Abstract
Purpose
The combination of gemcitabine plus bevacizumab produced a 21% response rate and a median survival of 8.8 months in a multicenter phase II trial in patients with metastatic pancreatic cancer. These encouraging data led Cancer and Leukemia Group B (CALGB) to conduct a double-blind, placebo-controlled, randomized phase III trial of gemcitabine/bevacizumab versus gemcitabine/placebo in advanced pancreatic cancer patients.
Patients and Methods
Eligible patients had no prior therapy for advanced disease, Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, no tumor invasion of adjacent organs, and no increased bleeding risk. The primary end point was overall survival. Patients were stratified by performance status, extent of disease, and prior radiotherapy. Patients received gemcitabine at 1,000 mg/m2 over 30 minutes on days 1, 8, and 15 every 28 days and bevacizumab at 10 mg/kg or placebo on days 1 and 15 every 28 days.
Results
Between June 2004 and April 2006, 602 patients were enrolled onto the study and 535 were treated. Median overall survival was 5.8 months for gemcitabine/bevacizumab and 5.9 months for gemcitabine/placebo (P = .95). Median progression-free survival was 3.8 and 2.9 months, respectively (P = .07). Overall response rates were 13% and 10%, respectively. Patients with a performance status of 0, 1, and 2 survived a median of 7.9, 4.8, and 2.4 months, respectively. The only statistically significant differences in grades 3 and 4 toxicity occurred for hypertension (10% v 3%; P < .001) and proteinuria (5% v 1%; P = .002); venous thrombosis grade ≥ 3 was equivalent in both arms (14% and 15%, respectively).
Conclusion
The addition of bevacizumab to gemcitabine does not improve survival in advanced pancreatic cancer patients.
INTRODUCTION
Pancreatic cancer has the lowest survival by stage of any solid tumor.1 Gemcitabine, the cornerstone of chemotherapy, has a modest impact. In the landmark trial that compared gemcitabine to fluorouracil, gemcitabine produced a response rate of 5% and a median overall survival (OS) of 5.7 months.2 Although many phase II studies have reported promising activity for various cytotoxic and targeted agents administered with gemcitabine, phase III trials of these combinations were uniformly negative,3–10 until gemcitabine/erlotinib was shown to improve survival.11 Although the results were statistically significant, with a hazard ratio (HR) of 0.82, the absolute improvement in median OS of 5.9 months with gemcitabine versus 6.2 months with the combination was modest.11 Novel agents that have a greater impact are urgently needed.
Bevacizumab (Avastin, Genentech, South San Francisco, CA) is a recombinant humanized monoclonal antibody against vascular endothelial growth factor A (VEGF-A).12 In a phase II trial of gemcitabine/bevacizumab in metastatic pancreatic cancer patients, Kindler et al13 reported a response rate of 21%, a median OS of 8.8 months, and a 1-year survival of 29%. Because these data appeared promising when compared with data for historical controls, the Cancer and Leukemia Group B (CALGB) evaluated this regimen in a randomized phase III trial. This article describes the results of that clinical trial; correlative studies of angiogenic biomarkers, pharmacogenomics, and clinical economics will be reported separately.
PATIENTS AND METHODS
Patients
Eligible patients had histologically or cytologically confirmed pancreatic adenocarcinoma not amenable to curative surgery. Measurable disease was not required. Prior chemotherapy for metastatic disease was not permitted. Adjuvant chemotherapy was allowed if it did not contain gemcitabine or bevacizumab, if it was given > 4 weeks before enrollment, and if the patient had subsequent disease progression. Prior radiation was allowed if it was completed > 4 weeks before enrollment and there was disease outside the radiation port. An Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2 and adequate bone marrow (granulocytes ≥ 1,500/μL, platelets ≥ 100,000/μL), renal (creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 60 mL/min), and hepatic function (total bilirubin ≤ 1 × upper limit of normal, AST ≤ 2.5 × upper limit of normal) were required. An international normalized ratio (INR) ≤ 1.5 was required unless the patient was on warfarin; warfarin-treated patients needed to be on a stable dose with an INR between 2 and 3. A urine protein < 1+ or a 24-hour urine containing < 1 g/dL of protein was required. Eligible patients were at least 18 years of age and had a life expectancy of at least 12 weeks.
Patients could not have had a major surgery, open biopsy, or significant traumatic injury within 28 days before registration or a fine-needle aspirate within 7 days before registration. Patients who had significant bleeding within 6 months before registration, esophageal varices, computed tomography (CT) scan documentation of invasion of adjacent organs, clinically significant heart disease, or CNS disease were excluded. This protocol was reviewed by the institutional review board of each participating center. All patients provided written informed consent according to federal and institutional guidelines.
Treatment
Gemcitabine at 1,000 mg/m2 was given intravenously over 30 minutes on days 1, 8, and 15 of a 28-day cycle. Bevacizumab at 10 mg/kg or placebo was administered intravenously after gemcitabine on days 1 and 15 of each cycle. The initial bevacizumab/placebo dose was given over 90 minutes. If no infusion reaction developed, the second dose was given over 60 minutes, and subsequent doses were given over 30 minutes. Treatment was discontinued for progressive disease, unacceptable adverse events, or patient withdrawal of consent.
Dose Adjustments
Dose modifications were based on toxicities within 1 day of treatment. Adverse effects were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 3.0. A cycle was not started until the absolute neutrophil count (ANC) was > 1.5 × 109/L and the platelet count was > 100 × 109/L. The following dose levels of gemcitabine were used: level 0 (1,000 mg/m2), level −1 (750 mg/m2), level −2 (550 mg/m2), and level −3 (425 mg/m2).
Within a cycle, if the ANC was between 0.5 and 0.999 × 109/L or the platelet count was between 50 and 75 × 109/L on the treatment day, the gemcitabine dose was reduced by one dose level. The dose was held for an ANC < 0.5 × 109/L or platelets < 50 × 109/L. Febrile neutropenia required a one-dose-level reduction of gemcitabine for subsequent cycles. Gemcitabine was reduced by one dose level for grade 3 and held for grade 4, hepatic toxicity, or edema.
There were no dose modifications of bevacizumab/placebo. If gemcitabine was held at the beginning of a new cycle, the bevacizumab/placebo dose was held until gemcitabine could be given. Bevacizumab/placebo was held on day 15 for platelets < 50 × 109/L. Bevacizumab/placebo was held for bilirubin or hepatic transaminase elevations grade ≥ 3 and was not resumed until these were grade ≤ 2. Bevacizumab/placebo was discontinued for grade ≥ 3 bleeding. For a grade 3 thrombosis or an asymptomatic grade 4 pulmonary embolus, bevacizumab/placebo was held and was resumed once the patient met the following criteria: an INR between 2 and 3 on a stable warfarin or a stable heparin dose, no pathologic conditions that carried a high risk of bleeding, and no bleeding grade ≥ 3 on study. Bevacizumab/placebo was discontinued for a symptomatic pulmonary embolus or recurrent or worsening thromboembolic events after it was resumed. Bevacizumab/placebo was held for persistent or symptomatic hypertension; it was discontinued if this delayed treatment for > 4 weeks, or if grade 4 hypertension developed. A 24-hour urine was required for proteinuria ≥ 2+ on a dipstick. If the urine protein was ≥ 2 g/24 hours, bevacizumab/placebo was held until it recovered to < 2 g/24 hours and was discontinued if it was held > 12 weeks. It was also discontinued for coagulopathy grade ≥ 3, grade 4 hypersensitivity reactions, and grade 4 adverse events attributable to bevacizumab, including GI perforation, intra-abdominal hemorrhage, abscess, fistula, or wound dehiscence.
Study Evaluations
Pretreatment evaluation included a complete medical history and physical examination, complete blood count and differential (CBC), chemistry panel, prothrombin time/INR, urinalysis, carbohydrate antigen 19-9 (CA 19-9), pregnancy test (in women of childbearing potential), CT scan or magnetic resonance imaging of the abdomen, and a chest x-ray or CT scan of the chest. Plasma, urine, serum, and whole blood were obtained for research studies.
A history and physical examination were performed every 14 days. A CBC was performed weekly. Serum chemistries were obtained every 14 days. A urinalysis was performed every 28 days. Prothrombin time/INR was obtained weekly only in patients receiving therapeutic warfarin. Imaging scans, CA 19-9, and plasma and urine for research studies were obtained every two cycles.
Response Criteria and Toxicity
Patients were evaluated for response according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria every two cycles.14 Confirmatory scans were obtained at least 4 weeks following initial documentation of objective response.
Statistical Design and Analysis
The primary end point of this study was OS, measured from trial registration until death from any cause. Patients were randomly assigned with equal probability to gemcitabine plus bevacizumab or placebo, stratified by disease extent (locally advanced v metastatic), ECOG performance status (0/1 v 2), and prior radiation (no v yes). The study was powered to detect an HR of 1.35 with 90% confidence testing a two-sided log-rank hypothesis with α = .05. The design assumed an accrual rate of 20 patients per month and an additional 12 months of follow-up. The sample size was inflated approximately 10% to compensate for patient cancellations and withdrawals for a target accrual of 590 patients. The primary efficacy analysis population was the intent-to-treat population, defined as all patients randomly assigned, irrespective of whether the assigned treatment was actually received. For efficacy analyses, patients were grouped according to the treatment assigned at randomization.
Secondary end points included objective response rate and duration, progression-free survival (PFS), and adverse events. Response was defined as complete response (CR) or partial response (PR) per RECIST criteria.14 PFS was measured from trial entry until time of disease progression or death from any cause.
OS and adverse events were monitored with interim analyses during the trial with results reported to the CALGB Data and Safety Monitoring Board (DSMB) biannually. OS was monitored using the Lan-DeMets analog of the O'Brien-Fleming boundaries (two-sided α = .05) truncating at 2.5815 and began when 15% of information was available. At each interim analysis, the two-sided 99.5% CI was constructed for the observed HR, and if the targeted HR of 1.35 was not contained in this interval, consideration was given to accepting the hypothesis of no difference in median survival. Bevacizumab-specific adverse events were monitored for early stopping if the total rate difference for grade ≥ 3 bleeding, proteinuria, or thrombosis, or grade ≥ 4 hypertension was significantly greater on the bevacizumab arm. The Lan-DeMets analog of the Pocock boundaries15 was used to determine significance at each test of the hypothesis. Conducting these interim analyses did not substantially impact the overall significance level of the test.16
A stratified Cox proportional hazards regression17 was used to compare treatment arms controlling for the stratification factors at random assignment. Survival probability estimates were calculated using the Kaplan-Meier method.17 Rates and proportions were compared using Fisher's exact test, where appropriate, or a χ2 approximation. SAS 9.1 (SAS Institute, Cary, NC) was used for all statistical analyses. All analyses were based on the study database frozen on June 9, 2009.
Patient registration, data collection, and data analysis were performed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and the study chairperson. Quarterly reports were submitted by the CALGB Statistical Center to the Clinical Trials Evaluation Program of the NCI using the Clinical Data Update System.
As part of the CALGB quality assurance program, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. Auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in protocols at each institution. Such on-site medical record review was performed in 106 patients (18% of the 602 patients on this study).
RESULTS
Patient Characteristics
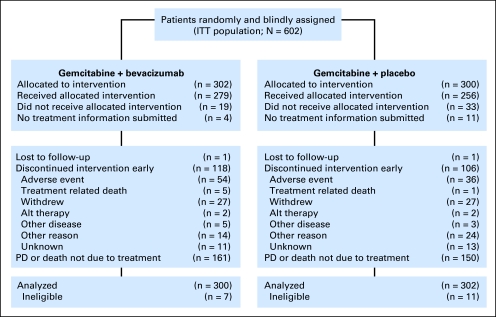
Between June 30, 2004, and April 14, 2006, 602 patients were randomly assigned (302 to gemcitabine/bevacizumab, 300 to gemcitabine/placebo); 279 and 256 patients, respectively, received study treatment. Of those who were randomly assigned but not treated, 52 patients withdrew consent, and no treatment information was submitted on 15 patients (Fig 1). Eighteen patients were ineligible. All randomly assigned patients were included in the intent-to-treat analysis.
Fig 1.
CONSORT diagram. ITT, intent to treat; Alt, alternative; PD, progressive disease.
Patient characteristics are listed in Table 1. There were no statistically significant differences between treatment arms with respect to age, sex, PS, extent of disease, or prior radiation. Approximately 85% of patients had metastatic disease, and 90% had a PS of 0 or 1. A slightly greater proportion of patients on the bevacizumab arm were male (58% v 51%); this difference was not statistically significant.
Table 1.
Patient Characteristics
| Characteristic | Gemcitabine + Bevacizumab |
Gemcitabine + Placebo |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 302 | 300 | ||
| Age, years | ||||
| Median | 63.7 | 65.0 | ||
| Range | 26-88 | 35-86 | ||
| Male sex | 58 | 51 | ||
| ECOG performance status | ||||
| 0 | 37 | 38 | ||
| 1 | 51 | 52 | ||
| 2 | 12 | 10 | ||
| White race | 88 | 88 | ||
| Extent of disease | ||||
| Locally advanced | 16 | 15 | ||
| Metastatic | 84 | 85 | ||
| Prior radiation | ||||
| Yes | 11 | 11 | ||
| No | 89 | 89 | ||
| Median baseline CA 19-9 | 1,146 | 1,726 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; CA 19-9, carbohydrate antigen 19-9.
Interim Analysis
In June 2006, on the basis of a protocol-specified planned interim analysis with 64% of the information available on OS, the CALGB DSMB released study data. At that point, 300 deaths (159 on the bevacizumab arm, 141 on the placebo arm) had been reported. Median OS was 4.99 and 5.45 months for the bevacizumab and placebo arms, respectively. The HR estimate was 0.90 in favor of placebo with 99.5% CI of 0.65 to 1.23. This interval did not contain the targeted HR of 1.35. Thus, the futility boundary was met. Since it was considered unlikely that there would be significant differences in OS between treatment arms with further follow-up, all patients on treatment were unblinded and notified of these results. Patients thought to be benefiting from bevacizumab were allowed to continue it with informed consent. Adverse event monitoring was conducted for any grade ≥ 3 bleeding or proteinuria, or grade ≥ 4 hypertension. No protocol-specified boundaries were crossed during interim toxicity monitoring for these end points. Monitoring for grade ≥ 3 thrombosis was conducted separately and was not significantly different between arms at any interim analysis.
Treatment Administration
A mean of 4.4 cycles of gemcitabine/bevacizumab and 3.9 cycles of gemcitabine/placebo were administered (P = .02).
Survival and Response
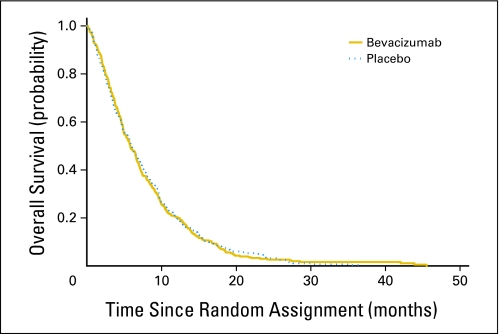
There was no statistically significant difference in median OS between study arms. The resulting stratified HR was 1.044 for placebo versus bevacizumab (95% CI, 0.88 to 1.24). The Kaplan-Meier curves for OS by treatment arm are shown in Figure 2. The median OS was 5.8 months (95% CI, 4.9 to 6.6) for gemcitabine/bevacizumab and 5.9 months (95% CI, 5.1 to 6.9) for gemcitabine/placebo (P = .95).
Fig 2.
Overall survival by treatment arm.
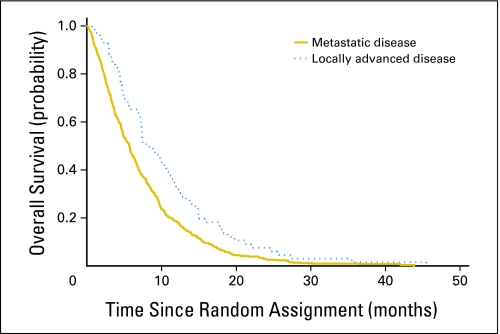
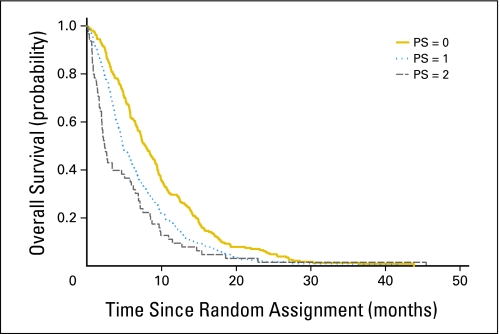
There were statistically significant differences in survival by extent of disease and PS. Patients with metastatic disease survived a median of 5.7 months compared with 9.9 months for patients with locally advanced disease (P = .009; Fig 3). Patients with a PS of 0 survived a median of 7.9 months compared with 4.8 and 2.4 months for PS 1 and PS 2 patients, respectively (P < .001; Fig 4). These differences were consistent between treatment arms.
Fig 3.
Overall survival by disease extent.
Fig 4.
Overall survival by performance status (PS).
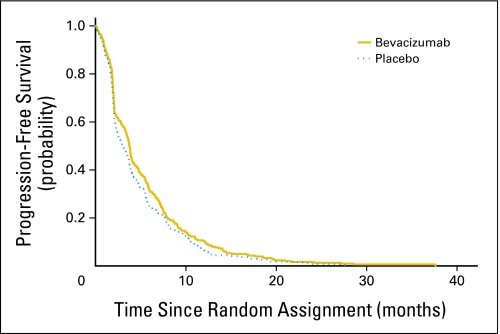
Figure 5 illustrates the Kaplan-Meier curves for PFS by treatment arm. The median PFS was 3.8 months (95% CI, 3.4 to 4.0 months) and 2.9 months (95% CI, 2.4 to 3.7 months) for the bevacizumab and placebo arms, respectively (P = .075).
Fig 5.
Progression-free survival by treatment arm.
Objective response rates were not significantly different: 13% for the bevacizumab arm (1% CR, 12% PR), and 10% for the placebo arm (1% CR, 9% PR). Stable disease occurred in 41% and 34% of patients on the bevacizumab and placebo arms, respectively.
Adverse Events
Grade 3 and 4 adverse events are summarized in Table 2. There were 13 treatment-related deaths. Five of the 10 deaths on the experimental arm were potentially attributable to bevacizumab (one hemorrhage, two pulmonary embolism, and two perforations). There were no differences in 30- and 60-day all-cause mortality between treatment arms.
Table 2.
Grades 3 and 4 Toxicities per Patient by Common Toxicity Criteria Version 3.0
| Toxicity | Gemcitabine + Bevacizumab (%) (n = 277) | Gemcitabine + Placebo (%) (n= 263) | P |
|---|---|---|---|
| Neutropenia | 33 | 29 | .35 |
| Anemia | 5 | 8 | .22 |
| Thrombocytopenia | 12 | 12 | 1.0 |
| Bleeding | 5 | 4 | .68 |
| Cerebrovascular accident | 2 | 2 | 1.0 |
| Hypertension | 10 | 3 | < .001 |
| Proteinuria | 5 | 1 | .002 |
| Venous thrombosis | 14 | 15 | .72 |
| Visceral perforation | 0.4 | 0 | 1.0 |
Hematologic adverse events were similar in both groups. Statistically significant differences in nonhematologic events occurred only for grade ≥ 3 hypertension (bevacizumab 10% v placebo 3%; P < .001) and proteinuria (5% v 1%; P = .002). Although high, rates of venous thrombosis grade ≥ 3 were nearly identical (14% and 15%) for the bevacizumab and placebo arms, respectively. There were 31 bleeding (grade ≥ 3), proteinuria (grade ≥ 3), or hypertension (grade ≥ 4) adverse events on the bevacizumab arm and 12 on the placebo arm (P = .006). This significant difference was principally due to the differences in proteinuria (15 v two events).
DISCUSSION
This randomized phase III study demonstrates that the addition of bevacizumab to gemcitabine does not improve survival in advanced pancreatic cancer patients. Not only does this trial fail to confirm the results of a prior phase II study of this regimen,13 these data also differ from the results achieved by the addition of bevacizumab to chemotherapy in several other malignancies.18–21 They are, however, similar to data from other randomized trials of the VEGF inhibtors bevacizumab, axitinib, and aflibercept in this disease.22–24
Patient selection is likely the most important factor in the disparate results of this phase III CALGB study and the phase II trial that provided the study rationale. The University of Chicago phase II trial13 reported a PR rate of 21% and a median OS of 8.8 months. The CALGB considered these results sufficiently promising for evaluation in a phase III study.
In retrospect, it is clear that the Chicago trial accrued a more fit population. Although all of the patients had metastatic disease and 83% had liver metastases, both of which augur a poor prognosis, that trial also contained more patients with a PS of 0, excluded patients with prior thrombosis, and had more patients who had received adjuvant therapy.
The striking differences in OS by PS observed in CALGB 80303 highlight the critical importance of this metric in predicting prognosis in advanced pancreatic cancer patients.25 Median survival was 7.9 months in PS 0 patients, 4.8 months in PS 1 patients, but only 2.4 months in PS 2 patients. It is likely that the 8.8-month median OS in the Chicago trial is partly attributable to the high proportion of PS 0 patients (60% v 38% in CALGB 80303) rather than to any bevacizumab effect. These data also suggest that future phase III trials evaluating new agents in advanced pancreatic cancer should be confined to patients with PS 0 and 1, since the limited survival of PS 2 patients is too brief to observe a potential drug effect.
Negative phase III results despite promising single-arm phase II data are, unfortunately, a common outcome in pancreatic cancer trials.3–10 These collective data suggest that in this disease, a single-arm, phase II trial design may not be not optimal.26 If the phase II study of gemcitabine/bevacizumab had employed a randomized phase II design, both arms would likely have contained similarly selected patients and shown little difference in outcome.
The addition of bevacizumab to chemotherapy increases OS and/or response rates and PFS in colorectal cancer, non–small-cell lung cancer, and breast cancer.18–21 When CALGB 80303 was initiated, it was plausible that these results would be replicated in pancreatic cancer. There are several possible explanations why this did not occur.
By normalizing tumor vasculature, VEGF inhibitors may enhance drug delivery, thereby increasing chemotherapy activity.27 Gemcitabine may be the most active agent for pancreatic cancer, but it is only modestly effective.2 Better delivery of a marginal agent yields marginal activity.
The preclinical models that suggested that VEGF inhibitors would be effective in pancreatic cancer28–33 may not replicate the human tumor microenvironment as well as newer genetically engineered models.34 Similar to human tumors, the dysfunctional vasculature of genetically engineered pancreatic models have a markedly diminished vessel density embedded in dense stroma, limiting drug delivery.34 Other potential mechanisms of resistance to VEGF inhibitors have been described.35–37
In conclusion, we have demonstrated that the addition of bevacizumab to gemcitabine does not improve survival in advanced pancreatic cancer. Our experience provides a rationale for favoring randomized phase II screening designs so that differences between the regimens rather than in the attributes of the study participants distinguish the differential impact of alternative treatment strategies.
Appendix
The following institutions participated in this study: Delaware Christiana Care Community Clinical Oncology Program (CCOP) and Helen F. Graham Cancer Center, Wilmington, DE: Stephen Grubbs, MD, supported by CA45418; Dana-Farber Cancer Institute, Boston, MA: Harold J. Burstein, MD, supported by CA32291; Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH: Konstantin Dragnev, MD, supported by CA04326; Duke University Medical Center, Durham, NC: Jeffrey Crawford, MD, supported by CA47577; Georgetown University Medical Center, Washington, DC: Edward P. Gelmann, MD, supported by CA77597; Cancer Centers of the Carolinas, Greenville, SC: Jeffrey K. Giguere, MD, supported by CA29165; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY: Jeffrey Kirshner, MD, supported by CA45389; Massachusetts General Hospital, Boston, MA: Jeffrey W. Clark, MD, supported by CA32291; Memorial Sloan-Kettering Cancer Center, New York, NY: Clifford A. Hudis, MD, supported by CA77651; Missouri Baptist Medical Center, St. Louis, MO: Alan P. Lyss, MD, supported by CA114558-02; Mount Sinai Medical Center, Miami, FL: Rogerio C. Lilenbaum, MD, supported by CA45564; Mount Sinai School of Medicine, New York, NY: Lewis R. Silverman, MD, supported by CA04457; Nevada Cancer Research Foundation CCOP, Las Vegas, NV: John A. Ellerton, MD, supported by CA35421; New Hampshire Oncology-Hematology Professional Association, Concord, NH: Douglas J. Weckstein; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN: Rafat Ansari, MD, supported by CA86726; Rhode Island Hospital, Providence, RI: William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY: Ellis Levine, MD, supported by CA59518; Southeast Cancer Control Consortium Inc., CCOP, Goldsboro, NC: James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY: Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH: Clara D. Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA: Barbara A. Parker, MD, supported by CA11789; University of Chicago, Chicago, IL: Hedy L. Kindler, MD, supported by CA41287; University of Illinois Minority-Based CCOP (MBCCOP), Chicago, IL: David J. Peace, MD, supported by CA74811; University of Iowa, Iowa City, IA: Daniel A. Vaena, MD, supported by CA47642; University of Massachusetts Medical School, Worcester, MA: William V. Walsh, MD, supported by CA37135; University of Minnesota, Minneapolis, MN: Bruce A. Peterson, MD, supported by CA164504; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO: Michael C. Perry, MD, supported by CA12046; University of North Carolina at Chapel Hill, Chapel Hill, NC: Thomas C. Shea, MD, supported by CA47559; University of Nebraska Medical Center, Omaha, NE: Anne Kessinger, MD, supported by CA77298; University of Vermont, Burlington, VT: Steven M. Grunberg, MD, supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC: David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC: Brendan M. Weiss, MD, supported by CA26806; Washington University School of Medicine, St. Louis, MO: Nancy Bartlett, MD, supported by CA77440.
Footnotes
Written on behalf of Cancer and Leukemia Group B.
Supported, in part, by Grants No. CA31946 from the National Cancer Institute to Cancer and Leukemia Group B 80303 (CALGB 80303) trial (R.L.S.), No. CA33601 to the CALGB Statistical Center (S.G.), and No. CA41287, CA 32291, CA47577, CA21115, CA17145, CA77651, CA45418, CA77440, and CA47559.
Presented, in part, at the 43rd Annual Meeting of the American Society of Clinical Oncology (ASCO), June 1-5, 2007, Chicago, IL, and the ASCO Gastrointestinal Cancers Symposium, January 19-21, 2007, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00088894.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Hedy Lee Kindler, Roche (C), OSI Pharmaceuticals (C); Herbert Hurwitz, Genentech (C), Roche (C); Joel Picus, Genentech (C); Richard M. Goldberg, Genentech (C) Stock Ownership: None Honoraria: Herbert Hurwitz, Roche Research Funding: Hedy Lee Kindler, Eli Lilly, Genentech; Herbert Hurwitz, Genentech, Roche; Richard M. Goldberg, Genentech Expert Testimony: None Other Remuneration: Pankaj Bhargava, AVEO Pharmaceuticals
AUTHOR CONTRIBUTIONS
Conception and design: Hedy Lee Kindler, Donna Niedzwiecki, Deborah Schrag, Herbert Hurwitz, Federico Innocenti, Robert J. Mayer, Richard L. Schilsky, Richard M. Goldberg
Administrative support: Donna Niedzwiecki, Donna Hollis, Susan Sutherland, Richard L. Schilsky
Provision of study materials or patients: Hedy Lee Kindler, Deborah Schrag, Herbert Hurwitz, Mary Frances Mulcahy, Eileen O'Reilly, Timothy F. Wozniak, Joel Picus, Pankaj Bhargava, Richard M. Goldberg
Collection and assembly of data: Hedy Lee Kindler, Donna Niedzwiecki, Donna Hollis, Susan Sutherland, Deborah Schrag, Herbert Hurwitz, Federico Innocenti
Data analysis and interpretation: Hedy Lee Kindler, Donna Niedzwiecki, Donna Hollis, Deborah Schrag, Herbert Hurwitz, Federico Innocenti, Eileen O'Reilly, Richard L. Schilsky, Richard M. Goldberg
Manuscript writing: Hedy Lee Kindler, Donna Niedzwiecki, Deborah Schrag, Herbert Hurwitz, Federico Innocenti, Richard L. Schilsky, Richard M. Goldberg
Final approval of manuscript: Hedy Lee Kindler, Donna Niedzwiecki, Donna Hollis, Susan Sutherland, Deborah Schrag, Herbert Hurwitz, Federico Innocenti, Mary Frances Mulcahy, Eileen O'Reilly, Timothy F. Wozniak, Joel Picus, Pankaj Bhargava, Robert J. Mayer, Richard L. Schilsky, Richard M. Goldberg
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, Moore MJ, Andersen J, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 4.Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 6.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncl. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol. 2006;24:4441–4447. doi: 10.1200/JCO.2006.07.0201. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Res and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 11.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 13.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 16.Freidlin B, Korn EL, George SL. Data monitoring committees and interim monitoring guidelines. Control Clin Trials. 1999;20:395–407. doi: 10.1016/s0197-2456(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 17.Collett D. Modelling Survival Data in Medical Research (ed 2) London, United Kingdom: Chapman & Hall; 2003. [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 21.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 23.Kindler HL, Ioka T, Richel DJ, et al. A double-blinded, placebo-controlled, randomised, phase III study of axitinib (AG-013736) plus gemcitabine versus placebo in advanced pancreatic cancer patients. Eur J Cancer Suppl. 2009;7:361. abstr 6502. [Google Scholar]
- 24.Phase 3 trial of aflibercept in metastatic pancreatic cancer discontinued. http://en.sanofi-aventis.com/press/press_releases/2009/ppc_26186.asp.
- 25.Boeck S, Hinke A, Wilkowski R, et al. Importance of performance status for treatment outcome in advanced pancreatic cancer. World J Gastroenterol. 2007;13:224–227. doi: 10.3748/wjg.v13.i2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannistra SA. Phase II trials in journal of clinical oncology. J Clin Oncol. 2009;27:3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 27.Fukumura D, Jain RK. Tumor microvasculature and micorenvironment: Targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solorzano CC, Baker CH, Bruns CJ, et al. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase. Cancer Biother Radiopharm. 2001;16:359–370. doi: 10.1089/108497801753354267. [DOI] [PubMed] [Google Scholar]
- 29.Bruns CJ, Shrader M, Harbison MT, et al. Effect of the vascular endothelial growth factor receptor-2 antibody DC-101 plus gemcitabine on growth, metastasis, and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer. 2002;102:101–108. doi: 10.1002/ijc.10681. [DOI] [PubMed] [Google Scholar]
- 30.Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer. 2003;2:8. doi: 10.1186/1476-4598-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J, Guo P, Matsuda K, et al. Pancreatic cancer cell-derived vascular endothelial growth factor is biologically active in vitro and enhances tumorigenicity in vivo. Int J Cancer. 2001;92:361–369. doi: 10.1002/ijc.1202. [DOI] [PubMed] [Google Scholar]
- 32.Bockhorn M, Tsuzuki Y, Xu L, et al. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res. 2003;9:4221–4226. [PubMed] [Google Scholar]
- 33.Tsuzuki Y, Mouta Carreira C, Bockhorn M, et al. Pancreas microenvironment promotes VEGF expression and tumor growth: Novel window models for pancreatic tumor angiogensis and microcirculation. Lab Invest. 2001;81:1439–1451. doi: 10.1038/labinvest.3780357. [DOI] [PubMed] [Google Scholar]
- 34.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebos JM, Lee CR, Kerbel RS. Tumor and host-mediated pathways of resistance and disease progression in response to anti-angiogenic therapy. Clinical Cancer Res. 2009;15:5020–5025. doi: 10.1158/1078-0432.CCR-09-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci. 2009;30:624–630. doi: 10.1016/j.tips.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: Implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]