Abstract
Introduction
Selenoprotein P (SelP) plays a critical role in neuronal survival and is associated with Alzheimer's pathology. We sought to determine a potential neuroprotective role for SelP in Alzheimer's disease.
Methods
We utilized RNAi to reduce SelP expression in neuronal N2A cells, and determined cell viability with flow cytometry. We subsequently measured neurotoxicity from exposure of aggregated amyloid beta (Aβ) peptides to SelP-knockdown and control N2A cells.
Results
We found that knockdown of SelP using siRNA in N2A cells reduced viability and increased apoptotic cell death. Additionally, knockdown of SelP using siRNA in N2A cells resulted in increased Aβ toxicity.
Conclusions
Our findings demonstrate that SelP protects neuronal cells from Aβ-induced toxicity, suggesting a neuroprotective role for SelP in preventing neurodegenerative disorders. (Ethn Dis. 2010;20[Suppl 1]:S1-92–S1-95)
Keywords: Selenium, Selenoprotein P, Alzheimer's Disease, Amyloid Beta, Oxidative Stress, Apoptosis
Introduction
Alzheimer's disease (AD) is the leading cause of dementia.1 The pathology of AD is characterized by large insoluble plaques made of amyloid beta (Aβ), which are thought to increase oxidative stress and promote neurode-generation,2 as well as production of intraneuronal protein aggregates termed neurofibrillary tangles.3
The trace element selenium, known for its antioxidant properties, is incorporated into proteins as the amino acid selenocysteine. Selenoproteins are a small class of proteins containing selenocysteine(s), and several are identified as protecting cells from oxidative damage.4 Selenoprotein P (SelP), a selenium transport protein, is produced predominantly in the liver and is thought to be transported to the brain via plasma.5 However, high concentrations of SelP produced in the brain suggest an important physiological role in the central nervous system. We previously demonstrated that SelP is associated with AD pathology.6 We therefore sought to determine the relationship between SelP in the brain and the oxidative damage induced by Aβ.
Methods
Cell Culture
A murine neuroblastoma cell line N2A was cultured in Dulbecco's Modified Eagle Medium (DMEM) (GIBCO, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA). The DMEM was replaced with Neurobasal Medium (NB) (Gibco, Carlsbad, CA) containing B27 supplement (Gibco, Carlsbad, CA) 48 hours before transfection or Aβ challenge.
Primer and siRNA
siRNA targeting SelP (Sepp1 siRNA, Sense sequence: 5′-GCCAUUAAGAUCGCUUACUtt-3′) and negative control siRNA (Silencer Negative Control #2 siRNA, sequence proprietary) were purchased from Ambion (Austin, TX). Integrated DNA Technologies-produced (Coralville, IA) qPCR oligonucleotide primers for both murine SelP and 18SrRNA were used as a housekeeping gene. Murine primer sequences used were SelP: forward 5′-TGT TGA AGA AGC CAT TAA GAT CG-3′ and reverse 5′-CAC AGT TTT ACA GAA GTC TCC ATC TTC-3′; and 18S rRNA: forward 5′-CGA TTG GAT GGT TTA GTG AGG-3′ and reverse 5′-AGT TCG ACC GTC TTC TCA GC-3′.
siRNA Transfection
The N2A cells were transiently transfected with SelP and control siRNA (Ambion, Austin, TX) using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA). Prior to transfection, cells were cultured to ~40% confluency in DMEM with 10% FBS. The DMEM was replaced with NB containing B27 supplement 48 hours before transfection. Cells were harvested 72 hours after transfection for flow cytometry. For the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, cultures were grown on cover slips in 48-well plates in DMEM with 10% FBS. Cells were then transfected with SelP and control siRNA using Arrest-In transfection re-agent (Open Biosystems, Huntsville, AL) containing rhodamine for identification of transfected cells.
Aβ challenge and TUNEL
Peptides for Aβ amino acids 25–35 (Aβ25–35), previously identified as a toxic region of Aβ, and the reverse order sequence (Aβ25–35), used as a control, were obtained from Bachem (Torrence, CA). Peptides were aggregated to oligomeric form in distilled water at 37°C for 72 hrs. Aβ was added to culture media at a concentration of 8.66 μM 4 hrs after siRNA transfection. The N2A cells were fixed with 4% paraformaldehyde after 48 hrs exposure to Aβ. The TUNEL assay was then conducted using the In Situ Cell Death Detection Kit system (Roche, Indianapolis, IN). Images were taken using a fluorescent microscope, and analyzed with ImageJ software (NIH, rsbweb.-nih.gov/ij/).
Flow Cytometry
Apoptosis and cell viability were assessed by annexin V (AV) binding and propidium iodine (PI) reactivity using the Vybrant Apoptosis Assay Kit #2 system (Invitrogen, Eugene, OR). Mean fluorescence was determined from samples of 10,000 cells using a Becton-Dickinson FACSCalibur flow cytometer.
RNA Isolation and qPCR
Whole RNA was harvested with TRIZOL reagent (Invitrogen, Carlsbad, CA) and purified with the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer's instructions. We synthesized cDNA from purified RNA using Applied Biosystem's (Foster City, CA) High Capacity cDNA Reverse Transcription Kit.
The SelP mRNA expression was quantified using qPCR. The qPCR reactions included Platinum SYBR Green qPCR SuperMix-UDG from Invitrogen (Carlsbad, CA), SelP and 18SrRNA oligonucleotide primers (IDT, Coralville, IA) and cDNA produced from extracted N2A mRNA. Experiments were conducted on a Roche LightCycler 480 II.
Statistics
Data were evaluated using Student's t-test or Two-Way ANOVA with Bonferroni's posthoc tests. P values less than .05 were considered significant.
Results
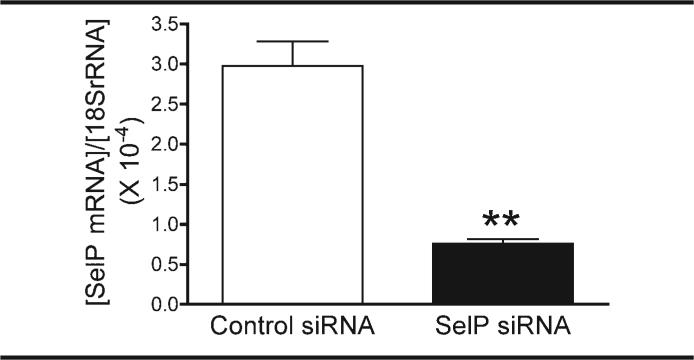
Successful knockdown of SelP mRNA expression was demonstrated with qPCR. Transfection with SelP siRNA significantly reduced SelP mRNA concentration in N2A cells to less than one third that of cells transfected with control siRNA, when normalized to cellular 18S rRNA concentrations (Figure 1).
Fig 1.
siRNA transfection reduces SelP mRNA expression. SelP gene expression measured with qPCR after transfection with SelP and control siRNA. ** P=.0021
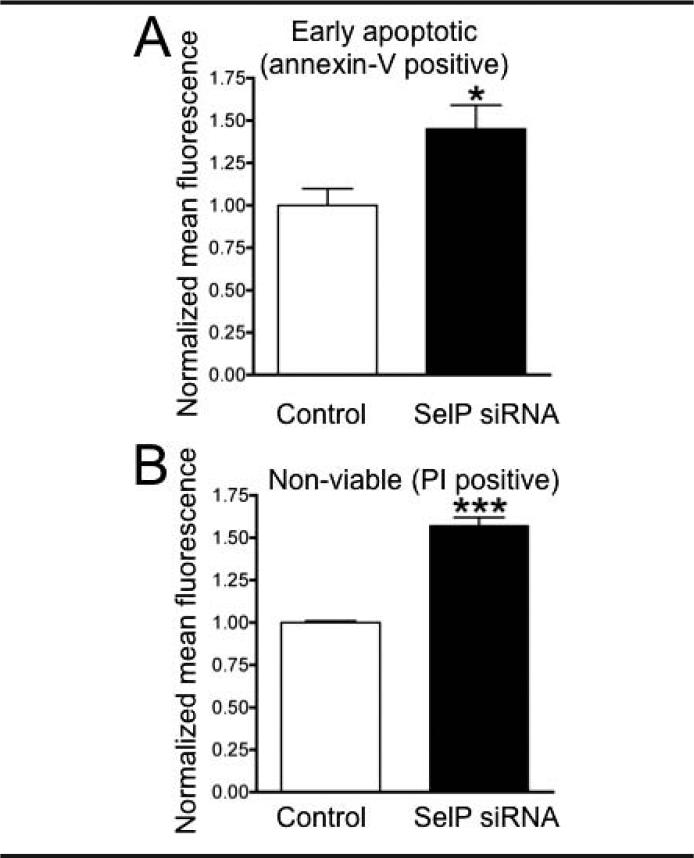
The effect of SelP knockdown on the viability of the cells was determined in an apoptosis and cell necrosis assay (Figure 2). Results of the mean fluorescence data of AV and PI labeling measured using flow cytometry revealed a significant increase in apoptotic cells and a significant decrease in viable cells in cultures following knockdown of SelP, as compared to controls.
Fig 2.
Knocking down SelP in N2A cells decreases cell viability. Apoptosis assay of knocked down N2A cells measuring AV (A) and PI (B) staining with flow cytometry 72 hours after transfection with control and SelP-targeting siRNA. * P<.05; *** P<.001
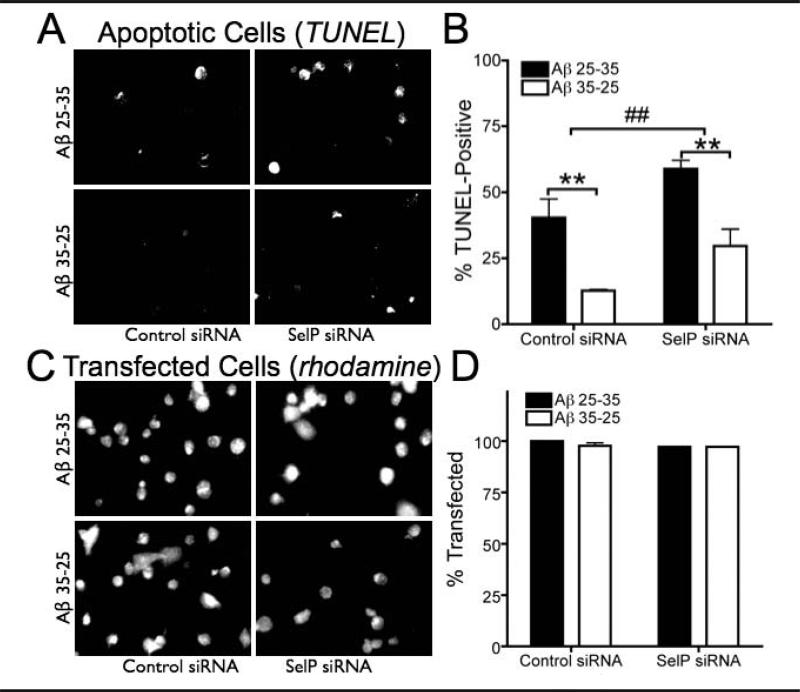
The susceptibility of SelP knockdown and control cells to Aβ-induced toxicity was determined using a TUNEL assay. We counted 50–100 cells from randomly chosen fields of view in three separate cultures from each group (control or SelP siRNA, Aβ25–35 or Aβ35–25). As shown in Figure 3A, the TUNEL assay demonstrated that SelP siRNA increased apoptotic cell death compared to control siRNA. The Aβ25–35 polypeptide resulted in significantly more apoptotic N2A cells than the Aβ35–25 controls. Exposure to Aβ25–35 induced more apoptosis in the absence of SelP, suggesting a protective role for SelP against Aβ toxicity. Transfection rates did not differ between treatment groups (Figure 3B). Additionally, the TUNEL was higher in non-transfected (rhodamine-negative) cells in all cultures treated with Aβ25-35 (48.3 ± 1.6%) compared to cultures treated with Aβ35-25 (25.0 ± 8.3%, P=.017), while there was no significant increase in TUNEL in non-transfected cells between control and SelP siRNA cultures (P=.57).
Fig 3.
Knockdown of selenoprotein P increases amyloid toxicity. Apoptosis measured in N2A cells by TUNEL assay following 48 hrs Aβ25–35 exposure compared to reverse order Aβ35–25 as negative control, after knockdown of SelP with siRNA. A: Examples of TUNEL in control and SelP siRNA transfected cultures exposed to either Aβ25–35 or Aβ35–25. Rhodamine in lipofection media labels transfected cells are red, while TUNEL-positive cells are green. B: Extent of apoptotic cell death indicated by TUNEL assay. Cells positive for both rhodamine and TUNEL are expressed as a percentage of rhodamine-labeled cells. ## indicates P=.008 for control vs siRNA, two-Way ANOVA; ** indicates P<.01, Bonferroni's posthoc tests. C: Examples of rhodamine-labeled cells in culture corresponding to examples shown in A. D: Rhodamine-labeled cells expressed as a percentage of total cells (determined by DAPI-labeling, not shown)
Discussion
Our data indicate that SelP has an important neuroprotective role both in general neuronal survival and in the case of Aβ-induced oxidative stress. Knockdown of SelP expression in N2A cells caused significantly more apoptotic and non-viable cells in the absence of oxidative challenge compared to control cells, indicating that SelP is essential to normal neuronal survival. Additionally, SelP deficient cells were more susceptible to apoptotic cell death in response to Aβ-induced oxidative challenge. These results are indicative of Aβ's increased toxicity to SelP knockdown cells and imply an antioxidative neuroprotective role for SelP.
The Aβ plaques are one of two markers for AD and are thought to produce oxidative damage to neurons, contributing to neurodegeneration.2 Selenoprotein P acts as an antioxidant via a redox motif involving its first selenocysteine residue,7 and can chelate potentially toxic metals.8 Selenoprotein P also has been shown to confer protection from oxidative stress to cultured human astrocytes. Additionally, SelP may be necessary for transport of selenium for the synthesis of other antioxidant selenoproteins such as the glutathione peroxidases and thioredoxin reductases.4
Our results suggest that SelP functions in regulating the redox environment of neuronal cells and confers protection from oxidative damage induced by Aβ. Selenoprotein P expression may play a role in AD pathology both directly as an antioxidant and indirectly as a Se donor in the synthesis of other selenoproteins. As oxidative damage is implicated in a number of neurodegenerative disorders, SelP-mediated neuroprotection may have important ramifications for other disorders in addition to AD.
Implications for Improving Health Disparities
This study increases our understanding of Alzheimer's disease and our biological defenses against neurodegeneration. Selenoprotein P expression increases with dietary selenium supplementation,5 suggesting supplementation might be used in treatment or prevention of Alzheimer's disease. Additionally, measurement of SelP levels in cerebral spinal fluid could potentially become a prognostic tool to better predict disease progression.
Acknowledgments
The authors thank Mariclair Reeves for help with TUNEL and flow cytometry, and Arjun Raman, Lucia Seale and Robert Nichols for helpful comments. Supported by Hawaii Community Foundation Grant 20061490 to FPB, NIH R01 NS40302 to MJB, and NIH G12 RR003061 for the JABSOM Microscopy and Imaging core facility.
References
- 1.Bennett DA. Part I. Epidemiology and public health impact of Alzheimer's disease. Dis Mon. 2000;46(10):657–665. doi: 10.1016/s0011-5029(00)90028-2. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002;36(12):1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 3.Maccioni RB, Lavados M, Maccioni CB, Mendoza-Naranjo A. Biological markers of Alzheimer's disease and mild cognitive impairment. Curr Alzheimer Res. 2004;1(4):307–314. doi: 10.2174/1567205043332018. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86(1):1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- 5.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–235. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 6.Bellinger FP, He QP, Bellinger MT, et al. Association of selenoprotein P with Alzheimer's pathology in human cortex. J Alzheimers Dis. 2008;15(3):465–472. doi: 10.3233/jad-2008-15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002;5(2):75–79. doi: 10.1046/j.1523-5408.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 8.Sasakura C, Suzuki KT. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem. 1998;71(3–4):159–162. doi: 10.1016/s0162-0134(98)10048-x. [DOI] [PubMed] [Google Scholar]