Abstract
Aminoacyl-tRNA (aa-tRNA), in a ternary complex with Elongation Factor-Tu (EF-Tu) and GTP, enters the aminoacyl (A) site of the ribosome via a multi-step, mRNA codon-dependent mechanism. This process gives rise to the preferential selection of cognate aa-tRNAs for each mRNA codon and consequently the fidelity of gene expression. The ribosome actively facilitates this process by recognizing structural features of the correct substrate, initiated in its decoding site, to accelerate the rates of EF-Tu-catalyzed GTP hydrolysis and ribosome-catalyzed peptide bond formation. Here, the order and timing of conformational events underpinning the aa-tRNA selection process were investigated from multiple structural perspectives using single-molecule fluorescence resonance energy transfer (smFRET). The time resolution of these measurements was extended to 2.5 and 10ms, a 10–50-fold improvement over previous studies. The data obtained reveal that aa-tRNA undergoes fast conformational sampling within the A site, both before and after GTP hydrolysis. This suggests that the alignment of aa-tRNA with respect to structural elements required for irreversible GTP hydrolysis and peptide bond formation plays a key role in the fidelity mechanism. These observations provide direct evidence that the selection process is governed by motions of aa-tRNA within the A site, adding new insights into the physical framework that helps explain how the rates of GTP hydrolysis and peptide bond formation are controlled by the mRNA codon and other fidelity determinants within the system.
Keywords: tRNA Selection, initial selection, induced fit, fidelity
INTRODUCTION
Messenger RNA (mRNA)-directed protein synthesis takes place on the two-subunit ~2.4MDa ribosome particle (70S in bacteria) during the elongation phase of translation. In this process, the ~25KDa, L-shaped aminoacyl-transfer RNA (aa-tRNA) molecule binds the ribosome within the Aminoacyl (A) site through base pairing interactions with the mRNA codon within the small subunit (30S) decoding site 1; 2. The ribosome’s recognition of the helical geometry arising from the paired mRNA codon and tRNA anticodon stimulates conformational events in the particle and tRNA that facilitate delivery of the amino acid linked to the distal 3′-CCA terminus of aa-tRNA into the peptidyltransferase center (PTC) of the large subunit (50S) more than 80Å away 1; 3; 4; 5; 6; 7. The selection process terminates with peptide bond formation catalyzed by elements of the PTC. The mechanism of aa-tRNA selection establishes the genetic code by ensuring that correct (cognate) aa-tRNA are rapidly incorporated into the ribosome while near- and non-cognate aa-tRNAs are rapidly and efficiently rejected 8; 9.
In vivo and in vitro measurements estimate the rate of translation at ~2–20 amino acids per second with error frequencies ranging from ~1×10−2–10−6 depending on experimental conditions 8; 10; 11. Watson-Crick codon-anticodon interactions are central to this fidelity in the aa-tRNA selection mechanism. However, thermodynamic differences in the pairing stabilities of the three nucleotide mini-helix can only afford ~10-fold discrimination 3. Biophysical studies have shown that the ribosome compensates for this disparity with a kinetically-driven 12; 13, induced-fit mechanism 14; 15 that allows two opportunities to discriminate aa-tRNAs based on the nature of the codon-anticodon interaction. Cognate (correctly paired) aa-tRNAs tend to rapidly progress through both discrimination steps, while near- (one mismatch) and non-cognate (more than one mismatch) aa-tRNAs tend to rapidly dissociate. The two steps, initial selection and proofreading 16; 17, are separated by irreversible GTP hydrolysis catalyzed by Elongation factor-Tu (EF-Tu). EF-Tu is bound in a stable ternary complex with the 3′-aminoacylated tRNA terminus and GTP (Figure 1A), serving additionally as a molecular bridge to the ribosome to increase the rate and fidelity of selection 18.
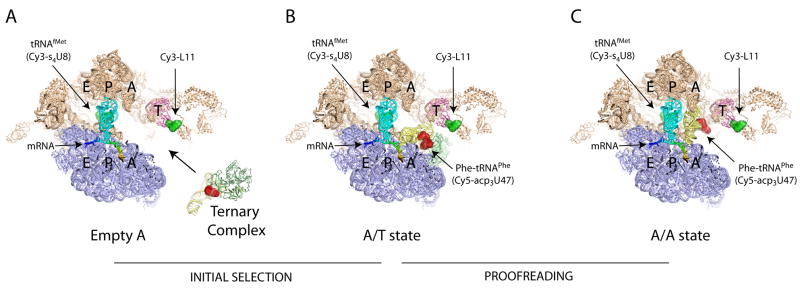
Figure 1. Established structural framework of the aa-tRNA selection process.
(A) Cross-section view revealing E, P and A, tRNA binding sites within the 70S particle showing large (tan) and small (blue) ribosomal subunits, mRNA (rainbow) P-site tRNAfMet (cyan), L11 (pink) and the ternary complex of tRNAPhe (yellow), EF-Tu (green) and GTP. Approximate dye positions show the specific sites of labeling on P-site tRNAfMet (Cy3-green), L11 (Cy3-green) and aa-tRNAPhe (Cy5-red) in sphere rendering. The Cy3 fluorophore on P-site tRNAfMet is partially obscured in this view by the tRNA body. (B) Structural model of the A/T state ribosome complex where ternary complex is stalled in the selection process immediately after GTP hydrolysis by the antibiotic kirromycin40. (C) Structural model of the pre-translocation ribosome complex showing the position of aa-tRNA in the classical (A/A) state.
Recent strides in cryo-electron microscopy, x-ray crystallography, rapid stopped-flow kinetic measurements and single-molecule fluorescence resonance energy transfer (smFRET) imaging have shed light on the selection mechanism 4; 5; 6; 7; 19; 20; 21. Initial binding of ternary complex to the ribosome is mediated by interactions between EF-Tu and the C-terminal domain of ribosomal protein L12 located on the 50S subunit. This codon independent contact localizes ternary complex to the leading edge of the ribosome to facilitate entry of the tRNA anticodon into the A-site decoding site. Subsequently, elements within the decoding site, including universally conserved residues A1492, A1493 of helix 44 (h44) and G530 of h18 recognize shape-specific features of the codon-anticodon pair by directly contacting the minor groove of the codon-anticodon minihelix. Kinetic studies aiming to probe features of the codon-recognition state have done so by stalling selection using the non-hydrolyzable GTP analogue, GDPNP. In so doing, ternary complex is stalled in the so-called A/T state. However, most structural studies of the A/T state have focused on systems biochemically-stalled using the antibiotic kirromycin4; 5. Kirromycin binds directly to EF-Tu to prevent aa-tRNA release, trapping ternary complex on the ribosome immediately after GTP hydrolysis. In this complex, the aa-tRNA anticodon is bound to the small subunit while the 3′-CCA remains tethered to ternary complex (Figure 1B). In the A/T state, aa-tRNA adopts a distinctly bent conformation22 stabilized by a domain closure of the small subunit specific for the cognate codon-anticodon interaction. In this configuration the aa-tRNA anticodon achieves a nearly fully-accommodated position in the A site. Contributing to the stability of this state, EF-Tu’s GTPase domain resides in a docked configuration with the GTPase Activating Center (GAC) of the large subunit, wherein EF-Tu forms close contacts with the Sarcin-Ricin Loop (SRL). GTP hydrolysis, catalyzed by residue histidine 84 of EF-Tu, allows the 3′-CCA terminus of aa-tRNA to dissociate from EF-Tu and enter the PTC followed immediately by peptide bond formation. Fully accommodated, the newly-formed peptidyl-tRNA resides in an A/A configuration within the pre-translocation ribosome complex (Figure 1C).
Pre-steady state bulk measurements, tracking relative fluorescence intensity changes in aa-tRNA accompanying its entry into the A site, have served as the primary means of investigating the kinetic parameters of the selection process 9; 23; 24. Such methods, combined with direct measurements of GTP hydrolysis and peptide bond formation, have afforded a comprehensive mechanistic framework of selection that has revealed the kinetically-controlled, induced-fit nature of the process. In this scheme, the forward reaction rates for cognate aa-tRNAs are accelerated with respect to those for near- and non-cognate during both initial selection and proofreading. Simultaneously, cognate aa-tRNA dissociation is slowed. Mechanistically, these kinetic features are understood to arise through conformational changes in EF-Tu, tRNA and ribosomal subunits that are precipitated by the nature of cognate mRNA-tRNA interactions occurring in the decoding site 9; 10; 25. Cognate aa-tRNAs trigger these conformational events, leading to efficient initial selection and proofreading processes. Near- and non-cognate aa-tRNAs fail to effectively do so, and in the absence of stabilizing interactions, dissociate from the ribosome.
While these findings have shed light on the allosteric pathways linking the sites responsible for decoding, GTP hydrolysis and peptide bond formation, important features of discrimination during initial selection and proofreading remain to be delineated in both structural and kinetic detail. For instance, it is not yet known precisely when codon-anticodon interactions occur during initial selection. The specific order and timing of conformational signaling events in the selection process, and how they are triggered by cognate aa-tRNA, also remain poorly understood. Towards the goal of delineating these open questions, structural and kinetic aspects of the selection mechanism have been investigated using highly-purified bacterial translation components under pre-steady state conditions using smFRET methods 26; 27; 28. By performing experiments from multiple structural perspectives at a time resolution (10ms) commensurate with the selection process (100–200ms) and at signal-to-noise ratios (>10:1) sufficient to detect sub-nanometer scale conformational events, direct observations of the nature and timing of structural events in the selection process have been made. These data demonstrate that aa-tRNA undergoes reversible, codon-dependent changes in position within the A-site during both initial selection and proofreading. Quantitative comparisons of cognate and near-cognate aa-tRNA interactions with the ribosome suggest that these conformational events, initiated from a highly-transient codon-recognition state, underpin the fidelity mechanism. Such insights shed new light into the allosteric communication pathway linking the functional centers of the small and large subunit, suggesting that the inherently dynamic natures of the system, as well as the physical separation between the active centers on the ribosome, are critical determinants of accuracy.
RESULTS
aa-tRNA accommodates into the A site via intermediate configurations
Single-molecule measurements of tRNA selection were performed under pre-steady state conditions by rapidly mixing (~50ms) 10nM ternary complex containing acceptor (Cy5-acp3U47)-labeled Phe-tRNAPhe with surface-immobilized ribosome complexes containing either donor (Cy3-s4U8)-labeled P-site tRNAfMet or (Cy3-C39)-labeled L11 protein (Figure 1). In so doing, individual ribosome particles could be directly imaged during the selection process at high –spatial and –time resolution (10ms). As previously described26; 27; 29,30, dye-labeled ribosome complexes were shown to be >90% competent for A-site Phe-tRNAPhe incorporation and translocation (Methods, Supplemental Figure 1). Such data demonstrate that translation elongation activities are neither compromised by dye labeling nor surface immobilization. Using a wide-field imaging configuration in combination with elevated illumination intensities (~3kW/cm2), optimized oxygen scavenging and triplet state quenching conditions31 (Cy5 photobleaching rate ≈ 1s−1; Cy3 photobleaching rate ≈ 0.2s−1), ~50 individual ribosome complexes could be imaged simultaneously at 10ms time resolution. Populations of individual aa-tRNA selection events (~250–1500) were obtained through repetition.
All aa-tRNA selection experiments were performed on ribosome complexes bearing deacylated tRNAfMet in the P site. Such complexes are reported to be fully active in aa-tRNA selection, indistinguishable from those bearing peptidyl-tRNAfMet in the P site32. Their use here has several distinct advantages: 1] the classical (A/A) tRNA configuration (τ ~ 350 ± 40ms)29 is relatively stable; 2] dynamics intrinsic to the system are independent of Mg2+ concentration33 and; 3] spontaneous aa-tRNA dissociation (drop-off) rates are slow (on the order of minutes) relative to those containing peptidyl-tRNA in the A site34. These features, combined with elevated Mg2+ ion conditions (15mM), help to restrict dynamics to those related to the selection process and slow such processes to allow quantitation without effecting the selection mechanism23; 35.
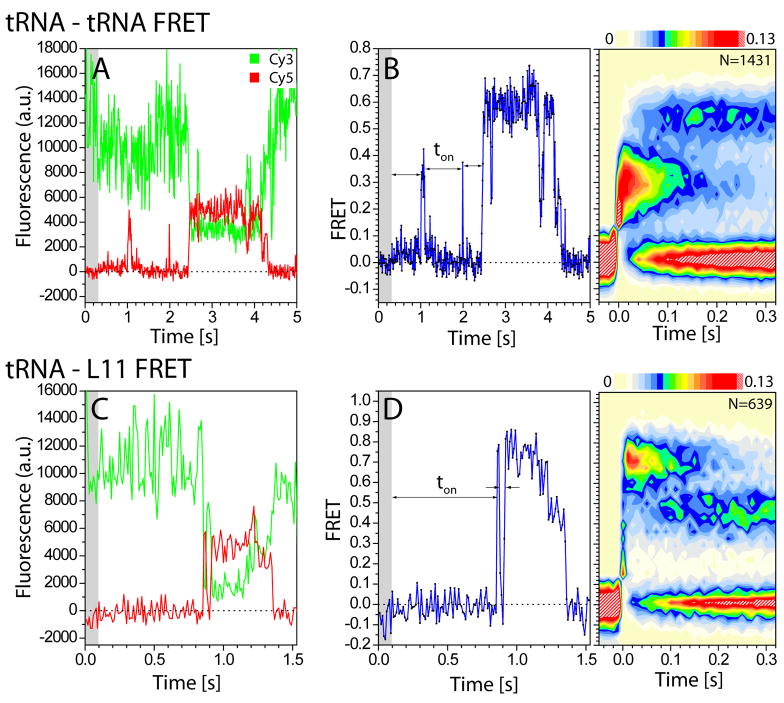
As anticipated from previous studies27, stopped-flow delivery of 10nM Phe-tRNAPhe(Cy5-acp3U47) ternary complex, demarked by a slight rise in Cy5 fluorescence background, yielded anticorrelated donor and acceptor fluorescence signatures characteristic of aa-tRNA entry into the A site of the ribosome (Figure 2A, C). From these data, time-dependent changes in FRET efficiencies were calculated for individual selection events based on the ratio of Cy3 and Cy5 fluorescence intensities (ICy5/(ICy3+ICy5)) (Figure 2B, D left panels). Visual inspection of individual FRET trajectories reporting on the performance of individual ribosome complexes in aa-tRNA selection revealed transient sampling events, followed by progression to a stable non-zero-FRET configuration via at least one, transient intermediate FRET state. Excursions to non-zero FRET states that quickly returned to zero FRET (average lifetime 70ms) represented approximately 45–60% of the total number of FRET observations. As transient events were observed to precede those leading to stable configurations, such events can be interpreted as “non-productive” ribosome encounters with the cognate ternary complex. Correspondingly, selection events leading to stable non-zero FRET configurations were considered “productive”. These behaviors are summarized in population-FRET histograms, where each FRET event observed was post-synchronized to its exit from the zero-FRET state (threshold ≥0.13 FRET) (Figure 2B, D right panels).
Figure 2. Single-molecule observations of aa-tRNA selection monitored from two distinct structural perspectives.
(A) Single-molecule fluorescence trajectories (Cy3, green; Cy5, red) obtained during the delivery of ternary complex with Cy5-labeled aa-tRNAPhe to ribosome complexes containing Cy3-labeled P-site tRNAfMet. (B, left panel) The corresponding FRET trajectory is shown in blue. The interval preceding stopped-flow injection of ternary complex is indicated (grey shaded area). The interval until the first observation of FRET (ton) reflects the bimolecular rate constant (~140μM−1s−1) at ~10nM aa-tRNA. (B, right panel) SmFRET trajectories are post-synchronized to the first observation of FRET above the noise threshold (0.13 FRET), and combined into two-dimensional histograms shown here as a contour plot. The contour plot color map reveals the probability distribution of FRET values at each time point (white-low probability; red-high probability). (C) Single molecule fluorescence trajectories (Cy3, green; Cy5, red) obtained during the delivery of ternary complex with Cy5-labeled aa-tRNAPhe to ribosome complexes containing Cy3-labeled L11. (D, left panel) The corresponding FRET trajectory is shown in blue. (D, right panel) Two-dimensional FRET histograms of the raw data created by superimposing each individual FRET trajectory to the data point at which FRET crossed the threshold of 0.13.
For P-site tRNA-labeled complexes, productive selection events were characterized by progression from an initial low-FRET state (~0.21) to an intermediate-FRET state (~0.32), followed by a relatively slow transition into a high-FRET state (~0.55). As previously described29, the high-FRET state corresponds to the fully-accommodated, classical (A/A) tRNA configuration (Figure 2B). For L11-labeled complexes, productive FRET events were characterized by a rapid progression into a high-FRET state (~0.73), followed by a relatively slow decay into an intermediate-FRET configuration (~0.45) (Figure 2D). The fully-accommodated (AC) aa-tRNA positions of both P-site- and L11-labeled complexes were further established by incubating with ternary complex for one minute -a time sufficient for complete pre-translocation complex formation -followed by buffer exchange (Supplemental Figure 2). In both cases, structural modeling of the high-FRET (~0.54) state for P-site tRNA-labeled complexes and intermediate-FRET (~0.45) state for L11-labeled complexes, confirmed their assignments as fully-accommodated, classical (A/A) tRNA configurations within the pre-translocation complex.
Preliminary assignment of codon-recognition and the GTPase-activated states
In order to assign the number and nature of FRET states on path to the accommodated (AC) state, distinct steps in the selection process were stabilized by biochemically stalling the system (Figure 3). As previously described27, the FRET state corresponding to the earliest detectable stage of the ternary complex-ribosome interaction, was assigned by performing selection experiments on ribosome complexes programmed with a near-cognate (CUU) mRNA codon in the A site (Figure 3A, B left panels). In such experiments, for both P-site tRNA and L11-labeled complexes, non-productive sampling events dominated the aa-tRNA selection process, (Supplemental Figure 3). For P-site tRNA-labeled complexes, dissociation primarily occurred from a low-FRET (~0.21) state; for L11-labeled complexes, dissociation occurred exclusively from a high-FRET (~0.73) state. Consistent with previous bulk measurements36, the bimolecular rate constant in both systems, estimated from the distribution of times (τon) observed between FRET events (≥0.13 FRET) at various ternary complex binding concentrations, was shown to be~100–140μM−1s−1and codon independent. Correspondingly, the low-FRET (~0.21) state for P-site tRNA-labeled complexes and the high-FRET (~0.73) state for L11-labeled complexes were given the preliminary assignment of the codon-recognition (CR) state.
Figure 3. Biochemical control experiments confirm the existence of three intermediate states during tRNA selection on the ribosome.
(A) Contour plots and histograms of FRET trajectories obtained during the delivery of Cy5-labeled ternary complex to ribosome complexes containing Cy3-labeled P-site tRNAfMet. Histograms were fit to the sum of three Gaussian distributions with means reflecting the AC state (~0.54), the GA state (~0.32), and the CR state (~ 0.21). (B) Contour plots and histograms obtained during the delivery of Cy5-labeled ternary complex to ribosome complexes containing Cy3-labeled L11. Histograms were fit to the sum of two Gaussian distributions with means reflecting the AC state (~0.46), and the GA and CR states (~0.73). (Left panels) ternary complex delivery to the near-cognate (CUU) complex. (center-left panels) ternary complex delivery to the cognate (UUC) complex in the presence of 1mM GDPNP, (center-right panels) ternary complex delivery to the cognate (UUC) complex in the presence of 20μM kirromycin (Right panels) ternary complex delivery to the cognate (UUC) complex.
In both systems, the A/T state was assigned by performing selection experiments in the presence of either the non-hydrolyzable GTP analogue, GDPNP, or the antibiotic kirromycin, which stall the selection process immediately prior to, and subsequent to, GTP hydrolysis, respectively. Both inhibitors were shown to efficiently stall P-site tRNA-labeled complexes in stable intermediate-FRET (~0.32–0.35) configurations (Figure 3A; second and third panels). Notably, the mean FRET value observed in the presence of GDPNP was slightly lower than that observed in the presence of kirromycin (ΔFRET ~0.05), suggesting that GTP hydrolysis within the A/T state leads to a small, but detectable change in aa-tRNA position in the A site. For the L11-labeled complex, both selection inhibitors were shown to stabilize cognate ternary complex in a high-FRET (~0.73) state (Figure 3B; second and third panels). Here, distinctions in FRET values between GDPNP- and kirromycin-stalled complexes were not observed.
Consistent with these assignments, in near-cognate, P-site tRNA-labeled complexes, which undergoes only slow GTP hydrolysis on the ribosome14, a low-FRET state was predominantly observed with only transiently sampling the intermediate-FRET state (Supplemental Figure 4). These observations suggest that contacts necessary for stable A/T state formation and GTP hydrolysis are inefficiently formed by near-cognate ternary complex even though the intermediate-FRET state can be transiently achieved. Correspondingly, the A/T state configuration achieved prior to GTP hydrolysis is heretofore referred to as the “GTPase-activated” (GA) state.
Both CR and GA state assignments were confirmed through uninhibited aa-tRNA selection experiments performed on P-site tRNA-labeled complexes. Here, FRET values consistent with the biochemically-stabilized CR, GA and AC states were readily returned (Supplemental Figure 5A). Such observations provided evidence that progression of aa-tRNA from CR to GA states (corresponding to initial selection) is accompanied by a ΔFRET ~0.12, while progression from GA to AC states (corresponding to proofreading) is accompanied by a ΔFRET ~0.22. By contrast, L11-labeled complexes displayed little or no change during the initial selection step, while proofreading was accompanied by a ΔFRET ~0.27. These data suggest that L11-ternary complex interactions may be formed in the CR state and maintained in the transition to the GA state. Taken together, such observations support a model in which FRET changes observed during the selection process can be principally ascribed to motions of aa-tRNA within the A site (Figure 1).
Quantitative treatment of smFRET observations of aa-tRNA selection
Quantitative assessments of the order and timing of FRET changes during the selection process were made by subjecting individual aa-tRNA selection events to statistical analyses using automated hidden Markov Modeling (HMM) procedures. To do so, each FRET event observed for P-site tRNA- and L11-labeled systems was idealized to specific kinetic models, where the FRET values of each state were defined according to the aforementioned biochemical assignments (Methods, Supplemental Figure 6). In the idealization procedure, the standard deviation of each FRET state was given by the width of the FRET states determined experimentally for rigid DNA oligonucleotide standards under identical conditions (Supplemental Figure 5B and C). To satisfy the principles of microscopic reversibility, and the finite probabilities of aa-tRNA dissociation and/or dye photobleaching, each state in the model was linked reversibly to each other and a zero-FRET state. After testing models of increasing complexity, a six-FRET state, linear reaction pathway was shown to provide the best fit to both the experimental data and the contemporary framework of aa-tRNA selection9 (Supplemental Figure 6C).
aa-tRNA reversibly progresses through intermediate states in the selection process
As suggested by contemporary models of aa-tRNA selection7 and previous smFRET studies27; 28, in the kinetic model employed here, transitions into FRET states where irreversible chemical steps occur (GA and AC states, respectively) could only be achieved via intermediate states (GA′ and AC′) with identical FRET values (Supplemental Figure 6C). In this scheme, GA′ and AC′ states are physically similar in nature to GA and AC states and reflect unsuccessful attempts to achieve stabilizing contacts necessary for chemistry. In so doing, the idealized data (Figure 4A, B, left panels) could provide estimates of: 1] the probabilities of specific transitions between FRET states i and j, Ti,j; and 2] the dwell times of each FRET state, τI. To limit this analysis to dynamics occurring during the selection process, idealizations were truncated after the observation of an AC-state dwell lasting ≥ 120ms. Similar results were obtained when this criterion was varied from 80–240ms. Such times are sufficient for peptide bond formation, but insufficient for hybrid state excursions that occur within the pre-translocation complex29.
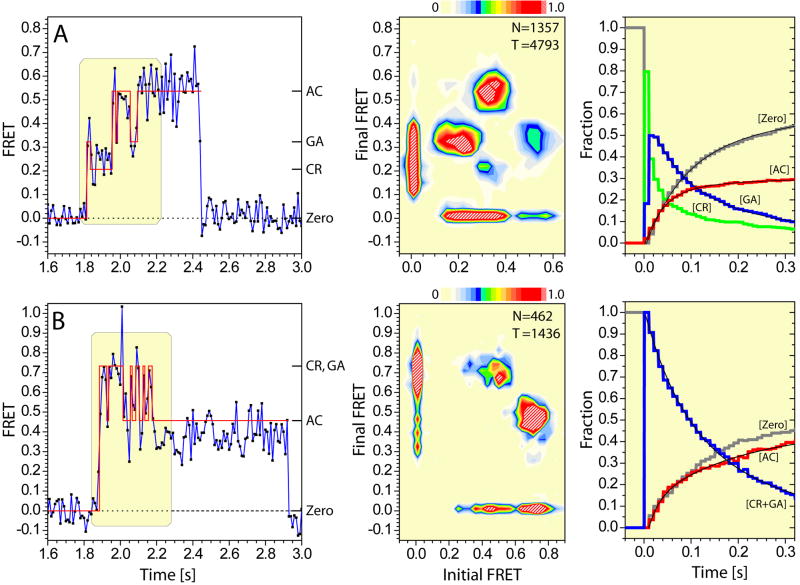
Figure 4. smFRET trajectories reveal the order and timing of structural transitions during aa-tRNA selection.
(A, left panel) smFRET trajectory (blue) resulting from the delivery of ternary complex containing Cy5-labeled aa-tRNAPhe to ribosomes containing labeled P-site tRNAfMet, with idealization generated using hidden Markov Modeling (HMM) overlaid in red. FRET values corresponding to the CR (low), GA (intermediate) and AC (high) states are indicated. The boxed region shown represents the completed tRNA selection event. (Center panel) The transition density plot (TDP) indicates the distribution of transitions between the three observed FRET states. The asymmetry of the TDP with respect to the diagonal axis reflects the pre-steady state nature of the observation. (Right panel) The time evolution of the occupancy in each observed FRET state reveal the order and timing of events in the selection process; zero FRET (grey); CR (green); GA (blue); AC (red). (B) The corresponding analysis of FRET trajectories observed on L11-labeled ribosome complexes.
The results of these analyses, summarized in transition density plots (TDP)37 (Figure 4A, B, center panels) and dwell time histograms (Figure 4A, B, right panels), revealed the order and timing of events in the selection process. Here, the asymmetric distribution of well-separated peaks in the TDP with respect to the diagonal axis that each has distinct intensities reflects both the accuracy of the idealization procedure and the non-equilibrium nature of the selection process. Consistent with the population FRET data, FRET transitions observed for P-site tRNA trended towards higher FRET states, while FRET transitions for L11-labeled complexes trended first to high- and then to intermediate-FRET states. Notably, the appearance of peaks in the TDP on both sides of the diagonal axis suggested the existence of reversible A-site tRNA motions during the selection process.
TDPs generated for P-site tRNA-labeled complexes revealed nine types of FRET transitions that could be assigned based on FRET value to specific events in the selection process: four transitions above the diagonal (T0,CR, T0,GA′/GA, TCR,GA′/GA, TGA,AC′/AC) and five transitions below the diagonal (TGA′/GA,CR, TAC,GA′/GA, TCR,0, TGA′/GA,0, TAC′/AC,0) (Figure 4A, center panel). Transitions above the diagonal represent motions towards the AC state and productive substrate incorporation; transitions below the diagonal reflect the reversible nature of initial selection and proofreading events (TGA′/GA,CR, TAC′/AC,GA, respectively) as well as the dissociation of aa-tRNA from the ribosome (TCR,0, TGA′/GA,0, TAC′/AC,0). Ternary complex binding events were predominantly initiated via a short-lived (~10–50ms) low-FRET CR state (T0,CR ~81%). Initial binding events appearing to proceed directly to intermediate- (T0,GA′/GA ~17%) and high-FRET states (T0,AC′/AC ~2%) could be explained by transitions through the CR state faster than the imaging time scale (Figure 4A, right panel). From the CR state, aa-tRNA was observed to either immediately dissociate (TCR,0 ~36%) or progress to the GA′/GA state (TCR,GA′/GA ~61%). From the GA′/GA state, aa-tRNA either returned to the CR state (TGA′/GA,CR n.d.), dissociated from the ribosome (TGA′/GA,0 ~35%), or progressed to the AC′/AC state (TGA′/GA,AC′/AC ~65%). From the AC′/AC state, aa-tRNA was observed to either transition back to the GA′/GA state (TAC′/AC,GA′/GA ~37%), dissociate from the ribosome (TAC′/AC,0 ~14%) or remain in this state (TAC′,AC ~48%).
Together with the dwell time information reporting on the rates of CR, GA and AC state accumulation and decay, these data directly revealed that aa-tRNA typically progressed through CR and GA states on path its fully-accommodated position in the A site. Notably however, reversibility was frequently observed in this progression (CR↔GA↔AC), as well as aa-tRNA dissociation during the process, resulting in only ~30–40% of all FRET events observed successfully achieving the AC state. As the translation components investigated were shown to be fully-active in aa-tRNA selection (Supplemental Figure 1) and fluorophore photobleaching rates were slow relative to the duration selection events, such inefficiencies could be attributed to the basic selection mechanism. Globally similar results were obtained through analyses of L11-labeled ribosome complexes (Figure 4B, center and right panels). Asymmetry in the TDP and transitions to zero FRET were again consistent with reversibility and dissociation from intermediates in the selection process. However, as stated above, the overlapping nature of CR and GA states (Figure 3), partially obscured the specific order and timing of events on path to the AC state.
Validation of codon-recognition state assignment
As a test of the fitness of the kinetic model and FRET state assignments, the rates exiting the codon-recognition state for P-site tRNA-labeled complexes were examined by analyzing the dwell-time information obtained through idealization. The known selection mechanism - including discrimination by rejection13; 38 and discrimination by induced-fit14 -predicts that the lifetime of the CR state should depend on the nature of the codon-anticodon interaction. Consistent with such models, a comparison of the rates of cognate and near-cognate aa-tRNA progression out of the CR state followed the anticipated trends (Supplemental Figure 7). Here, the lifetime of the cognate ternary complex codon-recognition state before dissociating from the ribosome was slowed relative to the near-cognate system. The lifetime of the cognate ternary complex codon-recognition state before progressing forward was accelerated with respect to the near-cognate system.
The energy landscape of aa-tRNA selection favors aa-tRNA rejection
The contribution of conformational dynamics to the aa-tRNA selection mechanism was further explored by computationally separating (Methods) and independently analyzing productive (Figure 5A) and non-productive (Figure 5B) events. FRET state lifetimes were derived by fitting the corresponding dwell time data. Productive events in P-site tRNA-labeled complexes, representing ~33% of all aa-tRNA-ribosome encounters observed, were characterized by a step-wise progression through CR and GA states on path to the fully accommodated AC state (Figure 5A, left and center panels). In agreement with the contemporary model of aa-tRNA selection in which aa-tRNA accommodation is rate-limited by proofreading events15, the observed rate of AC state accumulation ( ) was limited by the apparent rate of GA state exit ( ) (Figure 5A, right panel). The subpopulation of non-productive events (~69%) were characterized by extensive, reversible transitions during both initial selection and proofreading (TGA′/GA,CR, TAC′/AC,GA, respectively) (Figure 5B, center panel). Such events were ultimately rejected at a rate of ~13s−1, where dissociation pathways were distributed between CR, GA and AC states (~46%, 42% and 12% respectively), consistent with preferential dissociation of aa-tRNA prior to, or directly from, the state in which GTP hydrolysis occurs. Excursions to the AC/AC′ state, which returned directly to zero FRET, may either represent photobleaching or aa-tRNA rejection near the end of the proofreading process (Figure 5B, right panel).
Figure 5. Reversible FRET transitions during initial selection are codon dependent.
(Left panels) Contour plots and histograms of FRET trajectories, (Center panels) TDPs, and (Right panels) the time evolution of FRET-state occupancies obtained during the delivery of Cy5-labeled ternary complex to ribosome complexes containing Cy3-labeled P-site tRNAfMet and a (A and B) cognate or (C and D) near-cognate A-site codon. Trajectories were computationally sorted into two sub-populations: (A and C) productive accommodation events, characterized by stable formation of the AC state; and (B and D) non-productive events.
To confirm that the rejected subpopulation observed represents failed cognate aa-tRNA selection attempts, similar analyses were performed on ribosome complexes specifically programmed with a near-cognate (CUU) codon (Figure 5C, D). Here, both the distribution of FRET states and dynamic behaviors of productive and non-productive events were clearly distinct from those of the cognate system. As expected for a high-fidelity system, the vast majority of selection events were designated as non-productive selection events (~99%) according to the ≥120ms AC state occupancy selection criterion. Such events were dominated by reversible fluctuations between CR and GA′/GA states, where aa-tRNA dissociated from the CR state ~83% of the time at a rate of ~12s−1. Notably, the subpopulation meeting the criteria of a productive selection event (~1% of all events observed) were characterized by a broad distribution of FRET states as well as fast, reversible aa-tRNA dynamics. In contrast to productive cognate aa-tRNA incorporation, such events were typically observed (>50% of the time) to return to long-lived CR, GA and zero-FRET states following an AC′ state dwell, whose FRET value (~0.75) was significantly higher than observed for the cognate system (Supplemental Figure 8). Such dynamics could not be attributed to pre-translocation complexes bearing fully-accommodated near-cognate aa-tRNA in the A site (data not shown). Manual inspection of such FRET trajectories suggested that only a small fraction (1–10%) of near-cognate events classified as “productive” actually achieved the bona fide AC state. Correspondingly, only ~0.01–0.1% of the near-cognate A-site tRNA binding events was estimated to fully incorporate during the observation period.
EF-Tu-catalyzed GTP hydrolysis mediates progression from initial selection to proofreading
To examine whether EF-Tu-catalyzed GTP hydrolysis strictly partitions initial selection and proofreading events, similar kinetic analyses were performed on GDPNP- and kirromycin-stalled P-site tRNA-labeled complexes (Figure 3; Supplemental Figure 9). While ternary complex was observed to be highly-stabilized on the ribosome in a state globally consistent with the structurally-characterized A/T state6; 39; 40, both systems remained dynamic in nature, where excursions were observed to both CR- and AC-like states. Here, the mean FRET values, FRET state lifetimes and FRET dynamics differed only modestly. In GDPNP-stalled complexes, transitions characteristic of initial selection events (TGA′/GA,CR, TCR,GA′/GA) were more frequently observed. Kirromycin-stalled complexes were relatively stable in nature and exhibited dynamics characteristic of proofreading events (TGA′/GA,AC′AC, TAC′/AC,GA). Although consistent with the notion that GTP hydrolysis takes place in the intermediate-, GA-FRET state, these data suggest that forward progression out the GA state does not strictly depend on GTP hydrolysis.
RNA remodeling events underpin the selection mechanism
To examine the physical nature of conformational events underpinning aa-tRNA motions in the A site, experiments were performed over a range of magnesium ion concentrations [Mg2+] (2.5–15mM) known to alter the rate and fidelity of aa-tRNA selection15; 23. Here, FRET state lifetimes depending on RNA-RNA interactions and FRET state transitions stemming from RNA remodeling events are anticipated to be substantially altered41. Under each condition, evidence of CR and GA states were observed on path to the AC state as well as reversibility during initial selection and proofreading. Progression to the AC state was similarly limited by the rate exiting the GA state. Thus, consistent with previous results 14; 23, these data demonstrated that the basic mechanism of the selection process is globally similar at all Mg2+ concentrations.
The efficiency (productive events/total events) of productive AC state formation increased by ~10% at lower [Mg2+], achieving a maximum efficiency of (~43%) at ~5mM. The maximal rate of peptide bond formation ( ), was ~49s−1 at 2.5mM, increasing by ~3-fold upon lowering the [Mg2+] from 15mM (Supplemental Figure 10). As the average number of transitions observed per trace (T/N) stayed relatively constant over the titration (~4), while the total time for productive accommodation was reduced, the number of transitions observed per unit time must increase at least 3-fold upon lowering the [Mg2+] from 15mM to 2.5mM. Such observations suggest that aa-tRNA dynamics likely stem from Mg2+-dependent RNA remodeling events in the ribosome and/or tRNA, where the activation barriers for conformational events, and the dissociation of codon-anticodon interactions, are reduced by lowering the Mg2+ ion concentration.
Over the same titration, the dissociation rate of non-productive cognate selection events increased ~2-fold, reaching a maximum rate of ~26s−1 at 2.5mM Mg2+. Control experiments performed on the near-cognate system over the same titration showed that the total number of FRET events observed decreased at [Mg2+] ≤ 5mM, leading to an apparent decrease in the bimolecular rate constant. These data suggest that the lifetime of near-cognate selection events becomes shorter than the imaging time scale (ca. ≤10ms). In line with higher fidelity selection, “productive” events for the near-cognate system were reduced to a level below the detection limit. Thus, quantitative treatments of the low Mg2+ data could not be performed without “missed event” considerations27.
aa-tRNA motions associated with initial selection and proofreading exceed the imaging time scale
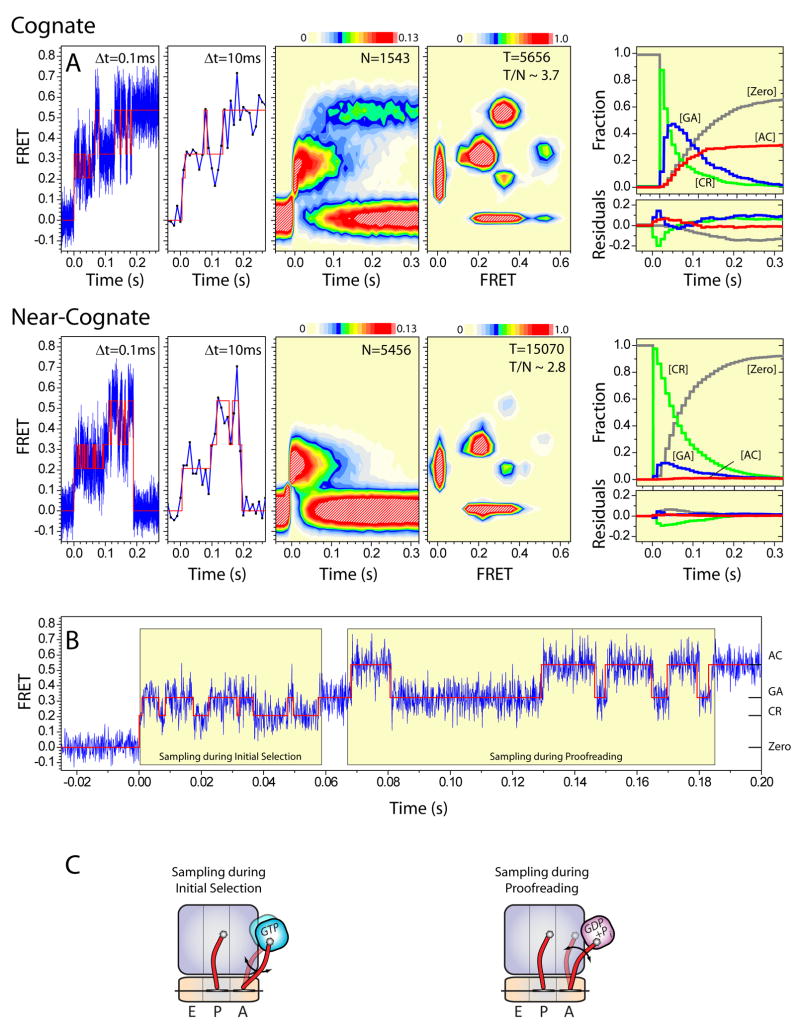
In order to address the finite imaging time scale and the potential existence of fast conformational dynamics that may be missed by the present analysis, the structural and kinetic parameters of selection were simulated at a time increment of 100μs. Here, initial estimates of the rate constants of each step in the kinetic model employed ( , k2, …, k9) (Supplemental Figure 6C) were estimated by global fitting procedures using the experimental dwell time information provided by idealization (Figure 4A). This initial treatment provided a reasonably good fit to the raw experimental data (population FRET histograms) for both the P-site tRNA- and L11-labeled systems (data not shown). Subsequent to this initial fit, only modest adjustments in kinetic parameters were needed (± 20%) to closely recapitulate each experimental observable: population-FRET histograms; TDPs; transitions per selection event (T/N); dwell time histograms; as well as the efficiencies of the selection process (Figure 6A; Supplemental Table 2).
Figure 6. Simulation of fast conformational dynamics recapitulates experimental smFRET data.
(A) smFRET trajectories (blue) of the selection process were simulated at 100μs time resolution for P-site tRNAfMet labeled ribosome complexes programmed with cognate (upper) and near-cognate (lower) mRNA codons in the A site according to the kinetic model shown in Supplemental Figure 6C. Idealized trajectories are overlaid in red. To compare directly with the experimental data (Figures 3, 4), simulated traces were binned to the experimental time resolution (10ms) and processed into two-dimensional FRET histograms, TDPs (center panels) and dwell time kinetics data (right panel). The residuals shown in the lower part of the panel show the deviation of the simulated state occupancies from the experimental data. (B) An individual smFET trace simulated at 100μs time resolution showing periods of conformational sampling during initial selection and proofreading (boxed regions) (C) Schematics showing a plausible molecular basis for the FRET fluctuations observed during the initial selection and proofreading, where A-site tRNA moves within the A site with respect to the ribosome and P-site tRNA.
Several important conclusions could be drawn from this analysis. First, such simulations support the notion that the aa-tRNA selection reaction coordinate is comprised of a series of linked, dynamic equilibria, where aa-tRNA undergoes fast conformational sampling within the A site during both initial selection and proofreading (Figure 6B) –a conclusion reinforced by direct inspection of individual FRET trajectories of the selection process (Figure 4). Second, the simulation data suggested that rates of aa-tRNA dynamics during initial selection and proofreading (k2 ≈260s−1; k−2 ≈200s−1 and k4 ≈90s−1; k−4≈30s−1, respectively) largely exceed the imaging time resolution (100 frames s−1). This observation suggests that the majority (80–90%) of conformational events in aa-tRNA selection are not observed at the experimental time scale (10ms). Correspondingly, the dynamics estimated through idealization of the FRET data (Figure 4) may be interpreted as vastly under-representing the true dynamics of the systems investigated. The fluctuations that are observed likely reflect only the tails of the exponentially distributed event lifetimes. Thus, the FRET states observed likely represent average aa-tRNA positions rather than static states from which low-probability, thermally-induced excursions occur28. Consistent with this notion, experiments performed on the cognate system at 2.5ms time resolution (400 frames s−1) demonstrated the existence of conformational dynamics on the order of 100–200s−1 occurring during both initial selection and proofreading processes (Supplemental Figure 11).
DISCUSSION
As shown through the experiments first establishing the genetic code, where aa-tRNA selection was performed in the absence of EF-Tu and energy expenditure, the process of aa-tRNA selection is inherently exergonic in nature42. These data demonstrate that EF-Tu and GTP hydrolysis serve only to increase the rate and fidelity of the process. Fidelity in aa-tRNA selection is ensured by the existence of substantial energetic barriers that restrict aa-tRNA entry into the A site of the ribosome. Conformational changes in the ribosome, aa-tRNA and EF-Tu triggered by codon-anticodon interactions enable cognate aa-tRNA to surmount these energetic barriers, whereas near- and non-cognate tRNAs, less able to do so, are efficiently rejected 43. Insights into the nature of selection events were first provided by the discovery of mutations in tRNA, the ribosome and EF-Tu distal to the site of codon-anticodon interaction that affect the fidelity mechanism 44; 45. Deeper mechanistic clues as to the role and timing of conformational processes in these components were provided by elegant structural and biochemical studies over the past two decades7; 43. Such studies have shown that the largest energetic barriers to aa-tRNA entry manifest during initial selection and proofreading processes.
Despite much progress, features of the selection mechanism critical to understanding fidelity remain poorly understood. Open questions include delineating the timing of codon recognition in the selection process as well as the physical basis of discrimination during initial selection and proofreading. Previous smFRET efforts to directly image selection events on individual ribosomes have shown a potential to answer these open questions by providing access to both structural and kinetic information simultaneously27; 28; 46. However, progress in this line of research has been hampered by the time resolution of imaging and fluorophore labeling positions that adequately inform on the process.
Through advancements in CCD technologies, strategies to mitigate fluorophore photophysics, and improvements in experimental throughput, the smFRET data presented here provide important clarifications as to when codon recognition occurs during selection as well as the nature of conformational events underpinning the selection mechanism. With the benefit of two independent structural perspectives and a 10-fold increase in time resolution over previous smFRET measurements, initial selection and proofreading are shown to be rate limited by microscopically reversible motions of aa-tRNA in the A site.
Global considerations and the trade-off between speed and accuracy in aa-tRNA selection
In line with the known trade-off between speed and accuracy in translation elongation47; 48, as well as a cellular demand for energy conservation, visual inspection of the smFRET data showed that aa-tRNA selection events partition into two distinct, “non-productive” and “productive”, sub-populations (Figure 2; Supplemental Figure 7). Each sub-population could be efficiently parsed through computational means49 (Figure 5). In this analysis, both cognate and near-cognate ternary complex were shown to favor rejection at early stages in the selection process, prior to achieving the configuration where GTP hydrolysis occurs (Supplemental Figure 4). As anticipated for a high-fidelity system, “productive events” were extremely rare for complexes programmed with a near-cognate mRNA codon (only ~20 out of 20,000 events observed). Consistent with the process being controlled by codon-anticodon interactions, productive events were ~300-times more frequent for those programmed with a cognate mRNA codon (~50–70% of the total number of events depending on [Mg2+]; Supplemental Figure 10). As elaborated below, such observations suggest that under cellular conditions (2.5–5mM Mg2+) wild-type ribosomes are only ~50% percent efficient in accepting cognate aa-tRNA after reaching the codon-recognition state. By contrast, contemporary models of selection9; 14, where measurements of codon-recognition state stability performed using non-hydrolyzable GTP analogues, imply efficiencies near unity.
As the biochemical components utilized were shown to be highly active in translation reactions (Supplemental Figure 1), barring the trivial explanation that inefficiencies reflect irregularities stemming from surface immobilization and/or the dye-labeling strategies used, inefficiencies in the aa-tRNA selection mechanism may be inherent to the system. In this view, the rejection of cognate aa-tRNA suggests the existence of dynamic range in both the speed and accuracy of the aa-tRNA selection mechanism. For instance, less-than-unity selection efficiencies may ensure greater overall accuracy at the expense of the rate of global translation. At physiological magnesium concentration (most closely reflected by the 2.5mM data set), the selection efficiencies and non-productive dissociation rates observed suggest bulk translation rates of ~5s−1 in the presence of excess near- and non-cognate aa-tRNA substrates (Supplemental Methods). Adjusting for the temperature, this value is in good agreement with the estimated translation rate (~20s−1) observed in vivo 10.
Existing models of aa-tRNA selection
Structural and kinetic characterization of the codon-recognition state is paramount to understanding the order of events in the aa-tRNA selection mechanism. Presently, two distinct models of codon recognition exist which differ in the order of early events in codon recognition - both are based on structures of the kirromycin-stalled ternary complex bound to the ribosome4; 5; 7. In the model described by Villa et al., interactions between the elbow region of aa-tRNA and the L11/GAC domain of the ribosome occur early in the ternary complex-ribosome encounter, preceding codon recognition. Spontaneous aa-tRNA bending events within the bound ternary complex subsequently allows aa-tRNA to sample the mRNA codon in the A site. Capture of the codon-anticodon complex by domain closure events in the 30S subunit leads to stabilization of the ternary complex on the ribosome. Distortions in the aa-tRNA-EF-Tu interaction brought about by domain closure stimulate GTP hydrolysis and relaxation from this high-energy state drives accommodation events. In the alternative model proposed by Schuette et al., ternary complex enters the ribosome via interactions with the L12 stalk protein and samples the mRNA codon in an undistorted conformation. Although a sub-optimal geometry for recognition by the decoding site, this transient state precipitates domain closure on the 30S subunit, which results with aa-tRNA bending and stabilization of ternary complex interactions with the GAC. As for Villa et al., distortions in aa-tRNA stemming from domain closure alter aa-tRNA’s interaction with EF-Tu to stimulate GTP hydrolysis and subsequent accommodation events. As recently highlighted7, smFRET studies are uniquely suited to uncovering transient states in the selection process. The smFRET data presented here, which provide further evidence of a transient codon-recognition state earlier in the selection process than previously appreciated, argue in favor of the model by Schuette et al., wherein early codon-recognition events trigger conformational changes in the ribosome and aa-tRNA that favor forward progression through the selection process.
Early events in codon-recognition
Although previous smFRET studies of biochemically-stalled systems have shed light on the existence of a transient, low-FRET codon-recognition state occurring early in the ternary complex-ribosome interaction27; 28, the time scale of these imaging experiments generally precluded its direct detection and quantification during uninhibited selection experiments. By contrast, at the time resolution of the present experiments (10ms) the timing of this state’s formation and decay has now been delineated as preceding, and on path to, GTP hydrolysis and peptide bond formation. For P-site tRNA-labeled complexes, this state corresponded to a low-FRET state (0.21 FRET), while for the L11-labeled complexes, this state corresponded to a high-FRET state (~0.73 FRET). As the rates entering this state were indistinguishable for cognate and near-cognate aa-tRNA and consistent with the known bimolecular rate constant of ternary complex interactions with the ribosome (~140μm−1s−1; Ref 23; Supplemental Figure 3), while displaying dramatically different rates of forward progression (Figures 3, 5 and Supplemental Figure 7), this state could be confidently assigned as the codon-recognition (CR) state. This assignment specifies that events required for selection, including GA state formation, occur subsequent to exiting this newly-defined codon recognition state.
Two important considerations must be given to this CR state assignment. First, the CR state, thus defined, is physically distinct from that previously defined through bulk investigations 9; 14; 50. Bulk interrogations aiming to probe the codon-recognition state have relied on biochemically stalling ternary complex prior to GTP hydrolysis using the non-hydrolyzable GTP analogue, GDPNP. Although this biochemical definition of the codon-recognition state is appropriate for near- and non-cognate systems, it does not completely reflect the codon-recognition state for the cognate system (Supplemental Figure 4). The GDPNP-stalled, cognate ternary complex predominantly resides in a highly-stabilized GA state, which is achieved after codon recognition, just prior to GTP hydrolysis, and near the endpoint of initial selection. Correspondingly, the “codon-recognition state” lifetime (reflected in contemporary selection models in the kinetic parameter, k−2) for the cognate ternary complex is reported to be long-lived (ca. 8–10 seconds). In this view, the transient CR state observed through the present experiments, therefore more closely resembles what has previously been defined as the “initial binding” complex32; 51. Consistent with the notion that the fluorescent signals used to derive contemporary selection models stem from distortions in the aa-tRNA molecule, this assessment specifies that ternary complex remains relatively undistorted when bound in the CR state and that bulk methods, which employ environment sensitive probes, may be relatively insensitive to initial selection events.
Second, this model specifies that biochemical steps preceding the formation of the newly-defined CR state must be fast relative to the observed bimolecular rate constant. In the contemporary model of tRNA selection23, this includes the kinetic parameters describing the non-specific interaction of EF-Tu with the L12 ribosomal stalk proteins (k1), the dissociation rate of this complex (k−1) and the forward rate at which the aa-tRNA anticodon enters the small subunit A site to interact with the mRNA codon (k2). As fast, reversible events leading into, and out of, the newly-defined CR state are not observed in the present experiments, even at dilute ternary complex concentrations (data not shown), the dissociation rate of the L12-EF-Tu interaction must be significantly greater than formation of the CR state.
Conformational dynamics of aa-tRNA in the A site and the fidelity mechanism
In good agreement with the notion that aa-tRNA itself participates in the selection process 25, both structural perspectives obtained suggest that structural transitions during the selection process report directly on aa-tRNA motions in the A site. Here, “productive” cognate and near-cognate aa-tRNA selection events were generally observed to follow a strict forward progression from CR→GA→AC states. Importantly however, each of these transitions was observed to be preceded by reversible steps, where the rates and probabilities of motion depended strongly on the nature of the mRNA codon-tRNA anticodon interaction (Figure 5). These reversible selection steps, corresponding to excursions to transient GA and AC states, were interpreted as structural events in the system, manifesting as aa-tRNA movements in the A site, that report directly on initial selection and proofreading processes, respectively7; 9.
As detailed in contemporary models of selection23, the rate-limiting features of initial selection and proofreading correspond to GTPase activation and accommodation, respectively. While thermal fluctuations in aa-tRNA position undoubtedly contribute to the motions observed28, the Mg2+-dependence of selection events (Supplemental Figure 10) suggests that the activation barriers to GTPase activation and accommodation include conformational processes directly related to RNA remodeling events. In this view, at least two conformational processes in the ribosome and/or tRNA underpin the fidelity mechanism: one that drives GTPase activation; another that drives accommodation. Such events may include rearrangements in ribosomal RNA elements of the small subunit decoding site, the large subunit GAC, as well as the tRNA molecule1; 50. Further experiments will be required to explore the relationship of these events to the domain closure model of selection proposed by Ogle and Ramakrishnan3. However, the present observations suggest that domain closure either occurs by a sequence of distinct structural events or that additional structural transitions occur within an RNA component of the system that also contribute to the selection process.
Initial insights into the nature and role of reversible events in aa-tRNA selection pathway were found through experiments in which cognate aa-tRNA selection was biochemically stalled with GDPNP or kirromycin. While both systems exhibited behaviors globally consistent with the structure and stability of ternary complex trapped in the A/T state22, they also displayed subtle structural differences on the population level (Figure 3), likely reflected in the substantial residual dynamics present in both systems (Supplemental Figure 9). As anticipated by the established biochemical sequence of events in the selection process, fluctuations characteristic of initial selection predominated in GDPNP-stalled complexes; fluctuations characteristic of proofreading events were favored in kirromycin-stalled complexes. Based on existing structural information, and contemporary models of the process, initial selection events (CR ↔ GA transitions) are likely dominated by reversible tRNA bending and unbending processes related to early domain closure events as well as EF-Tu docking and undocking events with the mobile GAC domain4; 5; 6; 22; 52. Proofreading events after GTP hydrolysis (GA ↔ AC transitions) may reflect further distortions within aa-tRNA leading to elbow displacement towards the P-site and/or reversible motions of the 3′-CCA end of aa-tRNA away from EF-Tu, towards the PTC 53.
Importantly, and as previously reported27, excursions characteristic of proofreading were evidenced in GDPNP-stalled complexes (high-FRET, AC-like states) and excursions characteristic of initial selection were evidenced in kirromycin-stalled systems (low-FRET, CR-like states). To the extent that such systems recapitulate mechanistic features of the highly-reversible, uninhibited selection process, these observations suggest that GTP hydrolysis does not strictly partition initial selection and proofreading dynamics: AC-like transitions can occur prior to GTP hydrolysis; CR-like excursions can occur after GTP hydrolysis.
Quantitative insights into the role of reversible aa-tRNA motions in the A site during selection
Quantitative insights into the rates of motions observed during uninhibited selection for cognate and near-cognate data sets were obtained by applying a highly-simplified kinetic model to individual selection events (Supplemental Figure 6C). In this model, only the rate-limiting determinants of aa-tRNA motion (observed as changes in FRET states) were included. Here, transient, reversible transitions between structurally-distinct states (CR↔GA; GA↔AC) manifest in the kinetic parameters describing GA′-(TCR,GA′; GTPase activation) and AC′-state excursions (TGA,AC′; accommodation), respectively. In this view, GA′ and AC′ states (like the CR state) are represent relatively high-energy configurations of the system that are interpreted physically as attempts to achieve GA and AC states, or intermediates resembling the transition states for GTP hydrolysis and peptide bond formation, respectively.
Consistent with discrimination by rejection13; 38 and induced-fit14, both experiment (Figures 3 and 5) and simulation (Figure 6; Supplemental Table 2) showed that structural transitions in aa-tRNA position during selection were rapid and codon dependent. Here, the simulated data suggested that the rates at which both the cognate and non-cognate aa-tRNA make forward attempts at GTPase activation (k2; GA′) and accommodation (k4; AC′) are similar in magnitude, while the reverse rates (k−2, k−4), and the chemical steps of GTP hydrolysis and peptide bond formation, differ by ~3–10 fold (Supplemental Table 2). In both cases, simulation of the selection process showed that reversible aa-tRNA motions during intial selection and proofreading were close to, or exceeding, the imaging time scale (ca. 30–600s−1). Limited datasets, obtained at 2.5ms time resolution, experimentally validated this finding(Supplemental Figure 11).
Substantial differences could be directly estimated from these rates in the effective probabilities of completing initial selection (P1) and proofreading (P2), which together determine the probability of “productive” selection events (P = P1*P2) (Supplemental Tables 1 & 2). In agreement with previously reported fidelity parameters (Supplemental Table 3), these probabilities suggest that the overall selectivity, S, is ~50, partitioned roughly equally between initial selection (f=10) and proofreading (F=5). Collectively, such observations are consistent with the ribosome’s “intrinsic selectivity” during selection arising from mechanisms that leverage small differences in the binding free energies of cognate and near-cognate tRNAs through: 1] “discard parameters” that maximize the accuracy and efficiency of the selection process through distinct rejection rates and; 2] an optimized evolution of the substrate-binding sites in the transition states for product formation47.
However, certain aspects of the model are difficult to reconcile with contemporary models and the known selectivity during proofreading steps23. First, the existence of premature aa-tRNA dissociation pathways in the selection process without spurious aa-tRNA re-entry raises important considerations in the theoretical and quantitative framework of fidelity which pertain to the principle of detailed balance11. Here, the existence of fast conformational processes in the ribosome associated with the selection process suggest that passive, thermally-accessible relaxation processes may occur that rapidly return the system to ground state configuration(s) following aa-tRNA dissociation. Second, the estimated probability of cognate aa-tRNA accommodation (P2=0.59) and the off-rate of near-cognate aa-tRNA during accommodation (k6) are not expected from the observed 1:1 stoichiometry of GTP hydrolysis and peptide bond formation measured in bulk14 or the anticipated propensity of near-cognate aa-tRNA to dissociate faster than cognate aa-tRNA. Both issues imply limitations of the simplified kinetic scheme used to simulate the selection process. While future experiments will be required to investigate these apparent discrepancies, both issues may be at least partially reconciled if in the near-cognate case, AC-state excursions do not actually correspond to productive selection events (Supplemental Figure 8) and if AC′-like excursions occur prior to GTP hydrolysis (Supplemental Figure 9).
aa-tRNA alignment in the A site determines the rates of chemistry steps in the selection process
Here, physical differences in transition state formation for cognate and near-cognate aa-tRNA manifest in the rates exiting GA′ and AC′ states as well as the estimated rates of GTP hydrolysis and peptide bond formation. These rates suggest a structural basis for the observed selectivity in both GTPase activation and accommodation steps that is supported by direct observations of “productive” near-cognate selection events (Figure 5B). Here, spurious alignment of aa-tRNA in the selection corridor is evidenced in the highly-broadened FRET state distributions observed 54. Structural defects of this nature likely effect efficiency of near-cognate aa-tRNA progression into GA- and AC-states and can help explain the observation of faster reverse rates (k−2 and k−4) for both GA′→CR and AC′→GA transitions as well as slower forward rates for chemistry steps (k3 and k5) (Supplemental Table 2).
While the rate information and probabilities (P1 and P2) obtained through simulation are only estimates of the true selection reaction scheme, they are consistent with a model where productive docking at the chemistry centers for GTP hydrolysis and peptide bond formation requires first, proper alignment of EF-Tu with respect to the GAC and subsequently the 3′-CCA end of aa-tRNA with respect to the PTC. Here, apparent rates of chemistry steps (k3 and k5) reflect the distinct probabilities, ρ, of achieving the proper orientation for chemistry multiplied by the rates of fast chemistry steps, kGTP and kpep, which are likely similar for both cognate and near-cognate systems (e.g. k3= ρGA·kGTP). Thus, differences in these rates principally reflect the distinct probabilities of cognate and near-cognate aa-tRNA alignment with respect to the chemistry centers of the ribosome. Near-cognate aa-tRNA misalignment resulting from improper, or incomplete, structural transitions of the tRNA anticodon55 or small distortions in the codon-anticodon interaction geometry in the decoding site are amplified over distance due to the physical separation from the chemistry centers (both the GAC and PTC) 28. The reversible nature of these events provides multiple opportunities for cognate aa-tRNA to achieve proper alignment prior to dissociation, while the reduced probability of productive binding events for near-cognate aa-tRNA slows chemistry, allowing time for off-rate discrimination. Both models are consistent with early studies showing that only intact aa-tRNAs can stimulate GTP hydrolysis and the proposal that tRNA acts as a molecular spring during the selection process 22. Such a model suggests that the physical dimensions of the ribosome and tRNA are critical determinants of the fidelity mechanism, where flexibility of the tRNA body contributes to aa-tRNA’s active role in the selection process 25; 56.
An integrated model of aa-tRNA selection
Figure 7 attempts to schematize these findings in the context of established features of the selection mechanism15. In the transition from state 0–1, ternary complex is escorted into the ribosome through the interaction of EF-Tu with the L12 ribosomal stalk proteins according to the bimolecular rate constant (~100–140μm−1s−1) 57. Dissociation from state 1 is estimated to occur on the order of 200–500s−1 to avoid unwanted translation inhibition by near- and non-cognate aa-tRNA binding10. This initial binding complex has a zero-FRET state and is not observed in the present experiments either due to its transient nature or due to the positioning of aa-tRNA. In the transition from state 1–2, ternary complex orients with respect to the ribosome as the aa-tRNA anticodon enters the small subunit decoding site and forms early interactions with the mRNA codon. This rapid step leads to formation of state 2, the low-FRET, CR state27 in which small subunit occupies an “open” configuration3, the L11-NTD interacts with ternary complex and residues A1492, A1493 and G530 of the decoding site begin to engage the codon-anticodon minihelix. Following codon-anticodon minihelix recognition, reversible transitions occur between state 2 and 3, resulting in aa-tRNA and EF-Tu movements towards the GA′ state and the A site. Such transitions represent transient attempts to properly position EF-Tu and the GAC with respect to the SRL for the GA state, where GTP hydrolysis can occur. Such movements may arise from conformational changes in the small subunit including “domain closure”-like events3 and/or aa-tRNA distortions, including tRNA bending, that may also be accompanied by L11 and GAC compaction 58. These initial selection events constitute a rate-limiting feature of the GTPase activation mechanism wherein the vast majority of near- and non-cognate ternary complexes dissociate, as well as a significant fraction of those that are cognate, prior to GTP hydrolysis. In the transition from state 3–4, EF-Tu productively docks with the SRL, domain closure events fully engage aa-tRNA and the GA state is successfully achieved. aa-tRNA is bound within the A site in a distorted conformation. Further conformational changes in EF-Tu and/or tRNA, perhaps accompanied by aa-tRNA excursions toward AC′-like positions, trigger conformational events enabling histidine 84 of EF-Tu to approach the GTP molecule. GTP hydrolysis completes the initial selection process. Following GTP hydrolysis, rapid, reversible transitions occur between states 4 and 5, precipitated by conformational changes in EF-Tu (GDP-Pi), aa-tRNA and the ribosome. These proofreading events, wherein aa-tRNA moves between GA-like and fully-accommodated positions, constitute a rate-limiting feature of the accommodation mechanism. Liberation of contacts between the 3′-CCA end of aa-tRNA and EF-Tu during this process enable the codon-anticodon interaction to again be tested. The vast majority of near- and non-cognate ternary complexes, as well as a small fraction of those that are cognate dissociate prior to peptide bond formation. During, or concomitant with the step 5–6 transition, inorganic phosphate is released and EF-Tu (GDP) releases from the ribosome. This step culminates with productive aa-tRNA incorporation into the large subunit PTC. Subsequent peptide bond formation completes accommodation and the process of aa-tRNA selection.
Figure 7. Kinetic model of aa-tRNA selection.
A simplified schematic model of the aa-tRNA selection process derived from the kinetic scheme of Rodnina and coworkers based on the the experimental observables obtained through smFRET.
Concluding remarks
While further experiments are required to examine the nature of conformational changes in the ribosome, aa-tRNA and EF-Tu in molecular detail, the experiments performed here provide a probabilistic and structural framework for the process that can be rigorously explored. As dynamic processes in the ribosome provide signatures of the fidelity mechanism, further explorations of this process will be greatly aided by improvements in the time scale of imaging, dye performance under intense illumination as well as the labeling strategies, and the overall experimental throughput of such investigations. Efforts to contextualize such studies with direct measurements of GTP hydrolysis and peptide bond formation must also be made to understand the precise timing of these chemical steps with respect to specific structural transitions. Ultimately, the true time scales and molecular events underpinning the fidelity mechanism must also be explored through orthogonal strategies including continued structure determination efforts and detailed molecular dynamics simulations 59. Interrogations of distinct ribosome complexes, within and across domains of life, may also shed light on conserved and divergent aspects of the selection mechanism that will doubtless enrich our understanding of how the ribosome has maintained its capacity to perform this complex process throughout evolution under varied conditions and changing demands.
MATERIALS AND METHODS
Sample Preparation
As previously described26, wild-type MRE600 ribosomes were purified from E. coli;. tRNAfMet and tRNAPhe (Sigma) were site-specifically labeled at position 8 (4-thiouridine (s4U)) and position 47 (3-amino-3-carboxypropyluridine (acp3U)), respectively. Ribosomal complexes containing deacylated P-site tRNAfMet(Cy3-s4U8) and programmed with cognate or near-cognate tRNAPhe codon in the A site were prepared as described previously26. The purification of L11 labeled ribosomes is described in Supplemental Methods. Biotinylated ribosomal particles were tethered to the surface via a biotin–streptavidin linkage. As previously described26, the ternary complex of EF-Tu-GTP·Phe-tRNAPhe (Cy5-acp3U47) was formed at a concentration of 0.95 μM and diluted into Tris-polymix buffer (pH 7.5; 50 mM Tris Acetate, pH 7.5, 100 mM KCl, 15 mM NH4OAc, 0.5 mM CaCl2, 0.1 mM EDTA, 5 mM putrescine, and 1 mM spermidine) to final concentration of ~10 nM.
Single-molecule fluorescence
smFRET data were acquired using a prism-based total internal reflection (TIR) microscope as previously described27. All experiments were performed in Tris-polymix buffer in the presence of an oxygen scavenging environment (1 unit/μl glucose oxidase, 8 units/μl catalase, 0.1% v/v glucose) containing a cocktail of triplet-state quenching compounds (1 mM Trolox, 1 mM cyclooctatetraene, 1 mM nitrobenzyl-alcohol)31. All experiments were performed in the presence of 15mM Mg(OAc)2 unless otherwise noted. Ribosome complexes (1 nM) containing either Cy3-labeled tRNAfMet in the P-site or Cy3-labled protein L11 and programmed with biotinylated mRNA were surface immobilized following brief incubation within PEG-passivated, strepatividin-coated quartz microfluidic devices. The ternary complex of EF-Tu(GTP)Phe-tRNAPhe(Cy5-acp3U47) was rapidly stop-flow delivered (mixing <50ms) while actively imaging the field. Cy3 fluorophores were excited by the evanescent wave generated by total internal reflection of a single frequency light source (Ventus 532nm, Laser Quanta). Cy3 and Cy5 fluorescence traces were collected using a 1.2 NA 60× water-immersion objective (Nikon), where optical treatments were used to spatially separate Cy3 and Cy5 frequencies onto a cooled, back-thinned CCD (Cascade 128, Photometrics). Fluorescence data were acquired using MetaMorph acquisition software (Universal Imaging Corporation) at a rate of 100 frames per second (10 ms integration).
Data Analysis
All data analysis was performed using custom software implemented in MATLAB (The MathWorks). Cy3 and Cy5 fluorescence-time traces were extracted from wide-field images by finding peaks of intensity above a defined threshold and summing the 5 most intense pixels proximal to each intensity maximum. Traces were corrected to zero background intensity and used to generate corresponding FRET trajectories according to the equation FRET=ICy5/(ICy5+ICy3). Traces were excluded from analysis if the signal-background noise ratio was <6:1 or if the lifetime of Cy3 prior to photobleaching was <20 frames (200 ms). Each trace was separated into distinct FRET events using an automated procedure, where each event starts at the observation of FRET ≥0.13 and ends with a dwell in zero-FRET states lasting >10 frames (100 ms). To minimize the contribution of specific artifacts to analysis, events were excluded from analysis if any of the following was observed: 1) reversible transitions to a dark state of Cy3; 2) large, stepwise drops in total fluorescence intensity (ICy3+ICy5) indicative of multiple fluorophore pairs summed into a single trace; or 3) a lack of anti-correlation between donor and acceptor fluorescence traces; 4) initial FRET values ≥0.13.
FRET histograms were generated by synchronizing these individual events to the first data point at which FRET ≥0.1327 and fit to a sum of Gaussian functions using Origin (OriginLab). FRET traces were idealized using an in-house implementation of the segmental k-means algorithm (SKM)60, FRET parameters derived from histogram fitting, and rates from a specific kinetic model (Supplementary Figure 3, 4). All parameters were fixed at their initial values. In events that achieved a ≥120 ms dwell-time in the high-FRET state were assigned to the accommodated state for the remainder of the trace. Transition density plots were generated by adding density to histograms according to the average FRET value in the dwell before (x-axis) and after (y-axis) each transition and normalized to the total observation time in non-zero FRET states. Dwell-time histograms were derived by summing the number of dwells in a state lasting at least as long as the time on the x-axis. The total density was then normalized to unity.
Supplementary Material
Acknowledgments
The authors are grateful to Kevin Sanbonmatsu and Paul Whitford for structural models of the E. coli ribosome shown in Figure 1 and for critical comments and discussions during the preparation of this manuscript. The authors also wish to acknowledge Jacques Ninio for helpful discussions regarding the fidelity mechanism and Suparna Sanyal (Uppsala University, Sweden) for the graciously providing the L11 knockout strain FTP 6063. This work was supported by the National Institutes of Health GM079238.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramakrishnan V. Ribosome structure and the mechanism of translation. Cell. 2002;108:557–72. doi: 10.1016/s0092-8674(02)00619-0. [DOI] [PubMed] [Google Scholar]
- 2.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JH, Noller HF. Crystal structure of the ribosome at 5.5 A resolution. Science. 2001;292:883–96. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 3.Ogle JM, Carter AP, Ramakrishnan V. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem Sci. 2003;28:259–66. doi: 10.1016/S0968-0004(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 4.Schuette JC, Murphy FVt, Kelley AC, Weir JR, Giesebrecht J, Connell SR, Loerke J, Mielke T, Zhang W, Penczek PA, Ramakrishnan V, Spahn CM. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J. 2009 doi: 10.1038/emboj.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villa E, Sengupta J, Trabuco LG, LeBarron J, Baxter WT, Shaikh TR, Grassucci RA, Nissen P, Ehrenberg M, Schulten K, Frank J. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc Natl Acad Sci U S A. 2009;106:1063–8. doi: 10.1073/pnas.0811370106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmeing TM, Voorhees RM, Kelley AC, Gao YG, Murphy FVt, Weir JR, Ramakrishnan V. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326:688–94. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodnina MV. Long-range signalling in activation of the translational GTPase EF-Tu. Embo J. 2009;28:619–20. doi: 10.1038/emboj.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev. 1989;53:273–298. doi: 10.1128/mr.53.3.273-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodnina MV, Gromadski KB, Kothe U, Wieden HJ. Recognition and selection of tRNA in translation. FEBS Lett. 2005;579:938–42. doi: 10.1016/j.febslet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 10.Johansson M, Lovmar M, Ehrenberg M. Rate and accuracy of bacterial protein synthesis revisited. Curr Opin Microbiol. 2008;11:141–7. doi: 10.1016/j.mib.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Ninio J. Multiple stages in codon-anticodon recognition:double-trigger mechanisms and geometric constraints. Biochimie. 2006;88:963–992. doi: 10.1016/j.biochi.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Hopfield JJ. Kinetic Proofreading: New Mechanism for Reducing Errors in Biosynthetic Processes Requiring High Specificity. Proceedings of the National Academy of Sciences of the United States of America. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57:587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- 14.Pape T, Wintermeyer W, Rodnina M. Induced fit in initial selection and proofreading of aminoacyl-tRNA on the ribosome. Embo Journal. 1999;18:3800–3807. doi: 10.1093/emboj/18.13.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gromadski KB, Rodnina MV. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol Cell. 2004;13:191–200. doi: 10.1016/s1097-2765(04)00005-x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RC, Stone PJ. Proofreading of the codon-anticodon interaction on ribosomes. Proc Natl Acad Sci USA. 1977;74:198–202. doi: 10.1073/pnas.74.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruusala T, Ehrenberg M, Kurland CG. Is there proofreading during polypeptide synthesis? EMBO Journal. 1982;1:741–5. doi: 10.1002/j.1460-2075.1982.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RC, Dix DB, Karim AM. The reaction of ribosomes with elongation factor Tu.GTP complexes. Aminoacyl-tRNA-independent reactions in the elongation cycle determine the accuracy of protein synthesis. J Biol Chem. 1986;261:4868–74. [PubMed] [Google Scholar]
- 19.Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature. 2009;461:1234–42. doi: 10.1038/nature08403. [DOI] [PubMed] [Google Scholar]
- 20.Marshall RA, Aitken CE, Dorywalska M, Puglisi JD. Translation at the Single-Molecule Level. Annu Rev Biochem. 2008;77:177–203. doi: 10.1146/annurev.biochem.77.070606.101431. [DOI] [PubMed] [Google Scholar]
- 21.Rodnina MV, Wintermeyer W. Fidelity of aminoacyl-tRNA selection on the ribosome: Kinetic and Structural Mechanisms. Annu Rev Biochem. 2001;70:415–435. doi: 10.1146/annurev.biochem.70.1.415. [DOI] [PubMed] [Google Scholar]
- 22.Frank J, Sengupta J, Gao H, Li W, Valle M, Zavialov A, Ehrenberg M. The role of tRNA as a molecular spring in decoding, accommodation, and peptidyl transfer. FEBS Lett. 2005;579:959–962. doi: 10.1016/j.febslet.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 23.Gromadski KB, Daviter T, Rodnina MV. A uniform response to mismatches in codon-anticodon complexes ensures ribosomal fidelity. Mol Cell. 2006;21:369–77. doi: 10.1016/j.molcel.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Daviter T, Gromadski KB, Rodnina MV. The ribosome’s response to codon-anticodon mismatches. Biochimie. 2006 doi: 10.1016/j.biochi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Cochella L, Green R. An active role for tRNA in decoding beyond codon:anticodon pairing. Science. 2005;308:1178–80. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanchard SC, Kim HD, Gonzalez RL, Jr, Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc Natl Acad Sci USA. 2004;101:12893–8. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat Struct Mol Biol. 2004;11:1008–14. doi: 10.1038/nsmb831. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, Blanchard SC, Kim HD, Puglisi JD, Chu S. The role of fluctuations in tRNA selection by the ribosome. Proceedings of the National Academy of Sciences. 2007;104:13661–13665. doi: 10.1073/pnas.0705988104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munro JB, Altman RB, O’Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25:505–17. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro JB, Altman RB, Tung CS, Cate JHD, Sanbonmatsu KY, Blanchard SC. Formation of the unlocked state of the ribosme is a multistep process. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0908597107. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dave R, Terry DS, Munro JB, Blanchard SC. Mitigating Unwanted Photophysical Processes for Improved Single-Molecule Fluorescence Imaging. Biophys J. 2009;96:2371–2381. doi: 10.1016/j.bpj.2008.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodnina MV, Fricke R, Wintermeyer W. Transient conformational states of aminoacyl-tRNA during ribosome binding catalyzed by elongation factor Tu. Biochemistry. 1994;33:12267–12275. doi: 10.1021/bi00206a033. [DOI] [PubMed] [Google Scholar]
- 33.Kim HD, Puglisi JD, Chu S. Fluctuations of Transfer RNAs between Classical and Hybrid States. Biophys J. 2007;93:3575–3582. doi: 10.1529/biophysj.107.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenkov YP, Rodnina MV, Wintermeyer W. Energetic contribution of tRNA hybrid state formation to translocation catalysis on the ribosome. Nat Struct Biol. 2000;7:1027–31. doi: 10.1038/80938. [DOI] [PubMed] [Google Scholar]
- 35.Sherlin LD, Uhlenbeck OC. Hasty decisions on the ribosome. Nat Struct Mol Biol. 2004;11:206–8. doi: 10.1038/nsmb0304-206. [DOI] [PubMed] [Google Scholar]
- 36.Mohr D, Wintermeyer W, Rodnina MV. GTPase activation of elongation factors Tu and G on the ribosome. Biochemistry. 2002;41:12520–8. doi: 10.1021/bi026301y. [DOI] [PubMed] [Google Scholar]
- 37.McKinney SA, Joo C, Ha T. Analysis of Single-Molecule FRET Trajectories Using Hidden Markov Modeling. Biophys J. 2006;91:1941–1951. doi: 10.1529/biophysj.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hopfield JJ. Kinetic proofreading:a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valle M, Zavialov A, Li W, Stagg SM, Sengupta J, Nielsen RC, Nissen P, Harvey SC, Ehrenberg M, Frank J. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nature Structural Biology. 2003;10:899–906. doi: 10.1038/nsb1003. [DOI] [PubMed] [Google Scholar]
- 40.Stark H, Rodnina MV, Rinke-Appel J, Brimacombe R, Wintermeyer W, van Heel M. Visualization of elongation factor Tu on the Escherichia coli ribosome. Nature. 1997;389:403–6. doi: 10.1038/38770. [DOI] [PubMed] [Google Scholar]
- 41.Bartley LE, Zhuang X, Das R, Chu S, Herschlag D. Exploration of the transition state for tertiary structure formation between an RNA helix and a large structured RNA. J Mol Biol. 2003;328:1011–26. doi: 10.1016/s0022-2836(03)00272-9. [DOI] [PubMed] [Google Scholar]
- 42.Nirenberg M, Leder P. Rna Codewords and Protein Synthesis. The Effect of Trinucleotides Upon the Binding of Srna to Ribosomes. Science. 1964;145:1399–407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- 43.Ogle JM, Ramakrishnan V. Structural insights into translational fidelity. Annu Rev Biochem. 2005;74:129–77. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- 44.Qin D, Abdi NM, Fredrick K. Characterization of 16S rRNA mutations that decrease the fidelity of translation initiation. Rna. 2007;13:2348–55. doi: 10.1261/rna.715307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodnina MV, Wintermeyer W. Ribosome fidelity: tRNA discrimination, proofreading and induced fit. Trends Biochem Sci. 2001;26:124–30. doi: 10.1016/s0968-0004(00)01737-0. [DOI] [PubMed] [Google Scholar]
- 46.Li PT, Vieregg J, Tinoco I., Jr How RNA unfolds and refolds. Annu Rev Biochem. 2008;77:77–100. doi: 10.1146/annurev.biochem.77.061206.174353. [DOI] [PubMed] [Google Scholar]
- 47.Lovmar M, Ehrenberg M. Rate, accuracy and cost of ribosomes in bacterial cells. Biochimie. 2006;88:951–61. doi: 10.1016/j.biochi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Ehrenberg M, Kurland CG. Costs of accuracy determined by a maximal growth rate constraint. Q Rev Biophys. 1984;17:45–82. doi: 10.1017/s0033583500005254. [DOI] [PubMed] [Google Scholar]
- 49.Weiss S. Fluorescence spectroscopy of single biomolecules. Science. 1999;283:1676–83. doi: 10.1126/science.283.5408.1676. [DOI] [PubMed] [Google Scholar]
- 50.Pape T, Wintermeyer W, Rodnina MV. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E-coli ribosome. Embo Journal. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodnina MV, Pape T, Fricke R, Kuhn L, Wintermeyer W. Initial binding of the elongation factor Tu.GTP.aminoacyl-tRNA complex preceding codon recognition on the ribosome. J Biol Chem. 1996;271:646–52. doi: 10.1074/jbc.271.2.646. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Agirrezabala X, Lei J, Bouakaz L, Brunelle JL, Ortiz-Meoz RF, Green R, Sanyal S, Ehrenberg M, Frank J. Recognition of aminoacyl-tRNA: a common molecular mechanism revealed by cryo-EM. EMBO J. 2008;27:3322–31. doi: 10.1038/emboj.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitford PC, Geggier P, Altman RB, Blanchard SC, Onuchic JN, Sanbonmatsu KY. Accommodation of aminoacyl-tRNA into the ribosome involves reversible excursions along multiple pathways. RNA. 2010 doi: 10.1261/rna.2035410. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanbonmatsu KY. Alignment/misalignment hypothesis for tRNA selection by the ribosome. Biochimie. 2006;88:1075–89. doi: 10.1016/j.biochi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Kurland CG, Rigler R, Ehrenberg M, Blomberg C. Allosteric mechanism for codon-dependent tRNA selection on ribosomes. Proc Natl Acad Sci U S A. 1975;72:4248–51. doi: 10.1073/pnas.72.11.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith D, Yarus M. Transfer RNA structure and coding specificity. I. Evidence that a D-arm mutation reduces tRNA dissociation from the ribosome. J Mol Biol. 1989;206:489–501. doi: 10.1016/0022-2836(89)90496-8. [DOI] [PubMed] [Google Scholar]
- 57.Diaconu M, Kothe U, Schlunzen F, Fischer N, Harms JM, Tonevitsky AG, Stark H, Rodnina MV, Wahl MC. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell. 2005;121:991–1004. doi: 10.1016/j.cell.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Valle M, Sengupta J, Swami NK, Grassucci RA, Burkhardt N, Nierhaus KH, Agrawal RK, Frank J. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. Embo J. 2002;21:3557–67. doi: 10.1093/emboj/cdf326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanbonmatsu KY, Joseph S, Tung CS. Simulating movement of tRNA into the ribosome during decoding. Proc Natl Acad Sci U S A. 2005;102:15854–9. doi: 10.1073/pnas.0503456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J. 2004;86:1488–501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.