Abstract
Purpose
Patients with advanced pancreas cancer present with disease that is poorly responsive to conventional therapies. Preclinical and early clinical evidence has supported targeting the epidermal growth factor receptor (EGFR) signaling pathway in patients with pancreas cancer. This trial was conducted to evaluate the contribution of an EGFR-targeted agent to standard gemcitabine therapy. Cetuximab is a monoclonal antibody against the ligand-binding domain of the receptor.
Patients and Methods
Patients with unresectable locally advanced or metastatic pancreatic adenocarcinoma were randomly assigned to receive gemcitabine alone or gemcitabine plus cetuximab. The primary end point was overall survival. Secondary end points included progression-free survival, time to treatment failure, objective response, and toxicity.
Results
A total of 745 eligible patients were accrued. No significant difference was seen between the two arms of the study with respect to the median survival time (6.3 months for the gemcitabine plus cetuximab arm v 5.9 months for the gemcitabine alone arm; hazard ratio = 1.06; 95% CI, 0.91 to 1.23; P = .23, one-sided). Objective responses and progression-free survival were similar in both arms of the study. Although time to treatment failure was longer in patients on gemcitabine plus cetuximab (P = .006), the difference in length of treatment was only 2 weeks longer in the combination arm. Among patients who were studied for tumoral EGFR expression, 90% were positive, with no treatment benefit detected in this patient subset.
Conclusion
In patients with advanced pancreas cancer, the anti-EGFR monoclonal antibody cetuximab did not improve the outcome compared with patients treated with gemcitabine alone. Alternate targets other than EGFR should be evaluated for new drug development.
INTRODUCTION
The 5-year survival rate of patients with pancreas cancer remains less than 5% because of the metastatic nature of the disease at presentation in the majority of patients.1 Conventional systemic therapies have had a marginal impact on patient outcome; therefore, studies of newer regimens are needed to improve the survival of patients with this disease. Gemcitabine is the most commonly used cytotoxic drug in pancreas cancer based on a comparison with fluorouracil in a phase III trial.2 Numerous trials using single-agent gemcitabine in combination with different cytotoxic agents have resulted in no improvement compared with gemcitabine alone.3–5
The epidermal growth factor receptor (EGFR or HER1) is considered a key therapeutic target in many human cancers. EGFR-mediated cell signaling plays a major role in proliferation, angiogenesis, metastasis, and evasion of apoptosis.6 Moreover, EGFR expression with its ligands was shown to adversely impact the outcome of patients with resected pancreas cancer.7,8 Therapeutic targeting of EGFR by either monoclonal antibodies or tyrosine kinase inhibitors has been clinically validated in a number of human cancers.9 Erlotinib added to gemcitabine has demonstrated a marginal improvement compared with gemcitabine alone in a recent phase III study in advanced pancreas cancer.10 Preclinical evidence using human pancreas cancer xenograft in nude mice supported the strategy of disrupting the EGFR-mediated signaling using cetuximab, a monoclonal immunoglobulin G1 chimeric antibody directed against the receptor protein expressed on the surface of human pancreas cells.11 Moreover, the combination of cetuximab and gemcitabine demonstrated additive antitumor activity in orthotopically grown human pancreas cancer in nude mice.12 The growth-inhibitory, proapoptotic, and antiangiogenic activities of cetuximab were associated with downregulation of signaling through the EGFR pathway and reduced expression of proangiogenic growth factors, such as vascular endothelial growth factor and interleukin-8.
The established benefit of targeting the HER1/EGFR pathway in certain human cancers (eg, colorectal cancers) and the frequent expression of the EGFR protein in pancreatic cancer cells stimulated the investigation for a potential role of anti-EGFR therapy in pancreas cancer.13 On the basis of the preclinical data, a pilot phase II trial of cetuximab plus gemcitabine was launched in patients with advanced pancreas cancer that suggested an improvement in disease control and survival over historical controls.14 In the 41 patients with EGFR-positive tumors, median progression-free survival time, median overall survival time, and 1-year survival rate were 3.8 months, 7.1 months, and 31.7%, respectively. Partial response and stable disease were seen in 12.2% and 63.4% of patients, respectively. We report on the outcome of a phase III trial undertaken by the Southwest Oncology Group (protocol S0205; ClinicalTrials.gov identifier: NCT00075686). The primary objective of the study was to compare the overall survival in patients with advanced unresectable or metastatic pancreas cancer treated with either gemcitabine plus cetuximab or gemcitabine alone.
PATIENTS AND METHODS
Patients
Patients were eligible for the study if they fulfilled the following criteria: histologically or cytologically confirmed adenocarcinoma of the pancreas with distant metastases or locally advanced unresectable disease; presence of either measurable or evaluable disease; Zubrod performance status of 0 to 2; and adequate organ function defined as an absolute neutrophil count ≥ 1,500/μL, platelet count ≥ 100,000/μL, creatinine ≤ 2.0 mg/dL, serum bilirubin ≤ 2 × the upper limit of normal range for the institution, and serum AST and ALT ≤ 2.5 × the upper limit of normal for the institution. Prior radical surgery was allowed, and patients must have completed adjuvant (nongemcitabine) therapy at least 6 months before entry onto the study. Patients were excluded from the study if they had HIV-1 infection, brain metastases, prior systemic therapy for advanced disease, therapy with EGFR-targeting agents, or pregnancy. Institutional review boards or ethics committees approved the study. All patients provided a signed informed consent in accordance with institutional and federal guidelines that included permission for the submission of tissue for EGFR assay.
Study Design
Objectives.
The primary objective of this study was to compare the overall survival between the two study arms (gemcitabine v gemcitabine plus cetuximab) in patients with advanced pancreas cancer. Secondary objectives included comparisons of time to treatment failure, progression-free survival, toxicity profiles, and objective response rates. We also evaluated EGFR expression to compare the overall survival in patients treated with these two regimens in the EGFR-positive subset.
Drugs.
Gemcitabine (Gemzar; Eli Lilly, Indianapolis, IN) was administered intravenously at a dose of 1,000 mg/m2 over 30 minutes. During the first 8 weeks, gemcitabine was administered weekly for 7 weeks followed by 1 week off. In all remaining cycles, gemcitabine was administered for 3 weeks followed by a week of rest. Cetuximab (Erbitux; ImClone Systems, Bridgewater, NJ) was delivered intravenously at a loading dose of 400 mg/m2 (over 120 minutes) on week 1, followed by weekly maintenance doses of 250 mg/m2 (over 60 minutes). Premedications included antiemetics and diphenhydramine 50 mg intravenously or orally. Treatment with both gemcitabine and cetuximab was continued until disease progression, unacceptable toxicity, delay of treatment by more than 4 weeks, or patient request. Dose adjustments or interruptions of either drug were undertaken based on toxicity using standard criteria for both drugs.
Efficacy and Toxicity
Overall survival was measured from date of registration to death as a result of any cause. Progression-free survival was measured from the date of registration to date of first observation of progressive disease, death as a result of any cause, or symptomatic deterioration. Time to treatment failure was measured from the date of registration to date of first observation of progressive disease, death as a result of any cause, symptomatic deterioration, or discontinuation of therapy. For these three end points, patients who did not meet the defined criteria were censored at the time of last known patient contact. Objective response assessments were performed in patients with measurable disease using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.15 Radiologic monitoring of tumor areas was undertaken by either computed tomography or magnetic resonance imaging every 8 weeks.
Patients were monitored for toxicity at least weekly, with adverse events reported to the Southwest Oncology Group Statistical Center after every cycle of treatment (28 days). Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3). In addition, serious adverse events were reported to the National Cancer Institute via the Adverse Event Expedited Reporting System.
EGFR Assay
Diagnostic tumor tissues were obtained from patients as part of an eligibility criterion. A paraffin block, 10 unstained sections, or five unstained cytologic smears were used for the determination of EGFR expression by immunohistochemistry. Deparaffinized sections were run on a DAKO Autostainer using predilute anti-EGFR antibody and the DAKO pharmDX kit (DAKO, Carpinteria, CA). Primary antibody and detection reagent incubations were each 30 minutes, and staining was visualized by reacting with diaminobenzidine for 5 minutes. Slides were then counterstained with Mayer's hematoxylin and dipped in Scott's water (2% magnesium sulfate, 0.2% potassium bicarbonate) before being dehydrated through graded alcohols/xylenes and coverslipped. Any surface membrane staining of tumor cells was regarded as positive. Samples with no tumor cell staining were interpreted as negative if adjacent normal epithelial elements showed some staining. Samples showing no tumor staining and lacking normal epithelial elements were read as uninterpretable.
Statistical Analysis
The primary end point of the study was overall survival. Assuming a 6-month median survival time in the gemcitabine arm, the study was designed to detect an improvement in overall survival to 8 months in the cetuximab arm, corresponding to a 1.33 hazard ratio (HR). With a one-sided α = .0125, the study had an estimated 92% power based on a targeted accrual of 706 eligible patients accrued over a 5-year interval. It was estimated that approximately 90% or more of these patients would be EGFR positive, yielding enough patients to detect a 1.33 HR in this subset with 90% power.
Patients were randomly assigned to one of the two treatment arms using the dynamic balancing algorithm with stratification based on performance status (0 to 1 v 2), extent of disease (locally advanced v metastatic), and prior pancreatectomy (yes v no). The primary analysis used a stratified Cox regression analysis based on these stratification factors. Eligible patients were included in the analyses according to the intent-to-treat principle. For the clinical end points, all patients were included regardless of treatment status. For adverse event analyses, only treated patients were included. Analyses of objective response were only conducted in the subset of patients with measurable disease at baseline. Patients who did not have appropriate on-study assessments of disease measurements were assumed to have not responded. A sensitivity analysis was also conducted on all randomly assigned patients, yielding no difference from what is reported here. Data analyses for this study were performed by the Southwest Oncology Group Statistical Center.
This study was monitored by the Data and Safety Monitoring Committee of the Southwest Oncology Group, with two planned interim analyses after approximately one third and two thirds of the expected deaths had occurred. At each interim analysis, the Data and Safety Monitoring Committee assessed whether the trial could be terminated early according to protocol-specified guidelines. Both reviews resulted in the recommendation for continuation of the study until final data maturity.
RESULTS
Patients
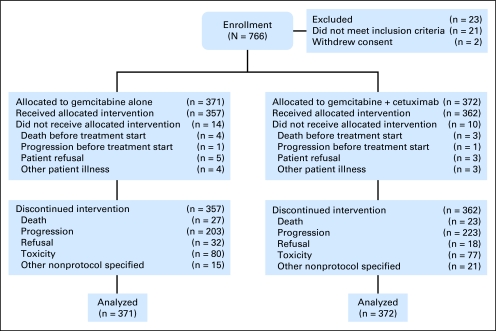
Seven hundred sixty-six patients were entered onto the study between January 1, 2004 and April 1, 2006 (Fig 1). Of these patients, 745 were clinically eligible for the study. Twenty-one patients were ineligible and excluded from analyses because of ampullary cancer (n = 10), adenocarcinoma originating from outside of the pancreas (n = 3), atrial fibrillation (n = 2), prior surgery (n = 1), neuroendocrine tumor (n = 2), prior chemotherapy (n = 2), and congestive cardiac failure (n = 1). Both arms were well balanced regarding the stratification factors and other patient characteristics (Table 1).
Fig 1.
CONSORT diagram.
Table 1.
Demographic and Clinical Characteristics of 743 Patients With Advanced Pancreas Cancer Treated With Gemcitabine Plus Cetuximab or Gemcitabine Alone
| Characteristic | % of Patients |
|
|---|---|---|
| Gemcitabine (n = 371) | Gemcitabine/Cetuximab (n = 372) | |
| Median age, years | 64.3 | 63.7 |
| Sex | ||
| Male | 54 | 51 |
| Female | 46 | 49 |
| Race | ||
| African American | 9 | 7 |
| White | 87 | 87 |
| Other | 4 | 6 |
| Hispanic | 2 | 5 |
| Performance status | ||
| 0/1 | 87 | 87 |
| 2 | 13 | 13 |
| Extent of disease | ||
| Localized | 22 | 21 |
| Metastatic | 78 | 79 |
| Liver metastases | 64 | 66 |
| Radical pancreatectomy | 11 | 10 |
| Adjuvant chemotherapy | 4 | 7 |
| Measurable disease | 88.3 | 86.3 |
Efficacy
Overall survival.
Eligible patients were included in the analysis of survival based on the intent-to-treat principle. Twenty-two patients did not receive any protocol treatment and were analyzed according to the assigned treatment arm. Two additional eligible patients withdrew consent for all treatment and follow-up after random assignment and were excluded from analyses. The median survival times for patients on gemcitabine alone and gemcitabine plus cetuximab were 5.9 and 6.3 months, respectively (HR = 1.06; 95% CI, 0.91 to 1.23; one-sided P = .19; Fig 2). There was no significant interaction between treatment and either sex or race (P = .48 and P = .57, respectively). Similarly, there were no detectable interactions between treatment and performance status (P = .38), prior pancreatectomy (P = .86), and disease extent at presentation (P = .78).
Fig 2.
Kaplan-Meier curves for overall survival in patients with advanced pancreas cancer treated with either gemcitabine alone or gemcitabine plus cetuximab.
Progression-free survival and time to treatment failure.
Two hundred twenty-four patients on the combination arm and 202 patients on the single-agent arm discontinued protocol treatment because of progression of disease. Median progression-free survival time was 3.0 months on the gemcitabine arm and 3.4 months on the gemcitabine plus cetuximab arm (HR = 1.07; 95% CI, 0.93 to 1.24; P = .18). The median time to treatment failure was 1.8 months on the gemcitabine arm compared with 2.3 months on the gemcitabine plus cetuximab arm (HR = 1.21; 95% CI, 1.04 to 1.40; P = .006). Although this difference was statistically significant in favor of patients treated with gemcitabine plus cetuximab compared with gemcitabine alone, the median difference was only 2 weeks.
Objective tumor response.
Objective tumor response was determined in 660 patients who had measurable disease at entry onto the study (Table 2). The objective response rate was similar in both arms of the study (P = .59). Fourteen percent of patients treated with gemcitabine alone had a confirmed or unconfirmed response compared with 12% of patients treated with the combination. Stable disease was observed in 30% and 37% of patients who received gemcitabine alone and the combination, respectively.
Table 2.
Objective Response Assessment in Patients With Locally Advanced or Metastatic Pancreas Cancer Treated With Gemcitabine Plus Cetuximab or Gemcitabine Alone
| Response | Gemcitabine/Cetuximab (n = 329) |
Gemcitabine (n = 331) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Complete response | 1 | 0 | 0 | 0 |
| Partial response | 27 | 8 | 23 | 7 |
| Unconfirmed complete response | 0 | 0 | 2 | 1 |
| Unconfirmed partial response | 13 | 4 | 21 | 6 |
| Stable disease | 122 | 37 | 100 | 30 |
| Progressive disease | 118 | 36 | 134 | 41 |
| Inadequate assessment* | 48 | 15 | 51 | 15 |
Included patients who had either early death or symptomatic deterioration but no objective evaluation.
Adverse Events
Seven hundred sixteen patients were evaluable for toxicity assessment. Table 3 compares the frequency of adverse events between the two arms of the study. Sixteen percent and 11% of patients experienced grade 4 or 5 toxicities on the gemcitabine plus cetuximab and gemcitabine alone arms, respectively. Eight grade 5 toxicities were reported, seven on the gemcitabine plus cetuximab arm and one on the gemcitabine arm. Four of the deaths on the gemcitabine plus cetuximab arm and one on the gemcitabine arm were disease related. Treatment-related deaths included hemolytic uremic syndrome (n = 1) and respiratory failure (n = 2). Cetuximab did not seem to worsen the gemcitabine-associated toxicities. However, cetuximab was associated with an increased frequency of allergic reactions and skin toxicities including acne and rash. Forty-eight percent and 8% of patients on the gemcitabine plus cetuximab arm experienced grade 2 and 3 skin toxicities, respectively. Ninety-seven patients on the combination arm and 116 patients on the gemcitabine alone arm were removed from study because of toxicity or patient refusal for other reasons.
Table 3.
Summary of Grade 3 or Greater Adverse Events in 716 Patients With Advanced Pancreas Cancer Treated With Gemcitabine Plus Cetuximab or Gemcitabine Alone
| Adverse Event | % of Patients With Grade ≥ 3 |
|
|---|---|---|
| Gemcitabine/Cetuximab (n = 361) | Gemcitabine (n = 355) | |
| ALT | 4.4 | 2.5 |
| Alkaline phosphatase | 4.7 | 3.1 |
| Allergic reaction | 4.7 | 3.1 |
| Anorexia | 6.4 | 7.3 |
| Bilirubin | 3.6 | 1.4 |
| Constipation | 3.0 | 2.5 |
| Dehydration | 3.9 | 2.3 |
| Diarrhea | 2.8 | 2.5 |
| Fatigue | 20.2 | 18.0 |
| Anemia | 9.7 | 6.2 |
| Hypokalemia | 3.9 | 1.4 |
| Leukocytes | 11.1 | 14.1 |
| Muscle weakness | 6.1 | 5.3 |
| Nausea | 9.1 | 6.2 |
| Neutropenia | 23.3 | 23.9 |
| Platelets | 6.6 | 8.5 |
| Rash | 6.9 | 0.0 |
| Vomiting | 6.6 | 2.2 |
EGFR Expression
Pretreatment pancreas cancer specimens were available in 702 eligible patients. EGFR expression was evaluable for 595 eligible patients. There were no differences in baseline characteristics or outcome in either EGFR assessable or nonassessable patients. Of the 595 patients evaluated, 547 (92%) stained positive. Among these EGFR-positive patients, the median survival was 6 months in each arm (HR = 0.98; 95% CI, 0.83 to 1.17; P = .42).
DISCUSSION
This report summarizes the largest clinical trial, to our knowledge, to study the impact of targeting the EGFR pathway in patients with advanced pancreas cancer. Despite promising preclinical and early clinical results, this study failed to demonstrate that cetuximab improved the outcome of therapy in patients treated with gemcitabine for advanced pancreas cancer. No significant difference was seen between the two arms of the study with respect to median survival, which was the primary end point (6.3 months for the gemcitabine plus cetuximab arm v 5.9 months for the gemcitabine alone arm; HR = 1.06, P = .23). Progression-free survival and objective response rates were also similar in both arms of the study. The slightly longer time to treatment failure in the cetuximab arm is likely a result of the potential bias by investigators and patients who, because of the unblinded nature of this trial, may have been willing to continue on the experimental arm for slightly longer.
Cetuximab has also failed to demonstrate improved patient outcome when paired with other chemotherapeutic regimens.16 Only the use of the tyrosine kinase inhibitor drug erlotinib has demonstrated modest improvement in patients with advanced pancreatic cancer. A phase III trial was undertaken by the National Cancer Institute of Canada (PA.3 Trial) to test the benefit of erlotinib, an oral tyrosine kinase inhibitor, in a comparable population of patients with advanced pancreas cancer when combined with gemcitabine.10 There was a significant difference in survival in favor of the gemcitabine plus erlotinib arm (HR = 0.82, P = .038). A major question is the lack of translation of the preclinical findings in pancreas cancer to the clinical setting and the discrepancy of outcome when compared with other adenocarcinomas (eg, colorectal cancer) in which cetuximab has shown clinical benefit.13
The current study supports the findings of other investigators regarding the lack of a predictive value of EGFR expression in tumors with respect to treatment outcome in patients treated with anti-EGFR agents.5 Specifically, EGFR expression, as quantified by immunohistochemistry, is unlikely to identify patients whose tumors are predominantly driven by the EGFR signaling pathway and thus will be responsive to cetuximab. EGFR mutations that predict response to therapy have not been identified in pancreas cancer.
Future research should improve our understanding of molecular mechanisms underlying resistance to the EGFR blockade and the identification of tumors that may be responsive to such therapy. K-Ras mutations influence response to cetuximab in patients with advanced colorectal cancer, but their contribution to cetuximab resistance has not been studied in pancreas cancer. The redundancy and cross talk in signaling pathways suggest that an effective blockade of proliferative and antiapoptotic signals in advanced pancreas cancer may require the testing of multitargeted strategies in the development of new therapies for this disease. A rational design of multitargeted therapies must ultimately be based on molecular profiling of tumors.
In conclusion, this phase III study of gemcitabine versus gemcitabine plus cetuximab failed to demonstrate a benefit for the addition of cetuximab in a molecularly unselected population of patients with advanced pancreas cancer. Results of this study do not exclude a potential benefit for an anti-EGFR therapeutic strategy in this disease because future work must focus on the identification of molecular predictors of response to this class of drugs.
Acknowledgment
Supported in part by the following Public Health Service Cooperative Agreement Grants awarded by the National Cancer Institute, Department of Health and Human Services: CA32102, CA38926, CA45808, CA22433, CA20319, CA11083, CA105409, CA04919, CA46441, CA35128, CA35431, CA67575, CA58416, CA45450, CA76429, CA58861, CA37981, CA63844, CA86780, CA45377, CA12644, CA58723, CA14028, CA46368, CA35119, CA27057, CA45807, CA95860, CA76448, CA35281, CA76447, CA35261, CA35178, CA68183 CA16385, CA42777, CA58882, CA35090, CA52654, CA67663, CA35176, CA45560, CA46113, CA35262, CA45461, CA35192, CA91105, CA46282 (Southwest Oncology Group); CA-25224, CA35195 (North Central Cancer Treatment Group); CA21115 (Eastern Cooperative Oncology Group); CA31946 (Cancer and Leukemia Group B [CALGB] Chairman's Grant), CA33601 (CALGB Statistical Center Grant); CA77651 (Memorial Sloan-Kettering Cancer Center Institutional Grant [CALGB]); and CA77202 (National Cancer Institute of Canada); also supported in part by ImClone Systems and Bristol-Myers Squibb.
Footnotes
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00075686.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Philip A. Philip, Curis (C), sanofi-aventis (C), Merck (C); Christopher L. Corless, Novartis (C), Pfizer (C), Molecular MD (C), Sequenom (C); Ralph Wong, Bristol-Myers Squibb (C); Charles D. Blanke, ImClone Systems (C) Stock Ownership: None Honoraria: Philip A. Philip, Roche, sanofi-aventis, Bayer Pharmaceuticals; Christopher L. Corless, Novartis, Sequenom; Patrick J. Flynn, ImClone Systems; Alok A. Khorana, Eli Lilly Research Funding: Philip A. Philip, sanofi-aventis, Bristol-Myers Squibb, Nektar; Christopher L. Corless, Novartis; Alok A. Khorana, Bristol-Myers Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Philip A. Philip, Jacqueline Benedetti, Cecilia M. Fenoglio-Preiser, James L. Abbruzzese, Charles D. Blanke
Administrative support: James L. Abbruzzese
Provision of study materials or patients: Philip A. Philip, Ralph Wong, Eileen M. O'Reilly, Patrick J. Flynn, Kendrith M. Rowland, James N. Atkins, Barry C. Mirtsching, Saul E. Rivkin, Alok A. Khorana
Collection and assembly of data: Philip A. Philip, Jacqueline Benedetti, Saul E. Rivkin, Bryan Goldman, Cecilia M. Fenoglio-Preiser, Charles D. Blanke
Data analysis and interpretation: Philip A. Philip, Jacqueline Benedetti, Eileen M. O'Reilly, Bryan Goldman, Charles D. Blanke
Manuscript writing: Philip A. Philip, Jacqueline Benedetti, Ralph Wong, Bryan Goldman, Cecilia M. Fenoglio-Preiser, James L. Abbruzzese, Charles D. Blanke
Final approval of manuscript: Philip A. Philip, Jacqueline Benedetti, Christopher L. Corless, Ralph Wong, Eileen M. O'Reilly, Patrick J. Flynn, Kendrith M. Rowland, James N. Atkins, Barry C. Mirtsching, Alok A. Khorana, Cecilia M. Fenoglio-Preiser, James L. Abbruzzese, Charles D. Blanke
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.el-Rayes BF, Shields AF, Vaitkevicius V, et al. Developments in the systemic therapy of pancreatic cancer. Cancer Invest. 2003;21:73–86. doi: 10.1081/cnv-120016406. [DOI] [PubMed] [Google Scholar]
- 4.Hochster HS, Haller DG, de Gramont A, et al. Consensus report of the International Society of Gastrointestinal Oncology on therapeutic progress in advanced pancreatic cancer. Cancer. 2006;107:676–685. doi: 10.1002/cncr.22036. [DOI] [PubMed] [Google Scholar]
- 5.Philip PA. Improving treatment of pancreatic cancer. Lancet Oncol. 2008;9:7–8. doi: 10.1016/S1470-2045(07)70391-1. [DOI] [PubMed] [Google Scholar]
- 6.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanaka Y, Friess H, Kobrin MS, et al. Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness. Anticancer Res. 1993;13:565–569. [PubMed] [Google Scholar]
- 8.Ueda S, Ogata S, Tsuda H, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: Poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–e8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 9.Milano G, Spano JP, Leyland-Jones B. EGFR-targeting drugs in combination with cytotoxic agents: From bench to bedside, a contrasted reality. Br J Cancer. 2008;99:1–5. doi: 10.1038/sj.bjc.6604373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 11.Overholser JP, Prewett MC, Hooper AT, et al. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89:74–82. [PubMed] [Google Scholar]
- 12.Bruns CJ, Solorzano CC, Harbison MT, et al. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 13.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 14.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: A randomized, multi-center, phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]