transplant Research. The median follow-up of survivors was 61 months. We performed multivariate analysis of pretransplantation variables and developed a predictive scoring system for survival.
Results
The 3-year overall survival (OS) rates were 19% for acute myeloid leukemia (AML) and 16% for acute lymphoblastic leukemia (ALL). For AML, five adverse pretransplantation variables significantly influenced survival: first CR duration less than 6 months, circulating blasts, donor other than HLA-identical sibling, Karnofsky or Lansky score less than 90, and poor-risk cytogenetics. For ALL, survival was worse with the following: first refractory or second or greater relapse, ≥ 25% marrow blasts, cytomegalovirus-seropositive donor, and age of 10 years or older. Patients with AML who had a predictive score of 0 had 42% OS at 3 years, whereas OS was 6% for a score ≥ 3. Patients with ALL who had a score of 0 or 1 had 46% 3-year OS but only 10% OS rate for a score ≥ 3.
Conclusion
Pretransplantation variables delineate subgroups with different outcomes. HSCT during relapse can achieve long-term survival in selected patients with acute leukemia.
INTRODUCTION
Patients with acute leukemia refractory to initial or reinduction chemotherapy have dismal prognoses if they do not undergo hematopoietic stem-cell transplantation (HSCT). The utility of transplantation for patients not in complete remission (CR), however, is controversial. Since 1990, 12 studies have reported series with more than 30 patients who underwent transplantation while not in CR,1–12 which included 39 to 230 (median, 63) patients per report. Disease-free survival (DFS) ranged from 2% to 32%. Selection bias for the healthiest patients and publication bias with more frequent reports for favorable series confound interpretation of these data. Eligibility criteria between series were variable, which precluded a meaningful comparison. The last study from the Center for International Blood and Marrow Transplant Research (CIBMTR) of refractory acute leukemia examined 126 patients who underwent transplantation from 1982 to 1989.1 Thus, the outcome of patients transplanted in the past 20 years without CR is largely unknown.
The prognostic factors for these transplantations in relapse are also controversial. Several factors have been associated with better outcome, though not consistently, and they include the following: absence of blasts in blood,1,4,6,9,10 fewer marrow blasts (ie, 5% or 30%),1,6,10,12 primary induction failure,8 untreated first relapse,2 cytogenetics,11 matched unrelated donor,4,8 matched sibling donor,5 female donor,7 female recipient,1 younger donor,4 younger recipient,1,2,7 better pretransplantation performance score,1,9 absence of significant infection at transplantation,1 middle-range tacrolimus levels,9 and presence of acute2,3,7 or chronic7 graft-versus-host disease (GVHD). In better-risk groups defined by these criteria, DFS ranged from 28% to 50%. However, these studies reported heterogeneous populations and generally included too few patients to perform a multivariate analysis of relevant pretransplantation risk factors.
To facilitate patient counseling and clinical decision making and to define the role of HSCT in patients without CR, we analyzed outcomes of 2,255 patients who underwent transplantation during relapse or primary induction failure reported to the CIBMTR from 1995 to 2004, and we developed a predictive scoring system for survival.
PATIENTS AND METHODS
Data Sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), the Autologous Blood and Marrow Transplant Registry (ABMTR), and the National Marrow Donor Program (NMDP) established in 2004, which collects data from more than 450 transplantation centers worldwide. The Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis collects, verifies, and audits data and performs observational analyses in compliance with the privacy rule (ie, Health Insurance Portability and Accountability Act) as a Public Health Authority after review and approval by the institutional review boards of the National Marrow Donor Program and the Medical College of Wisconsin. CIBMTR data includes Transplant Essential Data plus more detailed comprehensive disease and pre- and post-transplantation clinical information from a subset of patients selected by a weighted randomization scheme. Data are collected pretransplantation, 100 days post-transplantation, 6 months post-transplantation, and annually thereafter or until death.
Patients
The outcomes of 2,255 patients with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) who underwent a first myeloablative allogeneic bone marrow (BM) or peripheral-blood (PB) HSCT in relapse or primary induction failure between 1995 and 2004 are reported. Patients with AML and preceding myelodysplastic syndrome (MDS; n = 343) and patients with secondary leukemia were included, but those with chronic myeloid leukemia in blast crisis were not. Only patients who received total-body irradiation (TBI) or busulfan-based myeloablative conditioning regimens were analyzed. Patients receiving reduced-intensity or fludarabine-containing conditioning regimen (n = 488) were excluded. Patients undergoing syngeneic transplantations (n = 14) or cord-blood transplantations (n = 164) also were excluded.
Before analysis, we stratified disease status at HSCT into four categories. Primary induction failure was reported by the transplantation team. First untreated relapse was defined as first relapse without chemotherapy between relapse and the pre-HSCT conditioning regimen. First refractory relapse was defined as first relapse with chemotherapy (but no CR) between relapse and HSCT. Other patients were categorized as second and later relapse.
Patients with ALL were classified as having poor-risk cytogenetics with either t(4:11), t(9;22), t(8;14), hypodiploidy or near triploidy, or more than five cytogenetic abnormalities.13 Other ALL cytogenetic findings were classified as other abnormalities or normal. Patients with AML were classified according to the Eastern Cooperative Oncology Group/Southwest Oncology Group classification as good risk (inv16, t[8;21], t[15;17]), poor risk (−5/del[5q], −7/del[7q], inv[3q], abn11q, 20q or 21q, del[9q], t[6;9], t[9;22], abn17p, and complex karyotype defined as three or more abnormalities), or intermediate (other and normal karyotypes).14
Study End Points
The primary study end point was 3-year overall survival (OS). OS was defined as time from the date of transplantation to the date of death or last contact. For HSCT during relapse, because post-HSCT CR was not always achieved or reliably documented, it was therefore not possible to calculate the incidence of relapse, transplantation-related mortality, or disease-free survival (DFS). Neutrophil and platelet engraftment were defined as the first of 3 consecutive days with an absolute neutrophil count greater than 0.5 × 109/L or untransfused platelet count greater than 20 × 109/L. Acute GVHD (aGVHD) was defined by consensus criteria,15 whereas chronic GVHD (cGVHD) was classified according to the standard criteria in use before the recent National Institutes of Health consensus conference report.16
HLA typing methods and resolution varied over the period of study. Donor-recipient pairs, therefore, were reclassified as well matched, partially matched, or mismatched according to recently published CIBMTR criteria.17 Well-matched pairs had either no identified HLA mismatch and informative data at the four loci (ie, HLA-A, -B, and -C, and DRB1) or allele matching at the four loci. Partially matched pairs had a defined, single locus mismatch and/or or single locus that was missing HLA data. Mismatched pairs had two or more allele or antigen mismatches.
Statistical Analysis
Probability of 3-year OS was calculated by using the Kaplan-Meier estimator. Confidence intervals were calculated with a log transformation. Multivariate analyses were performed separately for AML and ALL. The probability of neutrophil and platelet recovery, and of aGVHD and cGVHD, were calculated by using cumulative incidence method to accommodate competing risks.
The effect of pretransplantation variables on OS at 3 years was compared by using a pseudovalue regression model.18 This technique reduces to a logistic regression model when there is no censoring and accounts for censored observations before 3 years. By using a log-log link function, this can be thought of as a pointwise Cox proportional hazards model that incorporates pretransplantation variables of interest. Factors that influenced outcomes were identified by stepwise forward selection multivariate model. Any covariate with a P value < .05 was considered significant. The following variables were considered in multivariate analyses: age at transplantation, donor and recipient sex, Karnofsky or Lansky performance score at HSCT, disease status at HSCT, circulating blasts, less than 25% marrow blasts at HSCT, time from relapse to transplantation, duration of first CR for patients in first relapse, pre-HSCT extramedullary leukemia, prior MDS, cytogenetics, prior fungal infection, conditioning regimen, donor-recipient sex and sex match, donor-recipient HLA match, donor-recipient cytomegalovirus (CMV) status, graft type, GVHD prophylaxis, and year of transplantation. Analyses were performed by using SAS software, version 9.1 (SAS Institute, Cary, NC).
Because multivariate analysis showed that some pretransplantation variables were associated with outcome, a scoring system was developed to link the significant pretransplantation risk factors with outcome. Several scoring models were tested and were based on placement of patients with similar risks in the same category on the basis of the fitted model. The scoring models were evaluated by using a Brier score approach, a function that is based on the calculation of the average squared deviation between predicted probabilities and outcomes.19 The scoring model that gave the lowest Brier score was picked as the best model.
RESULTS
Patients Characteristics
The 2,255 patients included 1,673 with AML from 221 centers in 34 countries and 582 with ALL from 180 centers in 33 countries. The median follow-up of survivors was 61 months in both disease groups (range, 2 to 137 months).
Patient, disease, and HSCT characteristics are listed in Table 1. The median age was 38 years for patients with AML and was 29 years for patients with ALL. Nearly half had a pre-HSCT Karnofsky score less than 90. Only 15% and 12% in the AML and ALL groups, respectively, had pre-HSCT fungal infections. More than 40% had greater than 25% marrow blasts at HSCT, whereas 57% of patients with AML and 38% of patients with ALL had circulating blasts. For the patients who underwent transplantation in first or later relapse, the duration of the first CR was less than 6 months in 55% of patients with AML and in 39% of patients with ALL.
Table 1.
Patient, Disease, and Transplantation Characteristics
| Characteristic | Patients by Disease |
|||||
|---|---|---|---|---|---|---|
| AML |
ALL |
|||||
| No. Evaluable | No. (n = 1,673) | % | No. Evaluable | No. (n = 582) | % | |
| No. of centers | 221 | 180 | ||||
| Age, years | ||||||
| Median | 38 | 29 | ||||
| Range | < 1-70 | < 1-60 | ||||
| Male sex | 880 | 53 | 371 | 64 | ||
| Karnofsky or Lansky score < 90 at transplantation | 1,595 | 759 | 48 | 553 | 262 | 47 |
| WBC at diagnosis, ×109/L | 1,443 | 484 | ||||
| Median | 11 | 21 | ||||
| Range | < 1-1,803 | < 1-990 | ||||
| < 50 | 1,079 | 75 | 329 | 68 | ||
| ≥ 50 | 364 | 25 | 155 | 32 | ||
| Prior history of extramedullary leukemia | 233 | 14 | 192 | 33 | ||
| Prior history of CNS disease | 862 | 80 | 9 | 394 | 104 | 26 |
| Prior history of myelodysplasia | 343 | 21 | NA | |||
| Marrow blasts at transplantation, % | 1,428 | 488 | ||||
| Median | 21 | 17 | ||||
| Range | 0-100 | 0-100 | ||||
| < 25 | 753 | 53 | 277 | 57 | ||
| ≥ 25 | 675 | 47 | 211 | 43 | ||
| Blasts in blood at transplantation, ×109/L | 1,524 | 518 | ||||
| Median | 4.2 | 0 | ||||
| Range | 0-12,798 | 0-24,116 | ||||
| 0 | 656 | 43 | 320 | 62 | ||
| ≥ 0 | 868 | 57 | 198 | 38 | ||
| Cytogenetics for AML | ||||||
| Good | 117 | 7 | ||||
| Intermediate/normal | 988 | 59 | ||||
| Poor | 273 | 16 | ||||
| Unknown | 295 | 18 | ||||
| Cytogenetics for ALL | ||||||
| High risk | 151 | 26 | ||||
| Other | 138 | 24 | ||||
| No abnormalities | 145 | 25 | ||||
| Unknown | 148 | 25 | ||||
| Disease status at transplantation | ||||||
| Primary induction failure | 636 | 38 | 144 | 25 | ||
| First untreated relapse | 322 | 19 | 67 | 12 | ||
| First refractory relapse | 428 | 26 | 251 | 43 | ||
| First relapse, unknown treatment | 9 | 1 | 9 | 2 | ||
| Second or additional relapse | 278 | 17 | 111 | 19 | ||
| Time from relapse to transplantation for HSCT in first refractory relapse, months | ||||||
| Median | 2.5 | 3.0 | ||||
| Range | < 1-23 | < 1-16 | ||||
| < 3 | 257 | 60 | 127 | 51 | ||
| ≥ 3 | 171 | 40 | 124 | 49 | ||
| Duration of first CR for patients in relapse, months | 1,566 | 501 | ||||
| Median | 5 | 8 | ||||
| Range | < 1-113 | < 1-97 | ||||
| < 6 | 515 | 55 | 140 | 39 | ||
| ≥ 6 | 415 | 45 | 217 | 61 | ||
| Prior fungal infection | 250 | 15 | 70 | 12 | ||
| Conditioning regimen | ||||||
| CyTBI + other | 456 | 27 | 241 | 41 | ||
| CyTBI | 489 | 29 | 167 | 29 | ||
| BuCy + other | 285 | 17 | 59 | 10 | ||
| BuCy | 320 | 19 | 40 | 7 | ||
| Bu or TBI ± other | 123 | 7 | 75 | 13 | ||
| Donor-recipient sex | ||||||
| M-M | 549 | 33 | 227 | 39 | ||
| M-F | 440 | 26 | 117 | 20 | ||
| F-M | 331 | 20 | 144 | 25 | ||
| F-F | 353 | 21 | 94 | 16 | ||
| Donor-recipient CMV serostatus | 1,615 | 564 | ||||
| −/− | 447 | 28 | 151 | 27 | ||
| +/− | 223 | 14 | 76 | 13 | ||
| −/+ | 426 | 26 | 145 | 26 | ||
| +/+ | 519 | 32 | 192 | 34 | ||
| Donor-recipient HLA match | ||||||
| HLA-identical sibling | 552 | 33 | 224 | 38 | ||
| Other related | 117 | 7 | 49 | 8 | ||
| Well-matched unrelated | 354 | 21 | 116 | 20 | ||
| Partially matched unrelated | 419 | 25 | 126 | 22 | ||
| Mismatched unrelated | 231 | 14 | 67 | 12 | ||
| Graft type | ||||||
| Bone marrow | 1,095 | 65 | 376 | 65 | ||
| PBSC | 578 | 35 | 206 | 35 | ||
| Year of transplantation | ||||||
| 1995-1996 | 429 | 26 | 163 | 28 | ||
| 1997-1998 | 382 | 23 | 122 | 21 | ||
| 1999-2000 | 292 | 17 | 105 | 18 | ||
| 2001-2002 | 288 | 17 | 106 | 18 | ||
| 2003-2004 | 282 | 17 | 86 | 15 | ||
| GVHD prophylaxis | ||||||
| T-cell depletion | 213 | 13 | 51 | 9 | ||
| (Tacrolimus or CsA) + MTX ± other | 1,198 | 72 | 436 | 75 | ||
| (Tacrolimus or CsA) ± other | 224 | 13 | 80 | 14 | ||
| Other | 38 | 2 | 15 | 3 | ||
NOTE. First refractory relapse is defined as transplantation in first relapse with chemotherapy between relapse and conditioning.
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; NA, not applicable; HSCT, hematopoietic stem-cell transplantation; CR, complete remission; Cy, cyclophosphamide; TBI, total-body irradiation; Bu, busulfan; CMV, cytomegalovirus; PBST, peripheral-blood stem cells; GVHD, graft-versus-host disease; CsA, cyclosporine; MTX, methotrexate.
At HSCT, 38% of patients with AML and 25% of patients with ALL were in primary induction failure, whereas 45% of patients with AML and 55% of patients with ALL were in first relapse; 19% and 12% of these patients with AML and ALL, respectively, in first relapse were in first untreated relapse. For those who underwent transplantation in first refractory relapse, the median times between relapse and HSCT were 2.5 and 3 months for patients with AML and ALL, respectively. Transplantations in second or later relapse were infrequent (17% and 19% in patients with AML and ALL, respectively).
Transplantation Characteristics
Cyclophosphamide plus TBI was included in pre-HSCT conditioning for 56% of patients with AML and for 70% of patients with ALL, whereas 36% of patients with AML and 17% of the patients with ALL received busulfan plus cyclophosphamide. Just greater than one third of patients received matched sibling donor grafts, and two thirds of all grafts were marrow. GVHD prophylaxis included methotrexate and a calcineurin inhibitor for 85% of patients with AML and 89% of patients with ALL. Ex vivo T-cell depletion was uncommon (13% of patients with AML and 9% of patients with ALL).
Engraftment and GVHD
As listed in Table 2, 90% had neutrophil and 66% had platelet recovery by day 100. Grades 3 to 4 aGVHD occurred in 23% of patients with AML and in 27% of patients with ALL. cGVHD occurred in 27% of the patients, nearly all within the first year after HSCT.
Table 2.
Univariate Probabilities of Outcome Among Patients Who Underwent Transplantation With Acute Leukemia in Relapse or Primary Induction Failure
| Variable | Univariate Probabilities of Outcome by Disease |
|||||
|---|---|---|---|---|---|---|
| AML |
ALL |
|||||
| No. of Patients Evaluable | Probability | 95% CI* | No. of Patients Evaluable | Probability | 95% CI* | |
| ANC > 0.5 × 109/L | 1,660 | 579 | ||||
| At 100 days | 90 | 87 to 92 | 89 | 83 to 93 | ||
| Platelets > 20 × 109/L | 1,627 | 561 | ||||
| At100 days | 66 | 63 to 69 | 66 | 61 to 69 | ||
| Acute GVHD, grades 2-4 | 1,653 | 570 | ||||
| At 100 days | 48 | 45 to 51 | 52 | 47 to 56 | ||
| Acute GVHD, grades 3-4 | 1,652 | 572 | ||||
| At 100 days | 23 | 21 to 26 | 27 | 24 to 31 | ||
| Chronic GVHD | 1,649 | 568 | ||||
| At 1 years | 25 | 23 to 28 | 26 | 22 to 30 | ||
| At 3 years | 27 | 25 to 29 | 27 | 23 to 32 | ||
| At 5 years | 27 | 25 to 30 | 27 | 23 to 32 | ||
| Overall survival | 1,673 | 582 | ||||
| At 6 months | 44 | 42 to 46 | 42 | 38 to 46 | ||
| At 1 year | 29 | 27 to 31 | 28 | 24 to 32 | ||
| At 3 years | 19 | 17 to 21 | 16 | 13 to 20 | ||
| At 5 years | 17 | 15 to 19 | 14 | 11 to 17 | ||
| Overall mortality | 1,673 | 582 | ||||
| At 100 days | 39 | 36 to 41 | 41 | 37 to 45 | ||
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; GVHD, graft-versus-host disease.
Kaplan-Meier or cumulative incidence, as appropriate.
Survival and Cause of Death
OS rates at 3 years were 19% for patients with AML (95% CI, 17% to 21%) and 16% for patients with ALL (95% CI, 13% to 20%). The mortality rate at 100 days after transplantation was 39% in AML, and it was 41% in ALL. As listed in Table 3, leukemia was the main cause of death and occurred at rates of 42% of AML and 37% of ALL. Other causes of death were similarly frequent in patients with ALL and AML.
Table 3.
Causes of Death Among Patients Who Underwent Transplantation With Acute Leukemia in Relapse or Primary Induction Failure
| Cause of Death | Patients by Disease | |||||
|---|---|---|---|---|---|---|
| AML |
ALL |
|||||
| No. Evaluable (n = 1,673) | No. | % | No. Evaluable (n = 582) | No. | % | |
| Overall No. of deaths | 1,370 | 491 | ||||
| Leukemia relapse or progression | 641 | 42 | 208 | 37 | ||
| Graft failure | 12 | 1 | 6 | 1 | ||
| Infection | 232 | 15 | 72 | 13 | ||
| Graft-versus-host disease | 111 | 7 | 45 | 8 | ||
| Organ failure | 180 | 12 | 88 | 16 | ||
| Hemorrhage | 43 | 3 | 15 | 3 | ||
| Idiopathic pneumonia/ARDS | 120 | 8 | 48 | 9 | ||
| Secondary malignancy | 9 | 1 | 3 | 1 | ||
| Other/unknown | 22 | 1 | 6 | 1 | ||
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; ARDS, acute respiratory distress syndrome.
Multivariate Analysis and Prognostic Scoring System
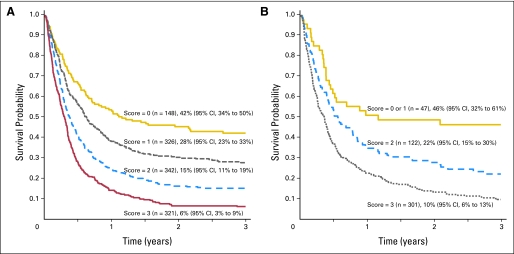
Multivariate analysis for OS at 3 years in AML is summarized in Table 4. Compared with HSCT after primary induction failure, survival after HSCT in first relapse was worse after short (ie, < 6 months) initial CR and better after longer (ie, > 6 months) initial CR. For patients who underwent transplantation in first relapse, survival was similar for refractory or untreated relapse. For patients with AML, survival was worse for patients with circulating blasts, a mismatched unrelated donor, a related donor other than an HLA-matched sibling, a Karnofsky or Lansky score less than 90, and poor-risk cytogenetics. By using these five risk factors (ie, first CR duration < 6 months, circulating blasts, non–HLA-identical sibling donor, performance score < 90%, poor-risk cytogenetics), a prognostic scoring system was established (Table 5). Patients with a score of 0 (n = 148) had a 42% 3-year OS (95% CI, 34% to 50%), whereas patients with a score ≥ 3 (n = 321) had only 6% 3-year OS (95% CI, 3% to 9%; Fig 1).
Table 4.
Multivariate Analysis for 3-Year Survival
| Variable by Disease | No. of Patients | Death Outcome |
P* | |
|---|---|---|---|---|
| Relative Risk | 95% CI | |||
| AML | ||||
| Disease status at transplantation | Poverall < .001 | |||
| Primary induction failure | 636 | 1.00 | ||
| Duration of first CR ≤ 6 months | 514 | 1.26 | 1.06 to 1.51 | .01† |
| Duration of first CR > 6 months | 414 | 0.83 | 0.69 to 0.99 | .0401 |
| Duration of first CR unknown | 100 | 0.92 | 0.68 to 1.24 | .5987 |
| Blasts in blood at transplantation | Poverall < .001 | |||
| Absent | 749 | 1.00 | ||
| Present | 673 | 1.48 | 1.28 to 1.72 | .0000 |
| Missing/negative | 242 | 1.27 | 0.98 to 1.64 | .0753 |
| Donor-recipient HLA match | Poverall < .001 | |||
| HLA-identical sibling | 544 | 1.00 | ||
| Other related | 117 | 2.21 | 1.51 to 3.23 | .0000* |
| Well-matched unrelated | 354 | 1.25 | 1.02 to 1.54 | .0279 |
| Partially matched unrelated | 419 | 1.15 | 0.96 to 1.38 | .1426 |
| Mismatched unrelated | 230 | 1.46 | 1.15 to 1.84 | .0017 |
| Karnofsky or Lansky score | Poverall < .001 | |||
| < 90 | 754 | 1.00 | ||
| 90-100 | 832 | 0.65 | 0.56 to 0.76 | .0000 |
| Missing | 78 | 0.58 | 0.42 to 0.81 | .0012 |
| Cytogenetics* | Poverall = .0226 | |||
| Good | 117 | |||
| Intermediate | 981 | 1.13 | 0.86 to 1.50 | .3791 |
| Poor | 272 | 1.47 | 1.06 to 2.04 | .0225 |
| Unknown | 294 | 1.37 | 1.00 to 1.89 | .0528 |
| ALL | ||||
| Disease status at transplantation | Poverall = .0003 | |||
| Primary induction failure | 144 | 1.00‡ | ||
| First untreated relapse | 67 | 1.34 | 0.83 to 2.15 | .2312 |
| First refractory relapse | 251 | 2.10 | 1.43 to 3.09 | .0002 |
| Second and additinoal relapse | 111 | 2.58 | 1.53 to 4.34 | .0004 |
| Blasts in marrow at transplantation, % | Poverall = .0014 | |||
| < 25 | 277 | 1.00‡ | ||
| ≥ 25 | 211 | 1.75 | 1.18 to 2.58 | .0053 |
| Missing | 94 | 2.21 | 1.34 to 3.66 | .0020 |
| Donor/recipient CMV status | Poverall = .0058 | |||
| −/− | 151 | 1.00‡ | ||
| +/− | 76 | 1.66 | 0.99 to 2.80 | .0555 |
| −/+ | 145 | 1.06 | 0.73 to 1.53 | .7718 |
| +/+ | 192 | 2.28 | 1.43 to 3.63 | .0005 |
| Missing | 18 | 0.95 | 0.43 to 2.10 | .9039 |
| Age, years | Poverall < .001 | |||
| < 1-9 | 55 | 1.00‡ | ||
| 10-19 | 102 | 1.76 | 1.03 to 3.02 | .0403 |
| 20-29 | 146 | 1.75 | 1.06 to 2.91 | .0296 |
| 30-39 | 127 | 1.57 | 0.95 to 2.60 | .0769 |
| 40-49 | 105 | 4.75 | 2.37 to 9.53 | < .001 |
| 50-59 | 46 | 1.46 | 0.79 to 2.70 | .2309 |
Abbreviations: AML, acute myeloid leukemia; CR, complete remission; ALL, acute lymphoblastic leukemia; CMV, cytomegalovirus.
P shown overall and as pairwise comparisons with reference group.
Pairwise comparison: duration of first CR < 6 months v > 6 months (P < .001).
Reference group.
Table 5.
Scoring System for Post-HSCT Outcome in AML and ALL
| Outcome by Disease | Score | No. of Patients |
|---|---|---|
| AML | ||
| Disease group | ||
| PIF or duration of first CR > 6 months | 0 | 763 |
| Duration of first CR < 6 months | 1 | 374 |
| Cytogenetics prior to HSCT | ||
| Good or intermediate | 0 | 901 |
| Poor | 1 | 236 |
| HLA match group | ||
| HLA identical sibling or well matched or partially matched unrelated | 0 | 900 |
| Mismatched unrelated | 1 | 156 |
| Related other than HLA identical sibling | 2 | 81 |
| Circulating blasts | ||
| Absent | 0 | 503 |
| Present | 1 | 634 |
| Karnofsky or Lansky score | ||
| 90-100 | 0 | 604 |
| < 90 | 1 | 533 |
| ALL | ||
| Disease group | ||
| PIF or first untreated relapse | 0 | 172 |
| First refractory relapse | 1 | 206 |
| Second and additional relapse | 2 | 92 |
| Donor CMV | ||
| Negative | 0 | 235 |
| Positive | 1 | 235 |
| Bone marrow blasts, % | ||
| < 25 | 0 | 268 |
| > 25 | 1 | 202 |
| Age, years | ||
| 1-9 | 0 | 45 |
| 10-39 | 1 | 302 |
| > 40 | 2 | 123 |
NOTE. Overall score is defined as the sum of the scores for each risk factor. For AML, four risk groups were defined as follows: score of 0, 1, 2, and ≥ 3. For ALL, three risks groups were defined as follows: score of 0 or 1; 2; and ≥ 3.
Abbreviations: HSCT, hematopoietic stem-cell transplantation; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; PIF, primary induction failure; CR, complete remission; CMV, cytomegalovirus.
Fig 1.
Probability of overall survival after transplantation with acute leukemia in relapse or primary induction failure according to risk score (ie, [A] acute myeloid leukemia score of 0, 1, 2, and ≥ 3; [B] acute lymphoblastic leukemia score of 0 and 1; 2; and ≥ 3).The 3-year survival rates and 95% CIs are indicated.
Multivariate analysis for patients with ALL (Table 4) demonstrated superior survival for HSCT in primary induction failure or first untreated relapse, with fewer than 25% marrow blasts, with a CMV-seronegative donor, and with age younger than 10 years. By using these four risk factors (ie, refractory first or later relapse, ≥ 25% marrow blasts, CMV-positive donor, age > 10 years), a prognostic scoring system also was established for patients with ALL (Table 5). Patients with a score of 0 or 1 (n = 47) had 46% survival at 3 years (95% CI, 32% to 61%), whereas patients with a score ≥ 3 (n = 301) had only 10% 3-year OS (95% CI, 6% to 13%; Fig 1).
DISCUSSION
This study demonstrated that HSCT can induce long-term survival in patients with acute leukemia who are not in CR: 19% with AML and 16% with ALL were alive at 3 years after transplantation. Outcome varied according to pretransplantation variables, and this allowed for the development of a predictive scoring system. Higher-risk patients had a 3-year survival of only 6% in AML and 10% in ALL, whereas 3-year survival for lower-risk patients was 42% in AML and 46% in ALL.
The absence of post-transplantation remission in some patients confounded the calculation of relapse rate, transplantation-related mortality, and DFS; thus, we used 3-year OS as the primary end point. In this setting, post-transplantation survival estimates can be used for clinical decision making and patient counseling. Use of 3-year survival as a proxy for DFS is additionally validated by the minimal decrease in survival between 3 and 5 years after transplantation—only 2% in each group. For these patients with advanced disease, leukemia was the most common single cause of all deaths—42% for AML and 37% for ALL—whereas 58% of AML and 63% of ALL deaths were attributable to nonrelapse causes.
Transplantation for patients with acute leukemia who are not in CR has been controversial.1–12 Nevertheless, 306 of the 371 CIBMTR allotransplant centers reported such patients, which represented 34% and 20% of the patients who underwent transplantation for AML and ALL, respectively, between 1995 and 2004. The OS rates of 16% and 19% in AML and ALL, respectively, are low but offer some hope when interpreted by using critical prognostic factors. Indeed, disease burden at HSCT was often considerable: 40% of the patients had more than 25% marrow blasts and more than half had circulating blasts. Performance score was also low, as nearly half had a Karnofsky or Lansky score less than 90.
To guide the choice whether to proceed to transplantation, we developed a predictive scoring system by using the significant and easily measured pretransplantation variables associated with outcome in a multivariate analysis. Patients with a score ≥ 3 have a dismal outcome, and alternative therapy or other HSCT approaches should be considered. Conversely, we suggest strong consideration of HSCT for patients with a risk score ≤ 2, because their predicted 3-year survival is between 15% and 46%.
Our multivariate analysis may also aid other transplantation-related decision making. The type of myeloablative conditioning regimen, TBI in the conditioning, GVHD prophylaxis, and graft source (ie, PB or bone marrow) did not affect outcome. Therefore, our data do not suggest critical importance for these variables in decision making. There was no impact of the donor type in ALL, whereas all closely matched donors yielded comparable survival in AML. These data suggest that, in the absence of an HLA-identical sibling, a well-matched or partially matched unrelated donor can result in satisfactory outcome. Robust data of umbilical cord blood for HSCT in this population are unavailable.
These data can also influence the choice between immediate transplantation or additional chemotherapy aimed at transplantation in CR. Patients who underwent transplantation with primary induction failure had the best prognosis, which suggests that HSCT should be considered early in patients resistant to initial induction chemotherapy. Indeed, additional chemotherapy could result in toxicity that might limit the success of transplantation. In patients with ALL in first relapse, the prognosis was better in untreated early relapse, which suggests that transplantation should be considered before reinduction chemotherapy. However, our data may reflect a selection of even more aggressive disease for patients still not achieving CR after reinduction chemotherapy. Thus, these data do not enable us to reliably compare transplantation in first relapse versus chemotherapy aimed towards transplantation in second CR.
This retrospective study did not include cord-blood transplantations or reduced-intensity conditioning regimens. Two studies of cord-blood transplantation reported small numbers of patients without CR with outcomes similar to those observed in this study.20,21A retrospective analysis showed that nonmyeloablative and myeloablative regimens had the same outcome in advanced AML and MDS.22 Three studies of reduced-intensity regimens in patients with relapsed or refractory disease reported rates of OS of 15%, 32%, and 45%, although inherent clinical selection of which advanced leukemia patients should receive reduced-intensity allografts seriously confound interpretation of these outcome data.23–25 We could not examine the impact of intensity and length of immunosuppression or the occurrence and severity of GVHD. Most importantly, this predictive model needs to be interpreted cautiously, as it has not been studied prospectively or confirmed with an independent validation cohort. Despite these limitations, our study provides powerful data to guide clinical decision making about HSCT for acute leukemia during relapse.
These data showed that the survival rates of lower-risk patients are almost comparable to those of series of patients who underwent transplantation in CR. However, they also highlight the need for improved treatment strategies for the higher-risk patients. The single most frequent cause of failure was leukemia progression. Additional research on newer antileukemic agents is thus needed to bring patients to CR before transplantation or to incorporate these pharmacologic or immunotherapeutic approaches into the peritransplantation therapy for patients with advanced acute leukemia.
Acknowledgment
We thank Philip McCarthy, Robert Peter Gale, Dipnarine Maharaj, Jurgen Finke, Peter Wiernik, Brian Bolwell, Jane Liesveld, Jordi Sierra, and Donald Bunjes for their helpful comments and insights.
Supported by Public Health Service Grant/Cooperative Agreement No. U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); Grant/Cooperative Agreement No. 5U01HL069294 from NHLBI and NCI; Contract No. HHSH234200637015C with Health Resources and Services Administration (Health Resources and Services Administration/Department of Health and Human Services); and Grants No. N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research. Also supported by grants from AABB, Aetna, American Society for Blood and Marrow Transplantation, Amgen, Medical College of Wisconsin (from anonymous donation), Astellas Pharma US, Baxter International, Bayer HealthCare Pharmaceuticals, Be the Match Foundation, Biogen IDEC, BioMarin Pharmaceutical, Biovitrum AB, BloodCenter of Wisconsin, Blue Cross and Blue Shield Association, Bone Marrow Foundation, Canadian Blood and Marrow Transplant Group, CaridianBCT, Celgene Corporation, CellGenix, GmbH, Centers for Disease Control and Prevention, Children's Leukemia Research Association, ClinImmune Labs, CTI Clinical Trial and Consulting Services, Cubist Pharmaceuticals, Cylex, CytoTherm, DOR BioPharma, Dynal Biotech (an Invitrogen Company); Eisai, Enzon Pharmaceuticals, European Group for Blood and Marrow Transplantation, Gamida Cell, GE Healthcare, Genentech, Genzyme Corporation, Histogenetics, HKS Medical Information Systems, Hospira, Infectious Diseases Society of America, Kiadis Pharma, Kirin Brewery, The Leukemia and Lymphoma Society, Merck and Company, The Medical College of Wisconsin, MGI Pharma, Michigan Community Blood Centers, Millennium Pharmaceuticals, Miller Pharmacal Group, Milliman USA, Miltenyi Biotec, National Marrow Donor Program, Nature Publishing Group, New York Blood Center, Novartis Oncology, Oncology Nursing Society, Osiris Therapeutics, Otsuka America Pharmaceutical, Pall Life Sciences, Pfizer, Saladax Biomedical, Schering Corporation, Society for Healthcare Epidemiology of America, Soligenix, StemCyte, StemSoft Software, Sysmex America, THERAKOS, Thermogenesis Corporation, Vidacare Corporation, Vion Pharmaceuticals, ViraCor Laboratories, ViroPharma, and Wellpoint.
Footnotes
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Gary Schiller, Genzyme Pharmaceuticals, Vion Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Michel Duval, Daniel Weisdorf, John P. Klein, Wensheng He, Jean-Yves Cahn, Mitchell Cairo, Bruce M. Camitta, Rammurti Kamble, Edward Copelan, Marcos de Lima, Vikas Gupta, Armand Keating, Hillard M. Lazarus, Mark R. Litzow, David I. Marks, Richard T. Maziarz, David A. Rizzieri, Gary Schiller, Kirk R. Schultz, Martin S. Tallman
Administrative support: Daniel Weisdorf, Wensheng He, Martin S. Tallman
Provision of study materials or patients: Michel Duval, Daniel Weisdorf, Edward Copelan, Hillard M. Lazarus, Mark R. Litzow, Richard T. Maziarz, David A. Rizzieri, Gary Schiller
Collection and assembly of data: Daniel Weisdorf, Wensheng He
Data analysis and interpretation: Michel Duval, Daniel Weisdorf, John P. Klein, Wensheng He, Jean-Yves Cahn, Mitchell Cairo, Bruce M. Camitta, Rammurti Kamble, Marcos de Lima, Vikas Gupta, Armand Keating, Hillard M. Lazarus, Mark R. Litzow, David I. Marks, Richard T. Maziarz, Kirk R. Schultz
Manuscript writing: Michel Duval, Daniel Weisdorf, Wensheng He, Jean-Yves Cahn, Mitchell Cairo, Bruce M. Camitta, Rammurti Kamble, Edward Copelan, Marcos de Lima, Vikas Gupta, Armand Keating, Hillard M. Lazarus, David I. Marks, Richard T. Maziarz, David A. Rizzieri, Kirk R. Schultz
Final approval of manuscript: Michel Duval, Daniel Weisdorf, John P. Klein, Wensheng He, Jean-Yves Cahn, Mitchell Cairo, Bruce M. Camitta, Rammurti Kamble, Edward Copelan, Marcos de Lima, Vikas Gupta, Armand Keating, Hillard M. Lazarus, Mark R. Litzow, David I. Marks, Richard T. Maziarz, David A. Rizzieri, Gary Schiller, Kirk R. Schultz, Martin S. Tallman
REFERENCES
- 1.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–1093. [PubMed] [Google Scholar]
- 2.Brown RA, Wolff SN, Fay JW, et al. High-dose etoposide, cyclophosphamide, and total body irradiation with allogeneic bone marrow transplantation for patients with acute myeloid leukemia in untreated first relapse: A study by the North American Marrow Transplant Group. Blood. 1995;85:1391–1395. [PubMed] [Google Scholar]
- 3.Brown RA, Wolff SN, Fay JW, et al. High-dose etoposide, cyclophosphamide and total body irradiation with allogeneic bone marrow transplantation for resistant acute myeloid leukemia: A study by the North American Marrow Transplant Group. Leuk Lymphoma. 1996;22:271–277. doi: 10.3109/10428199609051758. [DOI] [PubMed] [Google Scholar]
- 4.Godder KT, Hazlett LJ, Abhyankar SH, et al. Partially mismatched related-donor bone marrow transplantation for pediatric patients with acute leukemia: Younger donors and absence of peripheral blasts improve outcome. J Clin Oncol. 2000;18:1856–1866. doi: 10.1200/JCO.2000.18.9.1856. [DOI] [PubMed] [Google Scholar]
- 5.Goldman FD, Rumelhart SL, DeAlacron P, et al. Poor outcome in children with refractory/relapsed leukemia undergoing bone marrow transplantation with mismatched family member donors. Bone Marrow Transplant. 2000;25:943–948. doi: 10.1038/sj.bmt.1702373. [DOI] [PubMed] [Google Scholar]
- 6.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: The effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997;89:4226–4235. [PubMed] [Google Scholar]
- 7.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: A retrospective study of 379 patients reported to the Société Francaise de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 8.Greinix HT, Reiter E, Keil F, et al. Leukemia-free survival and mortality in patients with refractory or relapsed acute leukemia given marrow transplants from sibling and unrelated donors. Bone Marrow Transplant. 1998;21:673–678. doi: 10.1038/sj.bmt.1701152. [DOI] [PubMed] [Google Scholar]
- 9.Wong R, Shahjahan M, Wang X, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Hosing C, Saliba RM, Shahjahan M, et al. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone Marrow Transplant. 2005;36:157–162. doi: 10.1038/sj.bmt.1705011. [DOI] [PubMed] [Google Scholar]
- 11.Nemecek ER, Gooley TA, Woolfrey AE, et al. Outcome of allogeneic bone marrow transplantation for children with advanced acute myeloid leukemia. Bone Marrow Transplant. 2004;34:799–806. doi: 10.1038/sj.bmt.1704689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyekunle AA, Kröger N, Zabelina T, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: A long-term follow-up. Bone Marrow Transplant. 2006;37:45–50. doi: 10.1038/sj.bmt.1705207. [DOI] [PubMed] [Google Scholar]
- 13.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 14.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 16.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 17.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein JP, Logan B, Harhoff M, et al. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 19.Gerds TA, Schumacher M. Consistent estimation of the expected Brier score in general survival models with right-censored event times. Biom J. 2006;48:1029–1040. doi: 10.1002/bimj.200610301. [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Rocha V, Chastang C, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia: Eurocord-Cord Blood Transplant Group. Blood. 1999;93:3662–3671. [PubMed] [Google Scholar]
- 21.Michel G, Rocha V, Chevret S, et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: A Eurocord Group analysis. Blood. 2003;102:4290–4297. doi: 10.1182/blood-2003-04-1288. [DOI] [PubMed] [Google Scholar]
- 22.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Craddock C, Nagra S, Peniket A, et al. Factors predicting long-term survival after T-cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2009;95:989–995. doi: 10.3324/haematol.2009.013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks R, Potthoff K, Hahn J, et al. Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: Particular activity against advanced hematologic malignancies. Blood. 2008;112:415–425. doi: 10.1182/blood-2007-08-104745. [DOI] [PubMed] [Google Scholar]
- 25.Schmid C, Schleuning M, Schwerdtfeger R, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]