Abstract
Purpose
Emerging clinical evidence suggests intravenous bisphosphonates may inhibit breast cancer while oral bisphosphonates have received limited evaluation regarding breast cancer influence.
Patients and Methods
The association between oral bisphosphonate use and invasive breast cancer was examined in postmenopausal women enrolled onto the Women's Health Initiative (WHI). We compared a published hip fracture prediction model, which did not incorporate bone mineral density (BMD), with total hip BMD in 10,418 WHI participants who had both determinations. To adjust for potential BMD difference based on bisphosphonate use, the hip fracture prediction score was included in multivariant analyses as a BMD surrogate.
Results
Of the 154,768 participants, 2,816 were oral bisphosphonate users at entry (90% alendronate, 10% etidronate). As calculated hip fracture risk score was significantly associated with both BMD (regression line = 0.79 to 0.0478 log predicted fracture; P < .001; r = 0.43) and breast cancer incidence (P = .03), this variable was incorporated into regression analyses to adjust for BMD difference between users and nonusers of bisphopshonate. After 7.8 mean years of follow-up (standard deviation, 1.7), invasive breast cancer incidence was lower in bisphosphonate users (hazard ratio [HR], 0.68; 95% CI, 0.52 to 0.88; P < .01) as was incidence of estrogen receptor (ER) –positive invasive cancers (HR, 0.70; 95% CI, 0.52 to 0.94, P = .02). A similar but not significant trend was seen for ER-negative invasive cancers. The incidence of ductal carcinoma in situ was higher in bisphosphonate users (HR, 1.58; 95% CI, 1.08 to 2.31; P = .02).
Conclusion
Oral bisphosphonate use was associated with significantly lower invasive breast cancer incidence, suggesting bisphosphonates may have inhibiting effects on breast cancer.
INTRODUCTION
Bisphosphonates are commonly used for osteoporosis therapy1 and to reduce skeletal-related complications in patients with cancer with bone metastases.2,3 In addition, preclinical studies4,5 have led to clinical trials of bisphosphonates in early-stage breast cancer. Emerging evidence suggests both oral and intravenous bisphosphonates may reduce breast cancer recurrences6–8 and may also reduce locoregional recurrences.8,9
Lower bone mineral density (BMD) is both an indication for bisphosphonate use and is associated with lower breast cancer incidence.10,11 Perhaps as a result of an inability to control for BMD as a potential confounding factor, prior observational studies have not evaluated the association between oral bisphosphonate use and breast cancer incidence.
Results from analyses in the Women's Health Initiative (WHI) provide an opportunity to adjust for potential confounding by indication between bisphosphonate users and nonusers. Baseline BMD determinations were made in a subset of 10,693 WHI cohort participants. In analyses involving 9,941 of these women without a prior cancer history, hip BMD was significantly inversely related to breast cancer risk, a finding independent of the Gail breast cancer risk score.11 In addition, a model predictive of 5-year hip fracture risk, which did not include BMD, was developed and validated in the full WHI cohort.12 Thus, an association between BMD levels and 5-year hip fracture risk score in participants with both determinations could support use of the WHI hip fracture risk score as a surrogate for BMD in the full WHI cohort. Consequently, we examined the relationship between bisphosphonate use and breast cancer risk in the WHI cohort of postmenopausal women where adjustment for potential BMD differences between bisphosphonate users and nonusers could be addressed via the hip fracture risk score.
PATIENTS AND METHODS
Study Population
The WHI entered postmenopausal women into an observational study (n = 93,676) and four clinical trials (n = 68,132). Recruitment involved 40 clinical centers entering participants between 1993 and 1998. Postmenopausal women age 50 to 79 years, accessible for follow-up, and with an estimated survival of ≥ 3 years were eligible. The clinical trials had additional eligibility requirements related to medical history and adherence issues. The current analyses includes all enrolled women excluding those previously diagnosed with breast cancer or who used tamoxifen or raloxifene (n = 154,768). Detailed study methods have been previously described.13,14
All participants signed an informed consent. The institutional review boards at all participating institutions approved the protocols and procedures.
Measurement of Exposure
Participants completed questionnaires regarding personal demographics, medical, reproductive and family history, smoking and alcohol use, personal habits, and recreational physical activity. In calculation of the Gail 5-year breast cancer risk estimate,15 as benign biopsy details were not collected, women with prior biopsy were coded as unknown for the atypical hyperplasia variable.
Medication use including bisphosphonates was collected from an interview-administered questionnaire at baseline and at year 3 in all participants and additionally at year 1 in clinical trial participants. For those reporting bisphosphonate use, the type of compound and duration of use were recorded and validated by checking pill box labels. Current and previous use of menopausal hormone therapy and oral contraceptives were determined by interview as previously described.14,16
BMD determinations at baseline were made in 10,693 WHI women participating in a study conducted at three WHI clinical centers using dual-energy x-ray absorptiometry (DXA QR; Hologic Inc, Waltham, MA). The DXA scans followed standard protocols including weekly phantom scans at each clinic and use of calibration phantom periodically circulated between clinics which were implemented by technicians trained and certified by both Hologic Inc and the WHI Bone Density Coordinating Center at the University of California at San Francisco.11
Five-year risk of hip fracture was calculated using a published algorithm developed in the WHI cohort,12 which incorporates 11 clinical factors including age, self-reported health, weight, height, race/ethnicity, self-reported physical activity, history of fracture after age 54 years, parental hip fracture, current smoking, current corticosteroid use, and treated diabetes. The algorithm does not include BMD, and its predictive ability was not improved by BMD addition.12
Breast Cancer Screening and Diagnosis
Medical history was updated annually (in the observational study) or semi-annually (in the clinical trials) by questionnaire. Breast cancer self reports were verified at each clinic by medical record and pathology report review by centrally trained WHI physician adjudicators. Final central adjudication and coding of histology, stage, and hormone receptor status (estrogen receptor [ER] and progesterone receptor positive or negative after pathology report) was performed at the clinical coordinating center by adjudicators blind to clinical trial participation status or medication use.17
Mammogram and breast exam frequency were protocol defined in the clinical trials and were performed at baseline and annually in the hormone trials and at baseline and biannually in the dietary modification trial. Mammography and breast exam frequency were not protocol determined in the observational study. Information on clinical breast exam and mammography usage was collected annually from all participants.
Statistical Methods
Baseline characteristics of bisphosphonate users at baseline were compared with those of nonusers by χ2 tests (for categorical variables) or two-sample t tests (for continuous variables). Invasive breast cancer incidence rates per 1,000 person-years (PY) were calculated according to bisphosphonate use. Separate analyses were conducted for ER-positive and ER-negative invasive breast cancers as well as for ductal carcinoma in situ.
For the primary analyses, Cox regression models were used to compute hazard ratios (HRs) and 95% CIs for breast cancer incidence among the 2,816 bisphosphonate users at baseline versus the 151,952 nonusers at baseline. Cumulative incidence curves were estimated using the Kaplan-Meier method. In the age-adjusted analyses, the Cox proportional hazard analyses are adjusted for age and stratified on WHI trial component and randomization arm. In the multivariate-adjusted analyses, Cox proportional hazard analyses are adjusted for age, ethnicity, smoking, alcohol use, physical activity, body mass index, mammograms in the past 2 years, prior hormone use, total calcium intake, total vitamin D intake, calculated 5-year risk of hip fracture, calculated Gail 5-year risk of breast cancer, and stratified on WHI trial component and randomization arm. Women with missing values for a given covariate are excluded from analyses including the covariate.
In a secondary time-dependant Cox regression analysis, association with time since initiation of therapy was examined. In this model, bisphosphonate use by 2,816 women at baseline was updated at the year 1 (clinical trial participants only) and year 3 (all participants) visits to capture initiation of use during follow-up, resulting in a total of 9,741 exposed women. HRs for bisphosphonate use were estimated for the time intervals 0 to 2 years, 2 to 5 years, and more than 5 years since therapy initiation, accounting for duration of use at first report.
Tumor stage, grade, histology, and hormone receptor status were compared in baseline users and nonusers with χ2 statistics. Tumors with missing values were omitted from these analyses.
Interactions between baseline characteristics and bisphosphonate use were assessed in age-adjusted Cox proportional hazards analyses that included both the risk factor (in its continuous form) and bisphosphonate use as main effects. P values for assessing possible interactions are from Wald χ2 tests. Four subgroup comparisons were computed, less than 1 would be expected to be significant at the .05 level by chance alone. All analyses were conducted using SAS software, version 9.1 (SAS Institute, Cary, NC) and S-Plus version 8.0 (Insightful Corp, Seattle, WA). All statistical tests were two sided.
RESULTS
In this cohort of 154,768 participants, 2,816 were oral bisphosphonate users at entry and are included in the main analyses. Participants who were bisphosphonates users were more likely to be white, have a fracture family history, and higher calcium and vitamin D intake. The bisphosphonate users also had substantially higher calculated 5-year probability of hip fracture (for all mentioned variables P < .01).
Bisphosphonate users had a higher Gail model breast cancer risk and were older, more likely to have a prior benign breast biopsy and recent mammography and have a breast cancer family history. However, bisphosphonate users had higher physical activity and lower body mass index. Although many of the absolute differences in characteristics between bisphosphonate users and nonusers were small (with exceptions noted above) almost all were statistically significant given the large number of women in the cohort (Table 1).
Table 1.
Baseline Characteristics by Bisphosphonate Use
| Characteristic | Bisphosphonate Use |
P* | ||||
|---|---|---|---|---|---|---|
| No (n = 151,952) |
Yes (n = 2,816) |
|||||
| No. | % | No. | % | |||
| Age at screening, years | < .01 | |||||
| 50-59 | 51,421 | 33.8 | 351 | 12.5 | ||
| 60-69 | 68,190 | 44.9 | 1,318 | 46.8 | ||
| 70-79 | 32,341 | 21.3 | 1,147 | 40.7 | ||
| Race/ethnicity | < .01 | |||||
| White | 125127 | 82.3 | 2,535 | 90.0 | ||
| Black | 13,911 | 9.2 | 30 | 1.1 | ||
| Hispanic | 6,165 | 4.1 | 78 | 2.8 | ||
| American Indian | 677 | 0.4 | 1 | 0.0 | ||
| Asian/Pacific Islander | 3,941 | 2.6 | 137 | 4.9 | ||
| Unknown | 2,131 | 1.4 | 35 | 1.2 | ||
| Education | < .01 | |||||
| 0-8 years | 2,522 | 1.7 | 23 | 0.8 | ||
| Some high school | 5,641 | 3.7 | 72 | 2.6 | ||
| High school diploma/GED | 26,050 | 17.3 | 461 | 16.5 | ||
| School after high school | 57,399 | 38.1 | 924 | 33.1 | ||
| College degree or higher | 59,218 | 39.3 | 1,310 | 47.0 | ||
| Smoking | < .01 | |||||
| Never | 76,569 | 51.0 | 1,491 | 53.7 | ||
| Past | 62,959 | 41.9 | 1,158 | 41.7 | ||
| Current | 10,614 | 7.1 | 127 | 4.6 | ||
| Alcohol use | < .01 | |||||
| Non/past drinker | ||||||
| < 1 drink/month | 18,919 | 12.5 | 259 | 9.3 | ||
| < 1 drink/week | 30,996 | 20.5 | 600 | 21.5 | ||
| 1-< 7 drinks per week | 38,673 | 25.6 | 739 | 26.5 | ||
| 7+ drinks per week | 17,529 | 11.6 | 345 | 12.4 | ||
| Recreational physical activity, minutes/week | < .01 | |||||
| None | 23,187 | 16.0 | 337 | 12.1 | ||
| 10-< 115 | 40,462 | 27.9 | 649 | 23.3 | ||
| 115-< 255 | 43,383 | 29.9 | 931 | 33.4 | ||
| ≥ 255+ | 37,990 | 26.2 | 874 | 31.3 | ||
| Body mass index, kg/m2 | < .01 | |||||
| ≤ 23 | 28,412 | 18.9 | 921 | 32.9 | ||
| < 23-≤ 26 | 35,920 | 23.8 | 883 | 31.6 | ||
| > 26-≤ 30 | 40,247 | 26.7 | 621 | 22.2 | ||
| > 30 | 46,047 | 30.6 | 371 | 13.3 | ||
| Mammogram in the last 2 years | 122,497 | 83.2 | 2,484 | 91.0 | < .01 | |
| Have a current medical care provider | 140,896 | 93.6 | 2,713 | 97.6 | < .01 | |
| Age at menarche, years | < .01 | |||||
| < 11 | 33,370 | 22.0 | 508 | 18.1 | ||
| 12 | 39,455 | 26.0 | 741 | 26.4 | ||
| 13 | 43,796 | 28.9 | 858 | 30.5 | ||
| 14+ | 34,932 | 23.0 | 702 | 25.0 | ||
| Ever pregnant | 137,958 | 90.9 | 2,484 | 88.3 | < .01 | |
| Age at first birth, years | < .01 | |||||
| Never pregnant | 13,769 | 10.0 | 328 | 12.9 | ||
| No term pregnancy | 4,016 | 2.9 | 73 | 2.9 | ||
| < 20 | 19,755 | 14.3 | 183 | 7.2 | ||
| 20-24 | 57,290 | 41.6 | 967 | 38.2 | ||
| 25-29 | 31,809 | 23.1 | 738 | 29.1 | ||
| ≥ 30 | 11,085 | 8.0 | 244 | 9.6 | ||
| No. of live births | < .01 | |||||
| Never pregnant | 13,769 | 9.1 | 328 | 11.7 | ||
| None | 4,189 | 2.8 | 75 | 2.7 | ||
| 1 | 13,747 | 9.1 | 246 | 8.8 | ||
| 2 | 38,689 | 25.6 | 742 | 26.5 | ||
| 3 | 36,762 | 24.3 | 660 | 23.6 | ||
| ≥ 4 | 44,023 | 29.1 | 747 | 26.7 | ||
| Gravidity | ||||||
| Never pregnant | 13,769 | 9.1 | 328 | 11.7 | < .01 | |
| 1 | 10,555 | 7.0 | 198 | 7.1 | ||
| 2-4 | 89,268 | 58.9 | 1,679 | 59.8 | ||
| ≥ 5 | 37,839 | 25.0 | 602 | 21.4 | ||
| Parity | < .01 | |||||
| Never pregnant | 13,769 | 9.1 | 328 | 11.7 | ||
| Never had term pregnancy | 4,016 | 2.7 | 73 | 2.6 | ||
| 1 | 13,311 | 8.8 | 240 | 8.6 | ||
| 2 | 37,814 | 25.0 | 725 | 25.9 | ||
| 3 | 36,519 | 24.1 | 649 | 23.2 | ||
| ≥ 4 | 45,814 | 30.3 | 785 | 28.0 | ||
| Benign breast disease | < .01 | |||||
| No | 113,445 | 78.8 | 2,017 | 72.6 | ||
| Yes, 1 biopsy | 21,551 | 15.0 | 495 | 17.8 | ||
| Yes, ≥ 2 biopsies | 9,041 | 6.3 | 265 | 9.5 | ||
| Hysterectomy | 63,698 | 41.9 | 908 | 32.3 | < .01 | |
| Bilateral oophorectomy | 29,565 | 19.9 | 461 | 16.7 | < .01 | |
| Menopausal hormone therapy use | < .01 | |||||
| Never | 65,621 | 43.2 | 1,298 | 46.2 | ||
| Past | 23,351 | 15.4 | 594 | 21.1 | ||
| Current, years | ||||||
| 5 | 17,878 | 11.8 | 252 | 9.0 | ||
| 5-< 10 | 15,562 | 10.3 | 207 | 7.4 | ||
| ≥ 10 | 29,411 | 19.4 | 461 | 16.4 | ||
| Estrogen plus progestin use† | ||||||
| Yes‡ | 105,025 | 69.1 | 1,905 | 67.7 | ||
| No | 46,881 | 30.9 | 910 | 32.3 | ||
| Estrogen alone use | ||||||
| Yes§ | 94,572 | 62.3 | 1,936 | 68.8 | ||
| No | 57,281 | 37.7 | 877 | 31.2 | ||
| Oral contraceptive use, years | < .01 | |||||
| Never users | 88,335 | 58.2 | 1,990 | 70.7 | ||
| < 5 | 35,130 | 23.1 | 463 | 16.4 | ||
| 5-< 10 | 14,473 | 9.5 | 159 | 5.6 | ||
| ≥ 10 | 13,964 | 9.2 | 204 | 7.2 | ||
| Bisphosphonate type | — | |||||
| Alendronate sodium | — | — | 2,527 | 89.7 | ||
| Etidronate disodium | — | — | 285 | 10.1 | ||
| Pamidronate disodium | — | — | 1 | <0.1 | ||
| Tiludronate disodium | — | — | 1 | <0.1 | ||
| More than one | — | — | 2 | 0.1 | ||
| Years of bisphosphonate use | < .01 | |||||
| < 1 | 0 | 0.0 | 1,477 | 52.5 | ||
| 1-< 3 | 0 | 0.0 | 1,045 | 37.1 | ||
| ≥ 3 | 0 | 0.0 | 294 | 10.4 | ||
| Aspirin use | 31,757 | 20.9 | 702 | 24.9 | ||
| NSAID use | 51,918 | 34.2 | 1,021 | 36.3 | ||
| General health rating | < .01 | |||||
| Excellent | 26,176 | 17.3 | 381 | 13.6 | ||
| Very good | 62,061 | 41.1 | 1,079 | 38.6 | ||
| Good | 49,397 | 32.7 | 1,011 | 36.2 | ||
| Fair | 12,320 | 8.2 | 296 | 10.6 | ||
| Poor | 1114 | 0.7 | 29 | 1.0 | ||
| Gail risk > 1.7% | 57,581 | 37.9 | 1,633 | 58.0 | < .01 | |
| Family history of breast cancer | 26,123 | 18.2 | 586 | 22.1 | < .01 | |
| Family history of fracture after 40 years of age | 55,283 | 39.4 | 1,294 | 49.7 | < .01 | |
| History of fracture | < .01 | |||||
| At any age | 97,484 | 84.2 | 1,553 | 64.2 | ||
| At age ≥ 55 years | 18,330 | 15.8 | 867 | 35.8 | ||
| No. of falls in previous 12 months | < .01 | |||||
| None | 98,794 | 67.6 | 1,804 | 64.6 | ||
| 1 | 29,230 | 20.0 | 600 | 21.5 | ||
| 2 | 12,042 | 8.2 | 243 | 8.7 | ||
| ≥ 3 | 6,064 | 4.1 | 144 | 5.2 | ||
| Total calcium intake (supplements, diet, and medications), mg/d | < .01 | |||||
| Mean | 147,414 | 1,180.2 (727.8) | 2,718 | 1,604.3 (869.2) | ||
| > 800 | 38,806 | 26.3 | 304 | 11.2 | ||
| 800 to < 1,200 | 36,774 | 24.9 | 537 | 19.8 | ||
| ≥ 1,200 | 60,385 | 41.0 | 1,802 | 66.3 | ||
| Total vitamin D intake (supplements and diet), U/d | < .01 | |||||
| Mean | 147,414 | 368.5 (276.5) | 2,718 | 500.7 (310.6) | ||
| 200 | 56,130 | 38.1 | 554 | 20.4 | ||
| 200 to < 400 | 27,390 | 18.6 | 466 | 17.1 | ||
| 400 to < 600 | 36,117 | 24.5 | 738 | 27.2 | ||
| ≥ 600 | 27,777 | 18.8 | 960 | 35.3 | ||
| Total body BMD, g/cm2 | 10,303 | 1.02 (0.11) | 123 | 0.93 (0.09) | < .01 | |
| Total hip BMD, g/cm2 | 10,296 | 0.85 (0.14) | 122 | 0.72 (0.12) | < .01 | |
| 5-year probability of hip fracture tertiles, % | < .01 | |||||
| < 0.15564 | 50,771 | 33.4 | 301 | 10.7 | ||
| 0.15564-0.48349 | 51,853 | 34.1 | 770 | 27.3 | ||
| ≥ 0.48350 | 49,328 | 32.5 | 1745 | 62.0 | ||
Abbreviations: GED, general equivalency degree; NSAID, nonsteriodal anti-inflammatory drug; BMD, bone mineral density; E, estrogen; P, progestin; HT, hormone therapy; WHI, Women's Health Initiative.
P value is from a χ2 test of independence. All comparisons P < .01 except for enrollments onto clinical trials.
BMD determinations at baseline from women participating in the WHI at three clinical centers. Baseline characteristics of this subgroup were similar to the overall population and are detailed elsewhere.11
Includes use prior to entry and random assignment to E + P in HT trial.
Includes use prior to entry and random assignment to E alone in the HT trial.
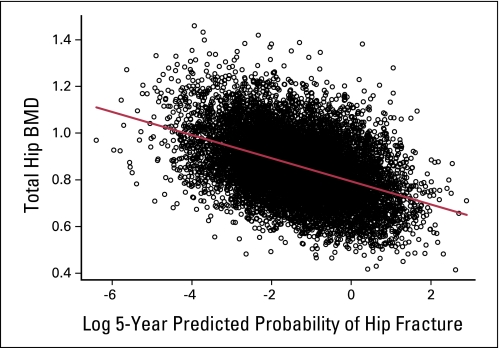
During the initial follow-up year, bisphosphonate users more commonly had mammograms than nonusers (71.8% v 61.6%, respectively; P < .001), but fewer breast biopsies (2.3% v 2.9%, respectively; P = .07). These trends continued throughout the follow-up period (Table 1; Appendix Table A1 , online only). In the 10,693 women with BMD determinations, total hip BMD was lower in bisphosphonate users compared to nonusers (0.72 ± 0.12 g/cm2 mean ± standard deviation v 0.85 ± 0.14, respectively; P < .01). To assess whether the 5-year hip fracture risk score could be used to adjust for BMD differences between bisphosphonate users and nonusers to control for potential confounding by indication, the relationship between the log of the calculated 5-year hip fracture risk was compared to total hip BMD in the 10,418 women who had both baseline total hip BMD determination and information on the 11 variables used for hip fracture prediction. A significant correlation (regression line = 0.79 + 0.0478 × log predicted hip fracture; P < .001; r = 0.43) was seen (Fig 1). In addition, in a Cox regression model examining the hip fracture risk score and breast cancer incidence in nonbisphosphonate users, an increasing probability of hip fracture was associated with decreased incidence of breast cancer (HR, 0.95; 95% CI, 0.90 to 0.99; P = .025). As a result, the hip fracture risk score was incorporated in the multivariate adjusted Cox proportional hazards analyses.
Fig 1.
The 5-year predicted probability of hip fracture was calculated from an 11-item algorithm, which does not incorporate bone mineral density (BMD). The log of the predicted probability of hip fracture is compared with total hip BMD at baseline in the 10,418 women who had both determinations. A significant correlation is seen (regression line = 0.79, 0478 log predicted hip fracture; P < .001; r = 0.43). The predicted probability of hip fracture was also significantly associated (hazard ratio, 0.95; 95% CI, 0.90 to 0.99; P = .025) with breast cancer incidence when considered as a continuous variable in a model adjusted for age and race/ethnicity and stratified by Women's Health Initiative trial component.
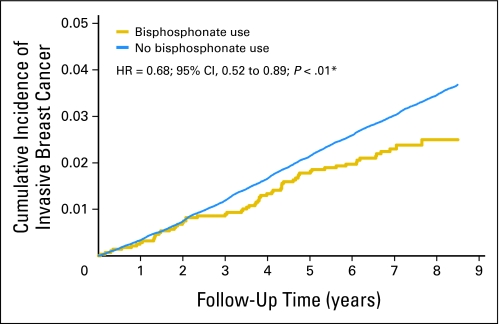
During a total of 1,202,865 PY of observation, 5,156 women were diagnosed with invasive breast cancer and 1,120 women were diagnosed with DCIS (Table 2). In age-adjusted analyses, the incidence of invasive breast cancer was 31% lower among women reporting bisphosphonate use at entry (64 cases, 3.29 per 1,000 PY) than among nonusers (5,092 cases, 4.38 per 1,000 PY) in analyses stratified on WHI trial component and randomization arm (HR, 0.69; 95% CI, 0.54 to 0.88; P < .01). Similarly, in the multivariate adjusted model, invasive breast cancer incidence was 32% lower in bisphosphonate users then in nonusers (HR, 0.68; 95% CI, 0.52 to 0.88; P < .01; Table 2). The cumulative breast cancer incidence over time in bisphosphonate users at entry versus nonusers is depicted in Figure 2.
Table 2.
Breast Cancer Incidence by Bisphosphonate Use
| Parameter | Bisphosphonate Use |
Multivariate Adjusted* |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||||
| No. of Cases | Person Years | Rate/1,000 Person Years | No. of Cases | Person Years | Rate/1,000 Person Years | Hazard Ratio | 95% CI | P | |
| Invasive breast cancer | 5,092 | 1,163,344 | 4.38 | 64 | 19,466 | 3.29 | 0.68 | 0.52 to 0.89 | < .01 |
| ER positive | 3,829 | 1,168,210 | 3.28 | 50 | 19,514 | 2.56 | 0.70 | 0.52 to 0.95 | .02 |
| ER negative | 717 | 1,180,485 | 0.61 | 8 | 19,666 | 0.41 | 0.66 | 0.31 to 1.39 | .27 |
| In situ breast cancer† | 1,090 | 1,178,967 | 0.92 | 30 | 19,601 | 1.53 | 1.59 | 1.09 to 2.33 | .02 |
Abbreviations: ER, estrogen receptor; E, estrogen; P, progestin.
From Cox proportional hazards regression models adjusted for age, ethnicity, smoking, alcohol use, physical activity, body mass index, mammogram in the last 2 years, prior E alone use, prior E+P use, total calcium, total vitamin D, 5-year risk of hip fracture, and Gail 5-year risk of breast cancer and stratified on Women's Health Initiative trial component and randomization arm.
Excludes lobular carcinoma in situ tumors.
Fig 2.
The cumulative incidence of invasive breast cancer after entry into the cohort by bisphosphonate use at baseline. Hazard ratio (HR) and 95% CI from a multivariate-adjusted Cox proportional hazards analysis.
The incidence of ER-positive cancers was 30% lower in bisphosphonate users (HR, 0.70; 95% CI, 0.52 to 0.94; P = .02). A similar trend for lower incidence of ER-negative cancers with bisphosphonate use was also seen but the latter was not statistically significant (HR, 0.66; 95% CI, 0.31 to 1.39; P = .27). There were no apparent differences in the stage or grade of breast cancers in bisphosphonate users. In bisphosphonate users the percentage of infiltrating ductal carcinomas was somewhat less and the percentage of combined infiltrating ductal and lobular carcinomas was somewhat greater than in nonusers (P for trend = .06; Table 3). Mortality related to invasive breast cancer was lower in bisphosphonate users (two deaths, 1.02 deaths/10,000 PY) than among nonusers (252 deaths, 2.13 deaths/10,000 PY) although the number of events was limited. For DCIS, the incidence was higher among women reporting bisphosphonate use (1.53 per 1,000 PY) than among nonusers (0.92 per 1,000 PY; HR, 1.58; 95% CI, 1.08 to 2.31; P = .02; Table 2).
Table 3.
Invasive Breast Cancer Tumor Characteristics by Bisphosphonate Use
| Characteristic | Bisphosphonate Use |
P | |||
|---|---|---|---|---|---|
| No |
Yes |
||||
| No. | % | No. | % | ||
| SEER stage | |||||
| Localized | 3,700 | 75.1 | 42 | 71.2 | |
| Regional | 1,176 | 23.9 | 16 | 27.1 | .74 |
| Distant | 52 | 1.1 | 1 | 1.7 | |
| Missing | 164 | 3.2 | 5 | 7.8 | .04 |
| Grade | |||||
| Well differentiated | 1,240 | 28.0 | 12 | 20.3 | |
| Moderately differentiated | 1,890 | 42.7 | 30 | 50.8 | .54 |
| Poorly differentiated | 1,164 | 26.3 | 15 | 25.4 | |
| Anaplastic | 137 | 3.1 | 2 | 3.4 | |
| Missing | 661 | 13.0 | 5 | 7.8 | .22 |
| Histology | |||||
| Infiltrating ductal | 3,182 | 63.4 | 33 | 53.2 | |
| Lobular | 487 | 9.7 | 4 | 6.5 | |
| Infiltrating ductal and lobular carcinoma | 705 | 14.0 | 16 | 25.8 | .06 |
| Tubular | 183 | 3.6 | 4 | 6.5 | |
| Other | 465 | 9.3 | 5 | 8.1 | |
| Missing | 70 | 1.4 | 2 | 3.1 | .22 |
| Hormone receptor status | |||||
| Estrogen receptor assay | |||||
| Positive | 3,829 | 84.0 | 50 | 86.2 | .85 |
| Negative | 717 | 15.7 | 8 | 13.8 | |
| Borderline | 13 | 0.3 | 0 | 0.0 | |
| Missing | 533 | 10.5 | 6 | 9.4 | .78 |
| Progesterone receptor assay | |||||
| Positive | 3,126 | 69.7 | 42 | 75.0 | .42 |
| Negative | 1,327 | 29.6 | 13 | 23.2 | |
| Borderline | 34 | 0.8 | 1 | 1.8 | |
| Missing | 605 | 11.9 | 8 | 12.5 | .88 |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Four subgroup analyses were performed (Table 4). There were more breast cancers diagnosed in bisphosphonate users compared to nonusers in women at higher hip fracture risk, but the interaction term was not significant.
Table 4.
Invasive Breast Cancer in Bisphosphonate Users v Nonusers by Baseline Risk Factors
| Risk Factor | No. of Patients | Hazard Ratio | 95% CI | P for Interaction |
|---|---|---|---|---|
| Gail risk | .63 | |||
| ≤ 1.7% | 2,646 | 0.48 | 0.28 to 0.84 | |
| > 1.7% | 2,446 | 0.78 | 0.58 to 1.06 | |
| Body mass index, kg/m2 | .68 | |||
| ≤ 26 | 2,091 | 0.76 | 0.55 to 1.04 | |
| > 26 | 2,961 | 0.54 | 0.34 to 0.88 | |
| Recreational physical activity, minutes/week | .59 | |||
| < 115 | 2,093 | 0.65 | 0.41 to 1.02 | |
| ≥ 115 | 2,729 | 0.70 | 0.51 to 0.97 | |
| 5-year probability of hip fracture tertiles, % | .26 | |||
| < 0.156 | 1,515 | 1.34 | 0.72 to 2.50 | |
| 0.156-0.483 | 1,788 | 0.74 | 0.45 to 1.21 | |
| ≥ 0.483 | 1,789 | 0.57 | 0.40 to 0.82 |
NOTE. From age-adjusted Cox proportional hazards analyses that included both the risk factor (in its continuous form) and bisphosphonate use as main effects. P values for assessing possible interactions are from Wald χ2 tests.
The association between time since initiation of bisphosphonate use and breast cancer incidence was examined in time-dependent analyses including the 9,741 women who reported bisphosphonate use either at entry or at the year 1 or year 3 clinic visits of the study period in comparison to the 145,027 women who never reported bisphosphonate use. A significant association was seen with lower breast cancer risk for women after short term use (P < .01; < 2 years use: HR, 0.50; 95% CI, 0.38 to 0.67; 2 to 5 years use: HR, 0.86; 95% CI, 0.64 to 1.17; > 5 years use, HR, 0.83; 95% CI, 0.53 to 1.27).
DISCUSSION
In a prospective cohort of postmenopausal women a statistically significant association was seen between oral bisphosphonate use and lower invasive breast cancer incidence. There were fewer ER-positive breast cancers diagnosed in bisphosphonate users and there was a trend for fewer ER-negative breast cancers in bisphosphonate users as well.
Low BMD has been associated with low breast cancer risk.10,11 However the strong significant association seen between a non-BMD containing, calculated 5-year hip fracture risk estimate17 and both BMD as well as breast cancer incidence, supports the use of the 5-year hip fracture score in the multivariate model to adjust for potential BMD difference between bisphosphonate users and nonusers.
A lower breast cancer incidence was seen in bisphosphonate users after relatively short-term use while a null association was seen with longer duration use. These findings are consistent with a direct effect of bisphosphonate on slowing or inhibiting growth of preclinical but already established breast cancers.
In this study, bisphosphonate use was associated with increased incidence of DCIS. The clinical significance of this finding is uncertain as much DCIS either does not develop into invasive breast cancer or does so with such delay to question clinical relevance.18,19 Women with DCIS receiving contemporary management, even those having mastectomy, remain at similar or higher risk of developing subsequent invasive breast cancer compared to women without that diagnosis.19,20 Thus, the excess of DCIS cases in bisphosphonate users would not lower the frequency of future invasive breast cancers in that group. In any event, if bisphosphonates prevent in situ cancers from progressing to an invasive stage or influence only invasive cancer, a relative increase in in situ cancers could result. Perhaps a similar differential effect was seen in the Breast Cancer Prevention Trial where tamoxifen and raloxifene both reduced invasive breast cancers despite a strong trend (P = .051) for more in situ cancers in the raloxifene group.21
Biologic plausibility for the study findings comes from preclinical and emerging clinical evidence which may be independent of the well-defined bone-mediated effects of bisphosphonates to reduce osteoclast activity and prevent release of factors that foster tumor growth.5,22 Angiogenesis inhibition23,24 and increased cancer surveillance via activation of gamma delta T cells represent other potential mediating mechanisms.25
Three adjuvant breast cancer studies have evaluated the oral bisphosphonates clodronate's influence on recurrence. In the largest trial, which randomly assigned 1,069 patients, those receiving clodronate 1,600 mg/d had significantly fewer bone metastases and longer survival compared to those in the placebo group.6 One of two smaller adjuvant trials also reported positive effects of clodronate on breast cancer outcomes.26–28 In another randomized adjuvant breast cancer trial, the oral bisphosphonate paimidronate did not significantly reduce bone metastases.29
Four adjuvant breast cancer studies have evaluated the intravenous bisphosphonate zoledronic acid.7–9,30 In an Austrian Breast Cancer Study Group trial, patients with breast cancer randomly assigned to receive zoledronic acid, 4 mg every 6 months, had significantly greater disease-free survival (HR, 0.69; 95% CI, 0.46 to 0.91; P = .012) and fewer locoregional recurrences and contralateral breast cancers.8 In a combined analysis from the similar ZFAST (Zometa-Femara Adjuvant Synergy Trial) and ZOFAST (Zoledronic Acid in the Prevention of Cancer Treatment–Induced Bone Loss in Postmenopausal Women Receiving Letrozole as Adjuvant Therapy for Early Breast Cancer) studies, postmenopausal patients with breast cancer randomly assigned to receive zoledronic acid, 4 mg intravenously every 6 months at random assignment, had 35% fewer breast cancer recurrences compared with women with delayed use (P = .04).7,9 Finally, neoadjuvant zoledronic acid doubled pathologic complete response frequency in an unplanned subgroup analysis of the AZURE (Adjuvant Zoledronic acid to redUce Recurrence) breast cancer trial.30 These results suggest bisphosphonates can reduce breast cancer recurrence and may have direct antibreast cancer effects as well.
After the WHI reports of net harm for combined estrogen plus progestin use,16,31 menopausal hormone therapy use declined from about 60 to 25 million prescriptions annually in the United States comparing 2001 to 2003 which coincided with a significant decrease in breast cancer incidence.32–34 In contrast, during the same period, bisphosphonate use was increasing at a relatively constant rate of about 3 million prescriptions annually with prescription numbers becoming similar to those for hormone therapy in 2003.35–37 While further study is needed, change in patterns of bisphosphonate use may have made a modest contribution to the recent reduction in breast cancer incidence seen in the United States.
Both tamoxifen and raloxifene, therapies approved for breast cancer risk reduction in the United States, almost exclusively influence hormone receptor–positive cancers,21,38 and no promising agents have been identified for receptor-negative breast cancer risk reduction.39 The suggestion that oral bisphosphonate use may lower receptor-negative breast cancer incidence therefore warrants further attention.
Study strengths include the prospective design, inclusion of a large, racially diverse population of well-characterized women, comprehensive assessment of breast cancer risk factors, prospective assessment of mammography and clinical breast exams, breast cancer adjudication using pathology report review and incorporation of a hip fracture risk prediction score associated with BMD to permit adjustment for the latter variable. A study limitation includes the observational design. In addition, there were substantial differences in the characteristics between bisphosphonate users and nonusers. Although we adjusted for many factors that could confound the association between bisphosphonate use and breast cancer risk, residual confounding nonetheless could have occurred.
In this large population of postmenopausal women, well characterized for breast cancer risk, oral bisphosphonate use was associated with lower invasive breast cancer incidence. These observational study findings require prospective confirmation. As oral bisphosphonates are in widespread and increasing use in clinical practice, these findings have public health implications. The influence of bisphosphonates in ongoing randomized, adjuvant therapy trials in women with early-stage breast cancer addressing outcomes including contralateral breast cancers will help clarify the clinical significance of the current findings.
Acknowledgment
We gratefully acknowledge the dedicated efforts of investigators and staff at the Women's Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, and the NHLBI program office (listing available at http://www.whi.org). Most importantly, we recognize the WHI participants for their extraordinary commitment to the WHI program.
Appendix
Table A1.
Women With Breast Screening by Bisphosphonate Use and Year of Study
| Bisphosphonate Use by Type of Breast Screening (%) | ||||||
|---|---|---|---|---|---|---|
| Mammogram | Breast Biopsy | |||||
| Bisphosphonate Use | Bisphosphonate Use | |||||
| Year | No | Yes | P* | No | Yes | P* |
| 1 | 61.6 | 71.8 | < .001 | 2.9 | 2.3 | .07 |
| 2 | 68.6 | 76.9 | < .001 | 3.3 | 2.6 | .03 |
| 3 | 70.1 | 77.1 | < .001 | 3.1 | 3.1 | .83 |
| 4 | 69.0 | 75.5 | < .001 | 3.1 | 2.8 | .28 |
| 5 | 69.9 | 74.3 | < .001 | 3.3 | 2.8 | .09 |
| 6 | 68.9 | 73.9 | < .001 | 2.4 | 2.7 | .35 |
| 7 | 67.1 | 70.0 | .004 | 3.0 | 2.5 | .12 |
| 8 | 65.2 | 63.0 | .13 | 2.2 | 2.4 | .76 |
P values are from χ2 tests of association.
Footnotes
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Rowan T. Chlebowski, AstraZeneca (C), Novartis (C), Pfizer (C), Amgen (C), Eli Lilly (C); Jane A Cauley, Novartis (C); Anne McTiernan, Merck (C); Robert B. Wallace, Merck (C), Novartis (C) Stock Ownership: Anne McTiernan, Merck Honoraria: Rowan T. Chlebowski, AstraZeneca, Novartis, Amgen Research Funding: Jane A Cauley, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Rowan T. Chlebowski, Jane A. Cauley, Garnet Anderson, Anne McTiernan
Administrative support: Rowan T. Chlebowski, Garnet Anderson
Provision of study materials or patients: Jane A. Cauley, Dorothy S. Lane, JoAnn E. Manson, Mary Jo O'Sullivan, Susan L. Hendrix, Robert B. Wallace
Collection and assembly of data: Garnet Anderson, Rebecca J. Rodabough
Data analysis and interpretation: Rowan T. Chlebowski, Zhao Chen, Jane A. Cauley, Garnet Anderson, Rebecca J. Rodabough, Anne McTiernan, JoAnn E. Manson, Linda Snetselaar, Shagufta Yasmeen, Robert B. Wallace
Manuscript writing: Rowan T. Chlebowski, Jane A. Cauley, Garnet Anderson, Rebecca J. Rodabough, Anne McTiernan
Final approval of manuscript: Rowan T. Chlebowski, Zhao Chen, Jane A. Cauley, Garnet Anderson, Rebecca J. Rodabough, Anne McTiernan, Dorothy S. Lane, JoAnn E. Manson, Linda Snetselaar, Shagufta Yasmeen, Mary Jo O'Sullivan, Monika Safford, Susan L. Hendrix, Robert B. Wallace
Funding/support.
The Women's Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118 to 32119, 32122, 42107-26, 42129-32, and 44221.
The funding organization had representation on the steering committee, which governed the design and conduct of the study, the interpretation of the data and the preparation and approval of manuscripts but did not participate in the manuscript preparation. The corresponding author has full access to the data and made the final decision when and where to submit the article for publication.
REFERENCES
- 1.Qaseem A, Snow V, Shekelle P, et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–415. [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate: Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 3.Hillner B, Ingle J, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Senaratne SG, Pirianov G, Mansi JL, et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–1468. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fromigue O, Kheddoumi N, Body JJ. Bisphosphonates antagonize bone growth factors' effects on human breast cancer cells survival. Br J Cancer. 2003;89:178–184. doi: 10.1038/sj.bjc.6601009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, Paterson S, Kanis JA, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol. 2002;20:3219–3224. doi: 10.1200/JCO.2002.11.080. [DOI] [PubMed] [Google Scholar]
- 7.Brufsky A, Bundred A, Coleman R, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13:503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 8.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 9.Eidtmann H, Bundred NJ, DeBoer R, et al. The effect of zoledronic acid on aromatase inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36 months follow-up of ZO-FAST. 31st Annual Meeting of the San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. abstr 44. [Google Scholar]
- 10.Cauley JA, Lucas FL, Kuller LH, et al. Bone mineral density and risk of breast cancer in older women: The study of osteoporotic fractures: Study of Osteoporotic Fractures Research Group. JAMA. 1996;276:1404–1408. [PubMed] [Google Scholar]
- 11.Chen Z, Arendell L, Aickin M, et al. Hip bone density predicts breast cancer risk independently of Gail score: Results from the Women's Health Initiative. Cancer. 2008;113:907–915. doi: 10.1002/cncr.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins J, Aragaki AK, Kooperberg C, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298:2389–2398. doi: 10.1001/jama.298.20.2389. [DOI] [PubMed] [Google Scholar]
- 13.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(suppl 9):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Hendrix SL, Langer RD, et al. Estrogen plus progestin influence on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. About SEER. http://www.seer.cancer.gov/
- 18.Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes of Health state of the science conference statement: Diagnosis and management of ductal carcinoma in situ. J Natl Cancer Inst. 2010;102:161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 19.Virnig BA, Tuttle TM, Shamilyan T, et al. Ductal carcinoma in situ of the breast: A systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast Cancer and Bowel Project B-24 randomized controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 21.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 22.Bedard PL, Body J, Piccart-Gebhart MJ. Sowing the soil for cure? Results of the ABCSG-12 trial open a new chapter in the evolving adjuvant bisphosphonate story in early breast cancer. J Clin Oncol. 2009;27:4043–4046. doi: 10.1200/JCO.2008.21.4908. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto K, Morishige K, Sawada K, et al. Aledronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem Biophys Res Commun. 2007;354:478–484. doi: 10.1016/j.bbrc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Santini D, Vincenzi B, Galluzzo S, et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13:4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 25.Caccamo N, Meraviglia S, Scarpa F, et al. Aminobisphosphonate-activated gamma delta T cells in immunotherapy of cancer: Doubts no more. Expert Opin Biol Ther. 2008;8:875–883. doi: 10.1517/14712598.8.7.875. [DOI] [PubMed] [Google Scholar]
- 26.Diel IJ, Solomayar EF, Costa SD, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 27.Diel IJ, Jaschke A, Solomayer EF, et al. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: A long-term follow-up. An Oncol. 2008;19:2007–2011. doi: 10.1093/annonc/mdn429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saarto T, Vehmanen L, Virkkumen P, et al. Ten year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43:650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen B, Ejlertsen B, Mouridsen HT, et al. Bisphosphonate treatment in primary breast cancer: Results from a randomized comparison of oral pamidronate versus no pamideonate in patients with primary breast cancer. Acta Oncol. 2008;47:740–746. doi: 10.1080/02841860801964988. [DOI] [PubMed] [Google Scholar]
- 30.Winter MC, Thorpe HC, Burkinshaw R, et al. The addition of zoledronic acid to neoadjuvant chemotherapy may influence pathological response – exploratory evidence for direct anti-tumor activity in breast cancer. 31st Annual Meeting of the San Antonio Breast Cancer Symposium; December 10-14, 2008; San Antonio, TX. abstr 5101. [Google Scholar]
- 31.Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Clarke CA, Glaser SL, Uratsu CS, et al. Recent declines in hormone therapy utilization and breast cancer incidence: Clinical and population-based evidence. J Clin Oncol. 2006;24:e49–e50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 33.Ravdin PM, Cronin KA, Howlander B, et al. The decrease in breast cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 34.Chlebowski RT, Kuller L, Prentice RL, et al. Breast cancer after estrogen plus progestin use in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udell JA, Fischer MA, Brookhart MA, et al. Effect of the Women's Health Initiative on osteoporosis and expenditure in Medicaid. J Bone Miner Res. 2006;21:765–771. doi: 10.1359/jbmr.060119. [DOI] [PubMed] [Google Scholar]
- 36.Watson J, Wise L, Green J. Prescribing of hormone therapy for menopause, tibolone, and bisphosphonates in women in the UK between 1991 and 2005. Eur J Clin Pharmacol. 2007;63:843–849. doi: 10.1007/s00228-007-0320-6. [DOI] [PubMed] [Google Scholar]
- 37.Huot L, Couris CM, Tainturier V, et al. Trends in HRT and anti-osteoporosis medication prescribing in a European population after the WHI study. Osteoporosis Int. 2008;19:1047–1054. doi: 10.1007/s00198-008-0587-1. [DOI] [PubMed] [Google Scholar]
- 38.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology 2008 clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Brown PH. Prevention of ER-negative breast cancer. Recent Results Cancer Res. 2009;181:121–134. doi: 10.1007/978-3-540-69297-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]