Abstract
Background
Our lab has previously demonstrated losses in contrast sensitivity to low spatial frequencies under scotopic conditions with older adults. It is not clear, however, whether the temporal frequency of a stimulus alters the relation between age and the spatial contrast sensitivity function (sCSF) under scotopic conditions.
Methods
A maximum-likelihood, two-alternative, temporal forced-choice QUEST procedure was used to measure threshold to spatially and temporally modulated stimuli in both young (mean = 26 years) and old (mean = 75 years) adults.
Results
In general, the shapes of the spatial and temporal CSFs were low-pass for both young and old observers; contrast sensitivity decreased at approximately the same rate with increasing spatial frequency and temporal frequency for both age groups, although the overall sensitivity of the old group was lower than that of the young group. The high-frequency resolution limit was lower for the old group compared to the young group.
Conclusions
The differences in contrast sensitivity between the young and old groups suggest a uniform loss in sensitivity of the channels mediating spatial and temporal vision. Because of this loss, the spatial and temporal window of visibility for the older adults is compromised relative to the younger adults.
Keywords: aging, scotopic vision, spatial contrast, temporal contrast
Introduction
Spatial vision and temporal vision have traditionally been quantified by measuring thresholds for contrast detection using luminance-varying sinusoidal gratings of various spatial and temporal frequencies; the reciprocal of threshold yields a contrast sensitivity function. The envelope of the spatial contrast sensitivity function (sCSF) is presumed to represent the response of several different spatial channels with narrow bandwidths and different peak sensitivities (Blakemore and Campbell, 1969; Watson and Robson, 1981), while the temporal contrast sensitivity function (tCSF) appears to be mediated by fewer and broader channels (Smith, 1971; Watson and Robson, 1981). Under photopic conditions, the foveal sCSF displays a peak sensitivity at 2–6 cycles per degree (cpd) with low frequency attenuation and a high-frequency cut-off of approximately 50 cpd (Campbell and Robson, 1968), and the foveal tCSF shows a peak sensitivity between 10 and 20 Hz with low frequency attenuation and a high-frequency cut-off > 50 Hz (Kelly, 1961). Thus, the foveal sCSF and tCSF measured under photopic conditions are band-pass.
The shapes of the sCSF and tCSF vary, however, within the specific experimental conditions. For example, when the sCSF and tCSF are measured in the peripheral retina under photopic conditions, both show a decrease in contrast sensitivity for higher frequencies compared to the fovea (Hilz and Cavonius, 1974; Kelly, 1984). There is also an interaction between spatial frequency and temporal frequency: under photopic conditions, the foveal sCSF loses its band-pass shape as temporal frequency increases, while the foveal tCSF becomes low-pass at higher spatial frequencies (Robson, 1966; Kelly, 1979).
When measurements in the peripheral retina are made under scotopic conditions, contrast sensitivity is reduced compared to those made under photopic conditions, and there is a luminance-dependent reduction in the high-frequency cut-off for both the sCSF and tCSF (Smith, 1973; Hess and Nordby, 1986; Savage and Banks, 1992). Many studies have also shown that as luminance decreases from photopic to scotopic levels, the sCSF and tCSF are transformed from band-pass to low-pass functions (van Nes et al., 1967; Daitch and Green, 1969; Roufs, 1972; Smith, 1973; Hess and Nordby, 1986; Swanson et al., 1987; Benedek et al., 2003), although this finding is not universal (Fiorentini and Maffei, 1973; Hess et al., 1987). There is also an interaction between temporal frequency and spatial frequency under scotopic conditions. As both frequencies increase, sensitivity is reduced and contrast sensitivity functions become low-pass (van Nes et al., 1967; Benedek et al., 2003).
In addition to these spatiotemporal frequency interactions, contrast sensitivity depends on the age of the observer. Losses in contrast sensitivity to the middle and high spatial frequencies under photopic conditions appear in the 30s and progressively decline into the 80s (Owsley et al., 1983); and as luminance decreases from the photopic to the scotopic range, older observers also show a loss in contrast sensitivity to the lower spatial frequencies (Sloane et al., 1988a,b). At least one study has reported that the senescent loss under scotopic conditions is more profound for the lower spatial frequencies than for the higher spatial frequencies (Schefrin et al., 1999), opposite to that observed under photopic conditions. Age-related losses have also been reported under photopic conditions for temporal contrast sensitivity, with older individuals showing a greater loss at the higher temporal frequencies than at the lower temporal frequencies (Wright and Drasdo, 1985; Mayer et al., 1988; Sloane et al., 1988b; Kim and Mayer, 1994). When the interaction between spatial frequency and temporal frequency is investigated with older observers under photopic conditions, there is an overall sensitivity decrease to both high spatial and temporal frequencies with a greater loss as a function of age (Tulunay-Keesey et al., 1988; Nameda et al., 1989). In one study, age did not alter the interaction between spatial frequency and temporal frequency (Tulunay-Keesey et al., 1988) and in another it did (Nameda et al., 1989).
Initially, senescent miosis, ocular media density differences, intraocular scatter and unspecified neural factors were suggested as contributing to the reduced sensitivity observed in older observers at the higher spatial frequencies under photopic conditions. An interferometry study which measured sCSF in young and old observers under photopic conditions has shown that pre-neural factors account for some of the high-spatial-frequency loss in older observers (Burton et al., 1993), and other studies have ruled out changes in pupil area with age as one of these factors (Sloane et al., 1988a; Elliott et al., 1990; c.f., Wright and Drasdo, 1985). Senescent miosis, however, has been suggested as contributing to temporal sensitivity losses at higher frequencies with age (Wright and Drasdo, 1985; Kim and Mayer, 1994). Higher-order aberrations have also been shown to contribute to age-related losses in photopic spatial contrast sensitivity (Elliot et al., 2009), while a decrease in sensitivity to low spatial frequencies with age under various luminance conditions has been ascribed to neural losses (Sloane et al., 1988a; Schefrin et al., 1999; c.f., Higgins et al., 1988).
Under scotopic conditions, losses in contrast sensitivity with age have been ascribed, in part, to changes in the rod neural pathway. Studies measuring scotopic thresholds (Gunkel and Gouras, 1963; Sturr et al., 1997; Jackson et al., 1998) have shown that lens density and pupil size cannot account entirely for losses in scotopic sensitivity with age, implying a neural loss. Similarly, increases in the area of complete spatial summation (Ricco’s area) with age under scotopic conditions have also been found to have a neural origin (Schefrin et al., 1998).
Few studies have measured scotopic sCSFs as a function of age (Sloane et al., 1988b; Schefrin et al., 1999), although one study has measured scotopic sCSFs with young adults under viewing conditions which mimick conditions in the older eye (Vidinova et al., 2009). In the Schefrin et al. (1999) study, stimuli were presented within a 1 s Gaussian temporal envelope in the nasal retina at 6° eccentricity. The results revealed an age-related loss in scotopic contrast sensitivity, with the largest losses at low spatial frequencies. To our knowledge, no studies have measured and compared scotopic tCSFs in younger and older observers. While studies have investigated the relationship between age, spatial frequency and temporal frequency under photopic conditions (Tulunay-Keesey et al., 1988; Nameda et al., 1989), a comparable study has not been conducted under scotopic conditions. For this reason, we have measured scotopic CSF for older and younger observers using spatially- and temporally-varying sinusoidal stimuli.
Method
Observers
Fifteen younger (mean: 26 years, range: 19–32 years) and 15 older (mean: 75 years, range: 67–81) adults participated in this experiment. Each age group included eight males and seven females, and participants within each age group were matched in terms of their prior experience with psychophysical testing, ranging from those with no experience to those with substantial experience. All observers were color normal as assessed by the Neitz anomaloscope, the Farnsworth Panel D-15 test, and the AO-HRR pseudoisochromatic plates. An experienced clinician performed direct and indirect ophthalmoscopy on each observer. Fundus photos were evaluated to rule out abnormalities of the optic nerve head, retina, and retinal vasculature. Participants were also excluded if they exhibited abnormalities of the anterior segment. Clinical grading of the lens was based on three dimensions of change (nuclear sclerosis, posterior subcapsular cataract, cortical spoking), with each rated on a scale from ‘clear’ to ‘4+’. Participants were included if they had normal age-related lens changes. In this study, all younger subjects were graded as ‘clear’ and all older subjects were graded as +1 nuclear sclerosis or better. Trial lenses were utilized to optimize each observer’s best-corrected distance acuity. Visual acuity in each corrected eye was determined with the Bailey-Lovie chart (Bailey and Lovie, 1976). Because the stimuli were viewed monocularly, the eye with the best-corrected acuity was used. All younger observers had a best-corrected acuity of 20/20 or better and all the older observers were 20/25 or better.
Prior to any data collection, participants provided written informed consent in accordance with the Declaration of Helsinki. Experimental procedures were reviewed and approved by the Institutional Review Board at the University of California, Davis.
Apparatus
A two-channel Maxwellian-view system combined, via a beamsplitter, the stimulus generated on a high-resolution CRT (CPD G200 17 inch; Sony Corporation, Tokyo, Japan) with a red LED fixation point. Stimuli from the CRT were viewed at optical infinity through a 7× astronomical telescope. A 4.31-mm field stop placed in front of the CRT, but before the telescope and beamsplitter, created an exit pupil of 1.9 mm, which is smaller than the smallest pupil size for participants in this age range under our experimental conditions (Birren et al., 1950). This pupil size has been shown to be optimal for visual acuity (Atchison et al., 1979) and is not affected by changes in spatial frequency and contrast under scotopic conditions (Young et al., 1995). Thus, the effective pupil size was the same for both the young and old observers. Neutral density filters placed after the field stop but before the beamsplitter and telescope maintained a mean retinal illuminance of −1.0 log scot td. Previous studies with sinusoidal gratings (e.g. Daitch and Green, 1969; D’Zmura and Lennie, 1986; Savage and Banks, 1992; Lennie and Fairchild, 1994) and absolute thresholds for uniform fields (Daitch and Green, 1969; Stabell and Stabell, 1976) have demonstrated that rods, and not cones, mediate detection at this retinal illuminance.
The CRT was controlled by a Macintosh G4 computer (Apple Inc, Cupertino, CA, USA) with an ATI Radeon 7500 video card (AMD, Sunnyvale, CA, USA) providing 10-bits of luminance resolution. This signal drove the green phosphor of the monitor (λd = 548 nm; 80 nm bandwidth at half power) and produced a maximum Michelson contrast of 86%. The frame rate was 85 Hz, and the voltage-luminance relationship was linearized. Radiometric output of the CRT was measured with a spectroradiometer/photometer (Model PR703-A; PhotoResearch Inc, Chatsworth, CA, USA) in 2 nm steps. The presentation software was developed in MATLAB 5.2.1 (Mathworks Inc, Natick, MA, USA) using Psychtoolbox extensions (Brainard, 1997; Pelli, 1997).
A pupil viewer was used to align participants to the optical system. A dental-impression bite-bar assembly permitted movement in three orthogonal dimensions.
Stimuli
The stimuli were vertically oriented Gabor patches presented at seven different spatial frequencies (0.25, 0.4, 0.8, 1.2, 1.8, 2.4 and 3.0 cpd) and sinusoidally modulated at six temporal frequencies (0.5, 1.0, 2.0, 4.0, 8.0, 16.0 Hz). Five cycles were shown for each spatial frequency due to the influence of the number of cycles on spatial contrast sensitivity (Howell and Hess, 1978; Savage and Banks, 1992). Gabor stimuli were presented with constant spatial bandwidth, with the sigma of the Gaussian envelope equal to four times the spatial period. To avoid spatial-adaptation effects, the phase of the sinusoidal grating was randomly varied on each trial. Temporal modulation was initiated in sine phase, and the temporal envelope was 1 s in duration. Stimulus presentations were synchronized with the frame rate, thereby minimizing harmonic artifacts. The fixation point was positioned so that the stimuli were imaged at 10° along the superior meridian, where rod density is maximal (Curcio et al., 1990).
Procedure
Observers adapted to the dark for 30 min followed by 1 min of adaptation to a blank screen with the same space-average luminance (−1.0 log scot td) as the test grating. Contrast sensitivity was then measured to the various combinations of temporal and spatial frequencies using a maximum-likelihood, two-alternative, temporal forced choice QUEST procedure (Watson and Pelli, 1983; Harvey, 1986). There were two interleaved staircases with each staircase consisting of a minimum of 45 trials and a maximum of 100 trials. Staircases were terminated when the standard deviation of the estimate fell below 0.05 log units. Threshold contrast corresponded to a detection probability of 82%. The various spatiotemporal frequency combinations were presented in a random order. Two thresholds were obtained for each spatiotemporal pairing that the participant could detect. Between 8 and 16 experimental sessions were required for each observer, with some older observers [mean: 10 sessions, range: 8–16 sessions] requiring more sessions than the younger participants [mean: 10 sessions, range: 8–12 sessions]. Each session lasted 60–90 min. Before the 30-min dark adaptation commenced, all observers engaged in practice trials under photopic conditions using a 0.4 cpd stimulus presented at 2 Hz to familiarize them with the experimental procedures.
Results
For each observer, mean log contrast threshold was computed for each spatiotemporal combination from the two staircases. At some spatiotemporal combinations, near the detection limit, only one threshold measurement was obtained from an observer. Mean contrast sensitivities for each spatiotemporal pairing were computed for each age group with the constraint that two or more observers in the age group were able to set a threshold for that pairing. (Table 1 notes the number of observers in each age group for each spatiotemporal combination.) Mean log contrast sensitivity was plotted as a function of spatial or temporal frequency in linear-log coordinates (spatial or temporal frequency, log contrast sensitivity) for each age group and fitted with a least-squares linear regression in Kaleidagraph 4.0 (Synergy Software, Reading, PA, USA).
Table 1.
Number of young [old] observers able to set thresholds for each spatial and temporal frequency combination
| Spatial Frequency (cpd) |
Temporal Frequency (Hz) |
|||||
|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 | |
| 0.25 | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 15 [15] |
| 0.40 | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 10 [1] |
| 0.80 | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 2 [0] |
| 1.20 | 15 [15] | 15 [15] | 15 [15] | 15 [15] | 15 [6] | 0 [0] |
| 1.80 | 15 [15] | 15 [14] | 15 [13] | 15 [12] | 5 [0] | 0 [0] |
| 2.40 | 14 [10] | 15 [10] | 13 [9] | 9 [4] | 0 [0] | 0 [0] |
| 3.00 | 12 [2] | 11 [1] | 8 [2] | 0 [0] | 0 [0] | 0 [0] |
Scotopic spatial contrast sensitivity
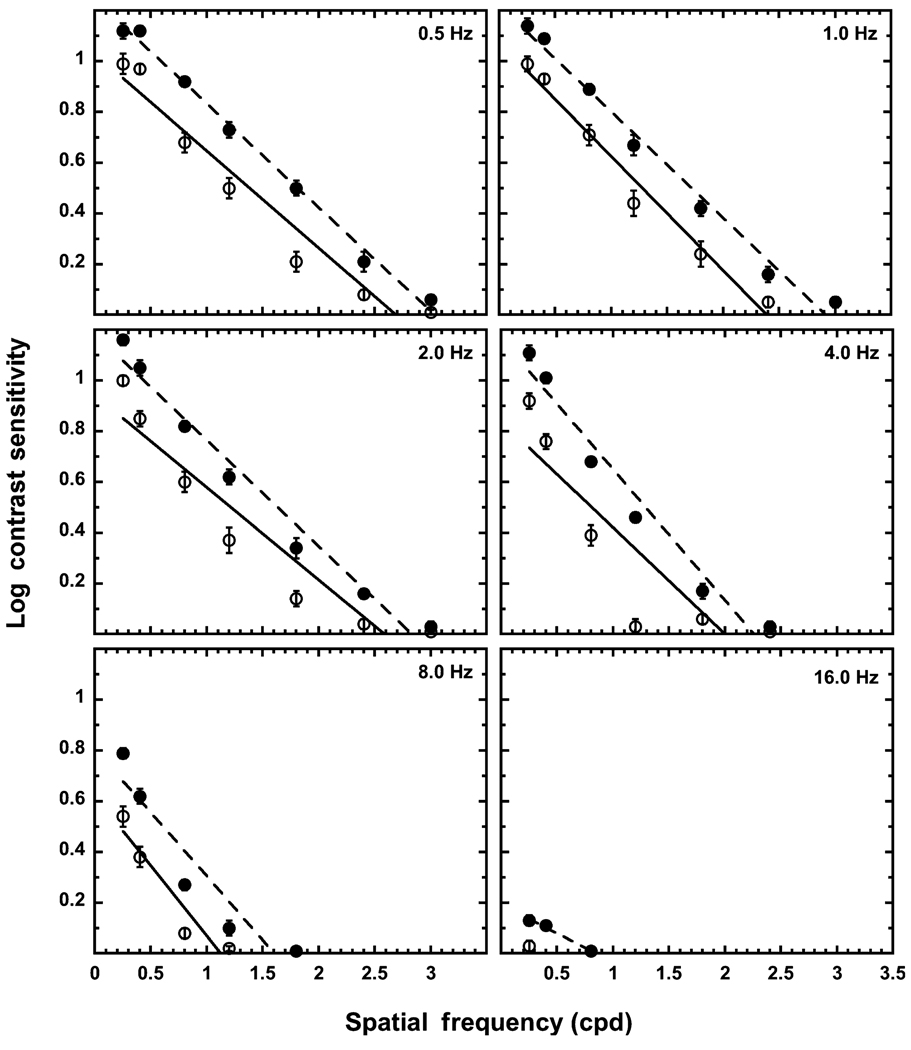
Figure 1 presents mean log contrast sensitivity plotted as a function of spatial frequency (cpd) for the young and old observers. Each panel denotes a different temporal frequency. Table 2 presents the slope, intercept and R values for the linear curve fits. The linear fits account for 90% or more of the variance in all but two experimental conditions (exception: 8 Hz-young and 4 Hz-old). The difference in slopes between the two age groups across temporal frequency is not statistically significant, while the difference in y-intercept values between the groups across temporal frequency is significant, t(4) = 7.17, p < 0.005.
Figure 1.
Spatial contrast sensitivity functions of the young (solid circles) and the old (open circles) observers. Each panel denotes a different temporal frequency. The error bars represent ± 1 standard error of the mean (S.E.M.). The dashed line is the best-fitting linear function at that temporal frequency for the young group, and the solid line is the best-fitting linear function for the old group.
Table 2.
Mean slopes and intercepts for sCSF
| Slope |
Intercept |
R |
||||
|---|---|---|---|---|---|---|
| Hz | Young | Old | Young | Old | Young | Old |
| 0.5 | −0.41 | −0.38 | 1.24 | 1.03 | 1.0 | 0.97 |
| 1.0 | −0.42 | −0.45 | 1.22 | 1.07 | 0.99 | 0.99 |
| 2.0 | −0.42 | −0.36 | 1.19 | 0.94 | 0.99 | 0.95 |
| 4.0 | −0.52 | −0.42 | 1.17 | 0.84 | 0.98 | 0.88 |
| 8.0 | −0.50 | −0.55 | 0.08 | 0.62 | 0.94 | 0.95 |
| 16.0 | −0.22 | – | 0.19 | – | 0.99 | |
At all temporal frequencies, the older adults are less sensitive to all spatial frequencies than the younger adults, and this is further verified by y-intercept values, which are lower for the old group than the young group. The overall decline in sCSF with increasing temporal frequency is similar for both age groups as indicated by the difference in y-intercept values from 0.5 Hz to 8.0 Hz (young: 0.44, old: 0.41), while the rate of decrease in contrast sensitivity from the lower to higher spatial frequencies is approximately the same for the two age groups as indicated by the similar slope values. Both age groups show an interaction between spatial frequency and temporal frequency. In particular, the slope at 16 Hz is shallower than at the other temporal frequencies for the young group, and the old group was only able to detect the lowest spatial frequency at 16 Hz. Also, both groups show a steeper decline in spatial contrast sensitivity for the 8.0-Hz stimulus relative to the other temporal frequencies. Within each age group, there is little difference in the sCSFs at the two lowest temporal frequencies (0.5 and 1.0 Hz), i.e. the slope and y-intercept values are comparable for these two temporal frequencies. In general, for both age groups, the inability to detect higher spatial frequencies at higher temporal frequencies occurs at 4 Hz. At 4 Hz, neither age group can detect the 3 cpd stimulus. As temporal frequency increases, this loss in sensitivity to higher spatial frequencies increases, with the older group unable to detect the 1.8–3.0 cpd stimuli at 8 Hz and the 0.4–3.0 cpd stimuli at 16 Hz. The young group also shows a loss in sensitivity at the higher spatial frequencies, but this loss does not extend as far as that of the old group. For example, at 16 Hz, the young group is still able to detect the three lowest spatial frequencies. All sCSFs are low-pass across temporal frequencies for both age groups.
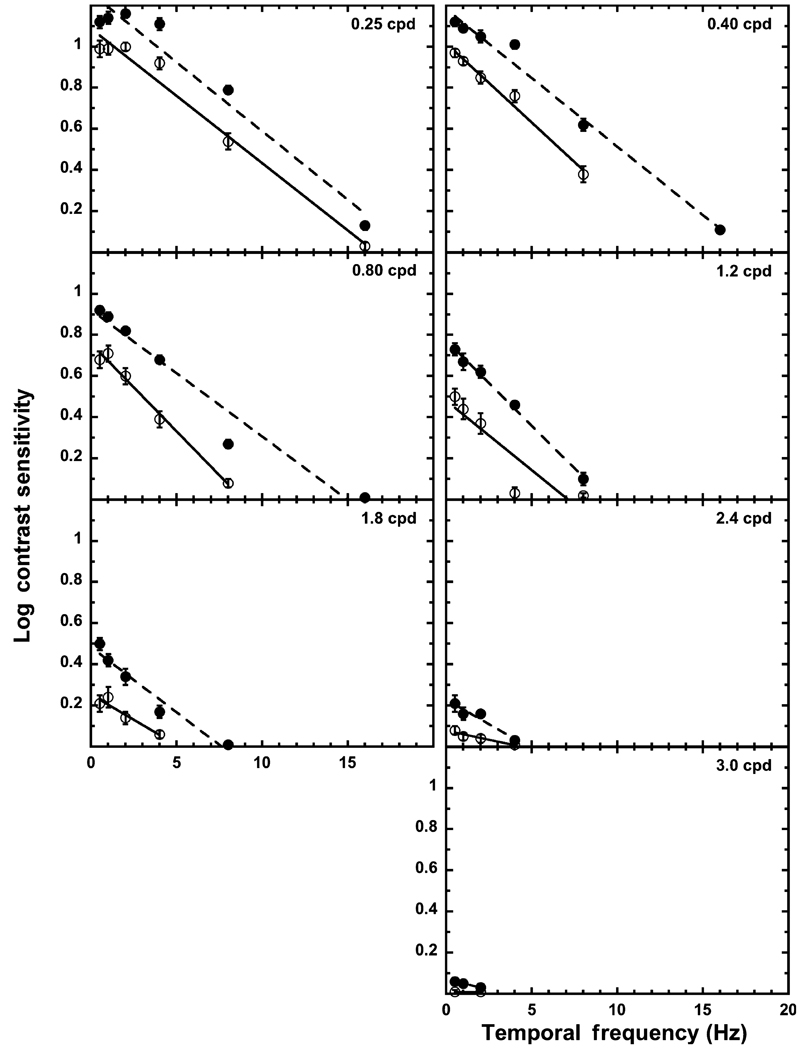
Scotopic temporal contrast sensitivity
Figure 2 displays tCSFs for the young and the old groups, with different panels representing the different spatial frequencies (cpd). Table 3 presents the slopes, y-intercepts, and R values for the linear fits to the data. (Note: values are only included when there are more than three data points at a particular spatial frequency.) The linear fits account for 90% or more of the variance in all but one experimental condition (1.20 cpd-old). The difference in slopes across spatial frequency for the two groups is not statistically significant, but the difference in y-intercepts across spatial frequency is significant, t(5) = 8.55, p < 0.001. The older adults have a lower y-intercept value than the younger adults.
Figure 2.
Temporal contrast sensitivity functions of the young (solid circles) and the old (open circles) observers. Each panel denotes a different spatial frequency. Error bars represent ± 1 S.E.M. The dashed line is the best-fitting linear function at that spatial frequency for the young group, and the solid line is the best-fitting linear function for the old group.
Table 3.
Mean slopes and intercepts for tCSF
| Slope |
Intercept |
R |
||||
|---|---|---|---|---|---|---|
| cpd | Young | Old | Young | Old | Young | Old |
| 0.25 | −0.07 | −0.07 | 1.26 | 1.09 | 0.98 | 0.99 |
| 0.40 | −0.07 | −0.08 | 1.18 | 1.02 | 0.99 | 0.99 |
| 0.80 | −0.06 | −0.09 | 0.92 | 0.76 | 0.98 | 0.99 |
| 1.20 | −0.08 | −0.07 | 0.77 | 0.48 | 1.0 | 0.89 |
| 1.80 | −0.06 | −0.05 | 0.48 | 0.25 | 0.98 | 0.95 |
| 2.40 | −0.05 | −0.02 | 0.23 | 0.08 | 0.96 | 0.95 |
| 3.00 | −0.03 | – | 0.075 | – | 1.0 | – |
As the tCSFs and y-intercepts illustrate, the old group is less sensitive than the young group at all temporal frequencies at each spatial frequency, but the overall decline in temporal contrast sensitivity from 0.25 cpd to 2.4 cpd is comparable for the two age groups (y-intercept difference for the young is 1.03 and for the old 1.01), with both groups showing a shallower slope at the higher spatial frequencies. As the slopes indicate, the decrease in sensitivity from the low to the high temporal frequencies is similar for the two age groups. Commencing at 0.40 cpd, the old group is unable to detect the 16-Hz stimulus while the young group is able to detect all temporal frequencies at the three lowest spatial frequencies. This inability to detect the higher temporal frequencies continues with the higher spatial frequencies until 3.0 cpd where the old group only detected the 0.5 Hz and 2.0 Hz frequencies and the young group only the 0.5 Hz, 1.0 Hz and 2.0 Hz stimuli. At the lowest spatial frequency (0.25 cpd), the tCSF for both groups appears to be band-pass with a decrease in sensitivity at the lower temporal frequencies, even though the linear fit is quite good for both age groups (see Table 3). For the spatial frequencies > 0.25 cpd, the tCSFs are low-pass.
Sensitivity differences
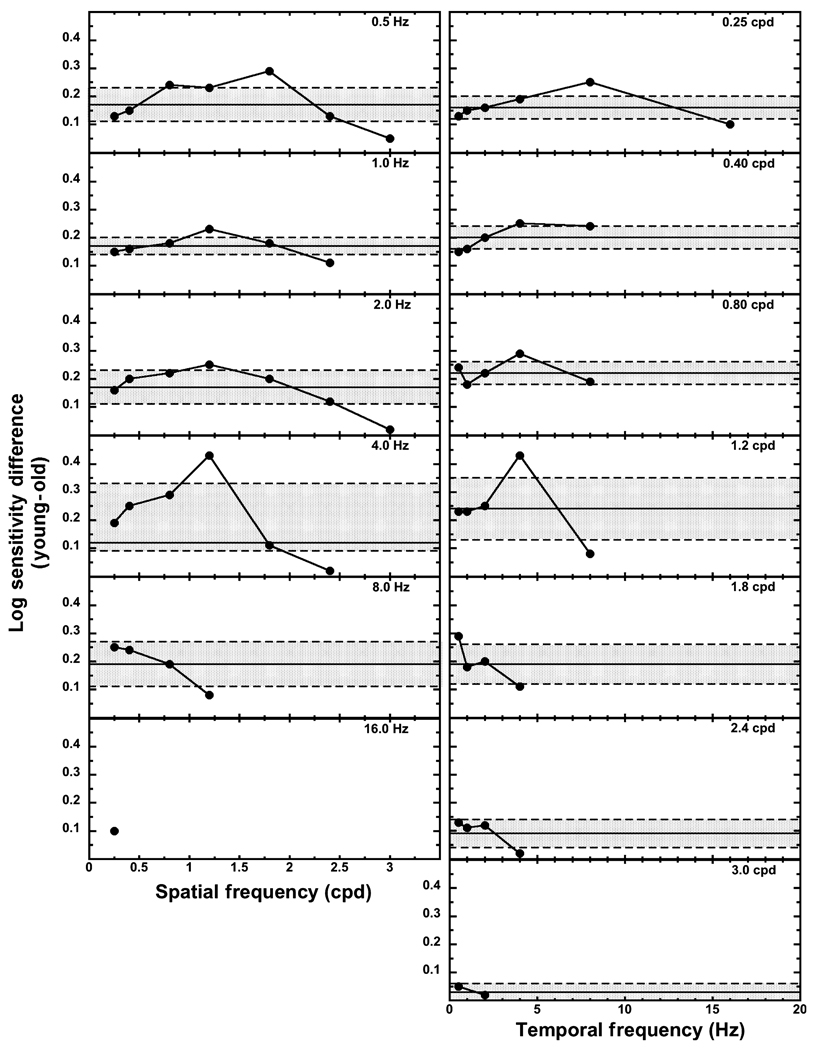
While the difference in slopes for the best-fitting curves in Figure 1 were not statistically significant between the two age groups, the slopes fitted to the spatial contrast data of the older group were shallower than those fitted to the data of the younger group at 0.5, 2.0, and 4.0 Hz. Similarly, the temporal contrast slopes were shallower for the old group than the young group at 1.20, 1.80 and 2.40 cpd. Such a difference in slopes would indicate that the losses in sensitivity would be larger for the old group at the lower frequencies than the higher frequencies. Log sensitivity differences between the young and old groups for spatial and temporal frequencies were determined to verify whether the data followed the predicted pattern from the curve fits.
Figure 3 presents the age sensitivity differences for spatial contrast in the left column of panels and the age sensitivity differences for temporal contrast in the right column of panels. The horizontal solid line represents the mean age sensitivity difference between the young and old groups in that panel, and the shaded region surrounded by the dashed lines is the 95% confidence interval about the mean. Any differences represented by the data points falling in the shaded region do not differ from the mean sensitivity difference under that particular temporal or spatial condition. Data points falling above the shaded region indicate differences greater than the mean difference and those falling below represent differences less than the mean difference. Within the top five panels for spatial contrast sensitivity (left column), the sensitivity difference at the highest spatial frequency is less than the mean sensitivity difference. There is at least one spatial sensitivity difference greater than the mean difference within the top four panels. This difference is at 1.8 cpd for the 0.5 Hz condition and 1.2 cpd for the 1.0, 2.0, and 4.0 Hz conditions. Overall, most of the data points fall within the shaded region of the figure indicating a similar difference in sensitivity between the young and old groups, regardless of spatial frequency.
Figure 3.
Log sensitivity differences between the young and the old groups plotted as a function of spatial frequency (left column) and temporal frequency (right column). The solid horizontal line denotes the mean difference for that particular experimental condition. The dashed lines and shaded region represent the 95% confidence interval around the mean difference.
For temporal contrast sensitivity differences at 0.25, 1.2, 1.8 and 2.4 cpd, the sensitivity difference at the highest temporal frequency is less than the mean sensitivity difference. In four panels, there is one temporal sensitivity difference greater than the mean difference. This occurs at 8 Hz with the 0.25 cpd stimulus, at 4 Hz with the 0.80 and 1.2 cpd stimuli, and at 0.5 Hz with the 1.8 cpd stimuli. Similar to spatial frequency, the temporal frequency sensitivity differences between the young and old groups show that most of the differences fall within the 95% confidence interval, indicating a similar difference in sensitivity between the young and old groups regardless of temporal frequency.
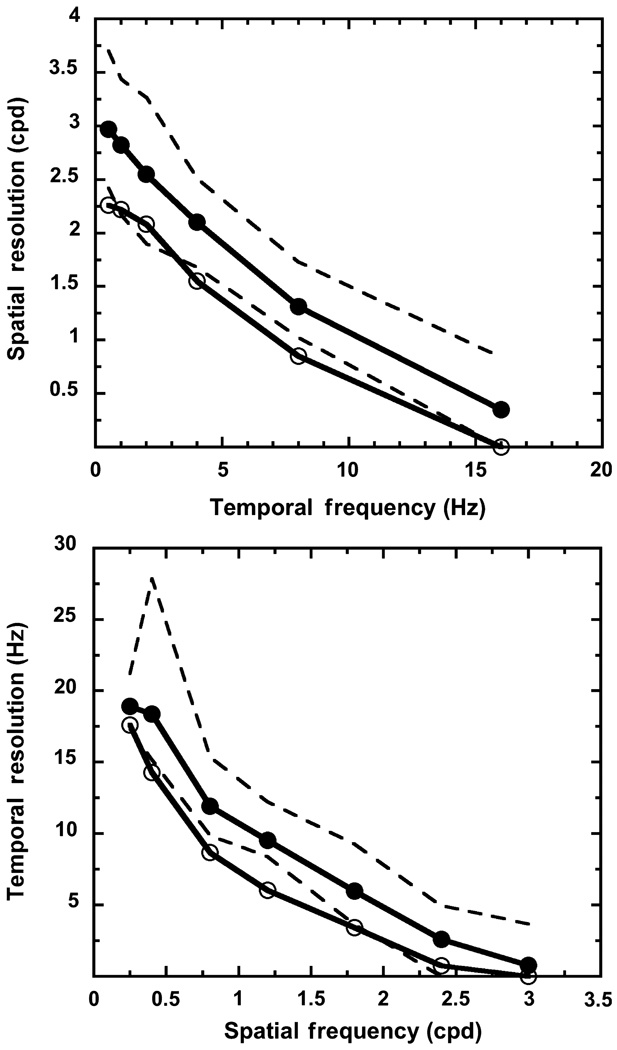
Spatial and temporal resolution
Spatial and temporal resolutions were derived for each individual by fitting a linear function to log contrast sensitivity plotted as a function of spatial or temporal frequency (Figure 4); the same procedure as was used to fit linear functions to the mean data (see above). For some of the higher frequencies, not all individuals were able to detect the stimulus; so that individual’s temporal or spatial resolution was set to 0. Figure 4 presents the results of this analysis. The upper and lower range of values for the young group are shown, and this range provides a means by which to judge if the resolution limits of the old group fall within the range of resolution limits for the young group. Figure 4 shows that spatial resolution under scotopic conditions decreases with temporal frequency, and temporal resolution under scotopic conditions decreases with spatial frequency for both age groups. The rate of loss appears to be similar for both age groups across spatial and temporal frequency. A linear fit to the resolution data points for each group (not shown in the figure) confirmed the rate of loss to be similar [slopes for spatial resolution: −0.17 (young observers) and −0.15 (old observers); slopes for temporal resolution: −6.75 (young observers) and −6.08 (old observers)]. The resolution limits are lower for the old group than the young group, and the mean spatial and temporal resolution values for the old group are similar to the lower range of resolution values for the young group. Thus, some individuals in the old group have spatial and temporal resolution limits similar to those of the young group.
Figure 4.
Mean spatial (upper panel) and temporal (lower panel) resolution. Solid and open circles denote the young and the old groups, respectively. The dashed lines represent the lower and upper range of resolution values for the young group.
Discussion
Results from this study demonstrated that sensitivity decreased with increasing spatial and temporal frequencies for both age groups, but the old group was less sensitive than the young group to all spatiotemporal frequencies. The old group was unable to detect more of the high spatial and temporal frequencies than the young group. There does not appear to be a three-way interaction among age, spatial frequency, and temporal frequency, i.e. the rate of contrast sensitivity loss with frequency is influenced by the interaction between spatial frequency and temporal frequency, while age generates an overall decline in sensitivity.
Comparison to previous studies
Previous studies often use younger adults to obtain sCSFs and tCSFs under scotopic conditions. When comparing studies with comparable mean retinal illuminances (approximately −1.0 log scot td) as used in our study, the sCSFs and tCSFs are low-pass (e.g. Savage and Banks, 1992) or show a transition from band-pass to low-pass with a retinal illuminance slightly greater than that used in this study to one slightly lower (e.g. Kelly, 1961; Daitch and Green, 1969; Smith, 1973; Hess and Nordby, 1986). Fiorentini and Maffei (1973), however, reported that both the sCSF and tCSF were band-pass at mean retinal luminances much lower than those used in the present study, and Nygaard and Frumkes (1985) measured band-pass tCSFs at retinal illuminances comparable to those in our study. It is not clear what factors among these studies produce the discrepant results, but it is surprising that sCSFs measured under scotopic conditions are ever band-pass because receptive fields appear to lose their inhibitory surround at low light levels (Barlow et al., 1957; Wiesel and Hubel, 1966).
The comparison of our high-frequency cut-offs to previous studies is a bit more challenging because retinal location, retinal luminance, and stimulus parameters affect the cut-off frequency. Using a 4-Hz stimulus with a mean retinal illuminance of −0.44 log scot td, Lennie and Fairchild (1994) reported a spatial high frequency cut-off of approximately 5 cpd at 5° and 10° in the temporal retina. The high-frequency cut-off decreased to 3 cpd at 30° retinal eccentricity. Savage and Banks (1992), using a stimulus with a mean retinal illuminance of −0.21 log scot td presented for 100 ms, showed a high frequency cut-off between 1 and 2 cpd in the nasal retinal at 20° temporal eccentricity. In our study, measurements were obtained at 10° in the superior retina at a mean retinal illuminance of −1.0 log scot td, and the stimulus was presented between 0.5 Hz and 16 Hz. The spatial high-frequency cut-offs for the young adults were contingent on temporal frequency, and the mean cut-off ranged from 2.97 cpd at 0.5 Hz to 0.35 cpd at 16 Hz. Part of these differences among studies may not only be attributed to experimental conditions, but to variations in rod (Curcio et al., 1990) and ganglion cell (Curcio and Allen, 1990) densities with retinal eccentricity and location. These differences determine the neural convergence of rod signals and limit spatial sampling.
Smith (1973) showed a temporal high-frequency cut-off of approximately 10 Hz for a 7°, 0.3 cpd stimulus presented at a mean retinal illuminance of −0.30 log scot td in the superior retina at 7° retinal eccentricity and between 6 and 10 Hz at a mean retinal illuminance of −1.30 log scot td. Nygaard and Frumkes (1985) also showed similar cut-off values to Smith (1973) for stimuli with mean retinal illuminances between −0.7 and −1.3 log scot td. The stimuli in the Nygaard and Frumkes study were 2° uniform fields and presented at 7° in the temporal retina. The mean temporal high-frequency cut-off values from our young adults ranged from 19 Hz at 0.25 cpd to 0.76 Hz at 3.0 cpd and were higher than those in other studies at the lower spatial frequencies. This may be attributed to the fact that our study presented five cycles of each spatial frequency; thus, our temporal stimulus at 0.25 cpd subtended 20° of visual angle and was much larger than those in the other two studies.
There have been few studies investigating age-related changes in sCSF and tCSF at low light levels. Sloane et al. (1988a,b) reported a loss of spatial contrast sensitivity for the older adults at mesopic and scotopic luminance levels. The older individuals in the Sloane et al. (1988a) study were unable to detect the 4, 8 and 11 cpd stimuli presented at 0.5 Hz with a mean luminance of 0.107 cd.m−2, but the younger adults could. The highest spatial frequency used in our study was 3 cpd, and two older individuals were able to detect this stimulus at 0.5 Hz. As Figure 1 illustrates, it is unlikely that any of the higher spatial frequencies used in the Sloane et al. (1988a) study would have been detected by the older group in this study. In the other study conducted by Sloane et al. (1988b), stimuli were presented at both 0.5 and 7.5 Hz. Similar to our study, the ability of the old group to detect higher spatial frequencies at the higher temporal frequency at mean luminances of 0.034 and 0.107 cd.m−2 was compromised relative to the young group. The sensitivity loss with age was relatively uniform across spatial frequency (Sloane et al., 1988a). Schefrin et al. (1999), however, reported the largest age-related losses in contrast sensitivity at the lower spatial frequencies rather than the higher spatial frequencies. As Figure 3 highlights, the difference in sensitivity between the younger and older groups in this study was smaller for the highest spatial frequency compared to the lower spatial frequencies measured at the various temporal frequencies (see below).
To our knowledge, no studies have measured tCSFs under scotopic conditions with an aging population, but Mayer et al. (1988) and Kim and Mayer (1994) have measured tCSFs under photopic conditions with various age groups. They reported a loss in foveal temporal sensitivity commencing at 45 years, with the loss being greater at the higher temporal frequencies than the lower temporal frequencies. In contrast, our study did not show a greater loss in sensitivity between the two age groups for higher temporal frequencies than lower temporal frequencies under scotopic conditions. The tCSFs measured under photopic conditions were also band-pass (Mayer et al., 1988; Kim and Mayer, 1994), whereas the tCSFs in our study under scotopic conditions were low-pass (except with the 0.25 cpd stimulus).
Our study systematically investigated the relationship among aging, spatial frequency and temporal frequency under scotopic conditions. Other studies (Tulunay-Keesey et al., 1988; Nameda et al., 1989) have investigated this dynamic under photopic conditions. They report a greater loss in both temporal and spatial contrast sensitivities for older adults than younger adults; and that the degree of loss with age is contingent on the spatial and temporal frequencies of the stimuli. For example, Tulunay-Keesey et al. (1988) noted that at the lower temporal frequencies the sensitivity to the low and medium spatial frequencies are not affected by age until approximately 45 years of age, whereas the spatiotemporal sensitivity to higher frequencies begins to show an age-related decline at approximately 30 years of age. These two studies (Tulunay-Keesey et al., 1988; Nameda et al., 1989) also showed the sCSF as band-pass at lower temporal frequencies and low-pass at higher temporal frequencies. In the Nameda et al. (1989) study, this transition from band-pass to low-pass in sCSFs occurred at lower temporal frequencies for the old group compared to the young group. Such a transition difference between age groups from band-pass to low-pass in the shape of the sCSF was not found in our study under scotopic conditions; all sCSFs were low-pass.
Losses in spatial and temporal processing have also been investigated in older non-human primates. While an electrophysiological study investigating both the parvocellular (P) and magnocellular (M) pathways in the LGN did not find any statistical differences between young and old rhesus monkeys, the optimal spatial and temporal frequency responses as well as the spatial and temporal high frequency cut-offs were lower in the M cells of the old monkeys compared to the young monkeys (Spear et al., 1994). Similarly, recordings from VI in young and old rhesus monkeys showed that cells in the old monkeys also responded optimally to lower spatial and temporal frequencies than cells in the young monkeys; and cells in the old monkeys also showed lower spatial and temporal acuities than cells in the young monkeys (Zhang et al., 2008). This loss in contrast sensitivity to higher frequencies was even more pronounced in recordings from MT cells (Yang et al., 2008).
Optical and neural mechanisms
At a retinal illuminance comparable to that used in this study, Schefrin et al. (1999) estimated that the ocular media differences between younger and older observers would be approximately 0.1. This difference in ocular media density may account for sensitivity differences observed between the young and old adults in our study at some of the high spatial and temporal frequencies (see Figure 3). At least one other factor, a floor effect, needs to be considered. The overall lower sensitivity associated with age guarantees that there will be fewer older adults able to detect the highest spatial and temporal frequencies. The number of young and old adults contributing to the means at these spatiotemporal frequencies in our study is < 15 (see Table 1): for some of the data points, there are only two older adults determining the mean. At the lower frequencies, differences in ocular media density between the two age groups can not completely explain the sensitivity differences, which are in general greater than 0.1 log scot td between the two age groups. This implies neural changes with age at either the receptoral or post-receptoral level.
Age-related losses have been reported in rod photoreceptor density (Curcio et al., 1993; Panda-Jonas et al., 1995) and ganglion cell density (Curcio and Drucker, 1993). Yet, due to the neural convergence of rod signals in the peripheral retina, it is the ganglion cell density, and not the photoreceptor density, that sets the upper limit on spatial contrast and resolution under scotopic conditions. Of course, further processing along the visual pathway in the lateral geniculate nucleus (LGN) and cortex can modify the limits set by ganglion cell sampling.
Because ganglion cell density sets the initial upper boundaries for spatial contrast sensitivity and resolution, the natural question arises as to which type of ganglion cell is receiving input from the rod system and limiting spatial perception. Previous anatomical (Grünert, 1997), electrophysiological (Purpura et al., 1988; Lee et al., 1997) and psychophysical (D’Zmura and Lennie, 1986; Lennie and Fairchild, 1994; Sun et al., 2001; Cao et al., 2008) studies have demonstrated that both the parvocellular (P) and magnocellular (M) pathways receive rod input; however, Purpura et al. (1988) determined that the contrast gain of M cells in the LGN could be measured at lower retinal illuminances than the P cells, approximately 0 log scot td for the M cells compared to 0.6 log scot td for the P cells. These results suggest that the M cells most likely mediate the detection of the stimuli used in our study. Moreover, the Nyquist limit computed for a 33.7 year old adult and a 70.5 year adult from the ganglion cell mosaic of M cells is consistent with the spatial high-frequency cut-off obtained psychophysically under scotopic conditions (Schefrin et al., 1999).
References
- Atchison DA, Smith G, Efron N. The effect of pupil size on visual acuity in uncorrected and corrected myopia. Am. J. Optom. Physiol. Opt. 1979;56:315–323. doi: 10.1097/00006324-197905000-00006. [DOI] [PubMed] [Google Scholar]
- Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am. J. Optom. Physiol. Opt. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J. Physiol. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek G, Benedek K, Keri S, Letoha L, Janáky M. Human scotopic spatiotemporal sensitivity: a comparison of psychophysical and electrophysiological data. Doc. Ophthalmol. 2003;106:201–207. doi: 10.1023/a:1022548013313. [DOI] [PubMed] [Google Scholar]
- Birren JE, Casperson RC, Botsinick J. Age changes in pupil size. J. Gerontol. 1950;5:216–221. doi: 10.1093/geronj/5.3.216. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Campbell FW. On the existence of neurons in the human visual system selectively sensitive to the orientation and size of retinal images. J. Physiol. 1969;203:237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat. Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Burton KB, Owsley C, Sloane ME. Aging and neural spatial contrast sensitivity: photopic vision. Vision Res. 1993;33:939–946. doi: 10.1016/0042-6989(93)90077-a. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J. Physiol. 1968;197:551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Pokorny J, Smith VC, Zele AJ. Rod contributions to color perception: linear with rod contrast. Vision Res. 2008;48:2856–2592. doi: 10.1016/j.visres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer’s disease and aging. Ann. Neurol. 1993;33:248–257. doi: 10.1002/ana.410330305. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest. Ophthalmol. Vis. Sci. 1993;34:3278–3296. [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J. Comp. Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Daitch JM, Green DG. Contrast sensitivity of the human peripheral retina. Vision Res. 1969;9:947–952. doi: 10.1016/0042-6989(69)90100-x. [DOI] [PubMed] [Google Scholar]
- D’Zmura M, Lennie P. Shared pathways for rod and cone vision. Vision Res. 1986;26:1273–1280. doi: 10.1016/0042-6989(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Elliott D, Whitaker D, MacVeigh D. Neural contribution to spatiotemporal contrast sensitivity decline in healthy ageing eyes. Vision Res. 1990;30:541–549. doi: 10.1016/0042-6989(90)90066-t. [DOI] [PubMed] [Google Scholar]
- Elliot SL, Choi SS, Doble N, Hardy JL, Evans JW, Werner JS. Role of high-order aberrations in senescent changes in spatial vision. J. Vis. 2009;9:1–16. doi: 10.1167/9.2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A, Maffei L. Contrast in night vision. Vision Res. 1973;13:73–80. doi: 10.1016/0042-6989(73)90165-x. [DOI] [PubMed] [Google Scholar]
- Grünert U. Anatomical evidence for rod input to the parvocellular pathway in the visual system of the primate retina. Eur. J. Neurosci. 1997;9:617–621. doi: 10.1111/j.1460-9568.1997.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Gunkel RD, Gouras P. Changes in scotopic visibility thresholds with age. Arch. Ophthalmol. 1963;69:4–9. doi: 10.1001/archopht.1963.00960040010003. [DOI] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav. Res. Methods Instrum. Comput. 1986;18:623–632. [Google Scholar]
- Hess RF, Nordby K. Spatial and temporal limits of vision in the achromat. J. Physiol. 1986;371:365–385. doi: 10.1113/jphysiol.1986.sp015981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Nordby K, Pointer JS. Regional variation of contrast sensitivity across the retina of the achromat: sensitivity of human rod vision. J. Physiol. 1987;388:101–119. doi: 10.1113/jphysiol.1987.sp016604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KE, Jaffe MJ, Caruso RC, de Monasterio FM. Spatial contrast sensitivity: effects of age, test-retest, and psychophysical method. J. Opt. Soc. Am. A. 1988;5:2173–2180. doi: 10.1364/josaa.5.002173. [DOI] [PubMed] [Google Scholar]
- Hilz R, Cavonius CR. Functional organization of the peripheral retina: sensitivity to periodic stimuli. Vision Res. 1974;14:1333–1337. doi: 10.1016/0042-6989(74)90006-6. [DOI] [PubMed] [Google Scholar]
- Howell ER, Hess RF. The functional area for summation to threshold for sinusoidal gratings. Vision Res. 1978;18:369–374. doi: 10.1016/0042-6989(78)90045-7. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, Cordle EP, Finley CD. Aging and scotopic sensitivity. Vision Res. 1998;38:3655–3662. doi: 10.1016/s0042-6989(98)00044-3. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Visual responses to time-dependent stimuli. I. Amplitude sensitivity measurements. J. Opt. Soc. Am. 1961;51:422–429. doi: 10.1364/josa.51.000422. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Motion and vision. II. Stabilized spatiotemporal threshold surface. J. Opt. Soc. Am. 1979;69:1340–1349. doi: 10.1364/josa.69.001340. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Retinal inhomogeneity. I. Spatiotemporal contrast sensitivity. J. Opt. Soc. Am. A. 1984;1:107–113. doi: 10.1364/josaa.1.000107. [DOI] [PubMed] [Google Scholar]
- Kim CB, Mayer MJ. Foveal flicker sensitivity in healthy aging eyes. II. Cross-sectional aging trends from 18 through 77 years of age. J. Opt. Soc. Am. A. 1994;11:1958–1969. doi: 10.1364/josaa.11.001958. [DOI] [PubMed] [Google Scholar]
- Lee BB, Smith VC, Pokorny J, Kremers J. Rod inputs to macaque ganglion cells. Vision Res. 1997;37:2813–2828. doi: 10.1016/s0042-6989(97)00108-9. [DOI] [PubMed] [Google Scholar]
- Lennie P, Fairchild MD. Ganglion cell pathways for rod vision. Vision Res. 1994;34:477–482. doi: 10.1016/0042-6989(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Mayer MJ, Kim CBY, Svingos A. Foveal flicker sensitivity in healthy aging eyes. I. Compensating for pupil variation. J. Opt. Soc. Am. A. 1988;5:2201–2209. doi: 10.1364/josaa.5.002201. [DOI] [PubMed] [Google Scholar]
- Nameda N, Kawara T, Ohzu H. Human visual spatio-temporal frequency performance as a function of age. Optom. Vis. Sci. 1989;66:760–765. doi: 10.1097/00006324-198911000-00007. [DOI] [PubMed] [Google Scholar]
- van Nes FL, Koenderink JJ, Nas H, Bouman MA. Spatiotemporal modulation transfer in the human eye. J. Opt. Soc. Am. 1967;57:1082–1088. doi: 10.1364/josa.57.001082. [DOI] [PubMed] [Google Scholar]
- Nygaard RW, Frumkes TE. Frequency dependence in scotopic flicker sensitivity. Vision Res. 1985;25:115–127. doi: 10.1016/0042-6989(85)90085-9. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal photoreceptor density decreases with age. Ophthalmology. 1995;102:1853–1859. doi: 10.1016/s0161-6420(95)30784-1. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Purpura K, Kaplan E, Shapley RM. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc. Natl Acad. Sci. USA. 1988;85:4534–4537. doi: 10.1073/pnas.85.12.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson JG. Spatial and temporal contrast-sensitivity function in the visual system. J. Opt. Soc. Am. 1966;56:1141–1142. [Google Scholar]
- Roufs JAJ. Dynamic properties of vision–I. Experimental relationships between flicker and flash thresholds. Vision Res. 1972;12:261–278. doi: 10.1016/0042-6989(72)90117-4. [DOI] [PubMed] [Google Scholar]
- Savage GL, Banks MS. Scotopic visual efficiency: constraints by optics, receptor properties, and rod pooling. Vision Res. 1992;32:645–656. doi: 10.1016/0042-6989(92)90181-h. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Bieber ML, McLean R, Werner JS. The area of complete scotopic spatial summation enlarges with age. J. Opt. Soc. Am. A. 1998;15:340–348. doi: 10.1364/josaa.15.000340. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Tregear SJ, Harvey LO, Werner JS. Senescent changes in scotopic contrast sensitivity. Vision Res. 1999;39:3728–3736. doi: 10.1016/s0042-6989(99)00072-3. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Alvarez SL. Aging, senile miosis and spatial contrast sensitivity at low luminance. Vision Res. 1988a;28:1235–1246. doi: 10.1016/0042-6989(88)90039-9. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects on spatial contrast sensitivity. J. Opt. Soc. Am. A. 1988b;5:2181–2190. doi: 10.1364/josaa.5.002181. [DOI] [PubMed] [Google Scholar]
- Smith RA. Studies of temporal frequency adaptation in visual contrast sensitivity. J. Physiol. 1971;216:531–552. doi: 10.1113/jphysiol.1971.sp009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA. Luminance-dependent changes in mesopic visual contrast sensitivity. J. Physiol. 1973;230:115–135. doi: 10.1113/jphysiol.1973.sp010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PD, Moore RJ, Kim CBY, Xue J-T, Tumosa N. Effects of aging on the primate visual system: spatial and temporal processing by lateral geniculate neurons in young adult and old rhesus monkeys. J. Neurophysiol. 1994;72:402–420. doi: 10.1152/jn.1994.72.1.402. [DOI] [PubMed] [Google Scholar]
- Stabell U, Stabell B. Absence of rod activity from peripheral vision. Vision Res. 1976;16:1433–1437. doi: 10.1016/0042-6989(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Sturr JF, Zhang L, Taub HA, Hannon DJ, Jackowski MM. Psychophysical evidence for losses in rod sensitivity in the aging visual system. Vision Res. 1997;37:475–481. doi: 10.1016/s0042-6989(96)00196-4. [DOI] [PubMed] [Google Scholar]
- Sun H, Pokorny J, Smith VC. Brightness induction from rods. J. Vis. 2001;1:32–41. doi: 10.1167/1.1.4. [DOI] [PubMed] [Google Scholar]
- Swanson WH, Ueno T, Smith VC, Pokorny J. Temporal modulation sensitivity and pulse-detection thresholds for chromatic and luminance perturbations. J. Opt. Soc. Am. A. 1987;4:1992–2005. doi: 10.1364/josaa.4.001992. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U, ver Hoeve JN, Terkla-McGrane C. Threshold and suprathreshold spatiotemporal response throughout adulthood. J. Opt. Soc. Am. A. 1988;5:2191–2200. doi: 10.1364/josaa.5.002191. [DOI] [PubMed] [Google Scholar]
- Vidinova MB, Beirne RO, Anderson RS, Williams R. Scotopic contrast sensitivity and the level of retinal stray light. Invest. Ophthalmol. Vis. Sci. 2009;50 E-abstract 1552. [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept. Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Watson AB, Robson JG. Discrimination at threshold: labeled detectors in human vision. Vision Res. 1981;21:1115–1122. doi: 10.1016/0042-6989(81)90014-6. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J. Neurophysiol. 1966;29:1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wright CE, Drasdo N. The influence of age on the spatial and temporal contrast sensitivity function. Doc. Ophthalmol. 1985;59:385–395. doi: 10.1007/BF00159172. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liang G, Li Y, Wang Y, Zhou Y, Leventhal AG. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neurosci. 2008;156:748–757. doi: 10.1016/j.neuroscience.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Young RSL, Kimura E, Delucia PR. A pupillometric correlate of scotopic visual acuity. Vision Res. 1995;35:2235–2241. doi: 10.1016/0042-6989(94)00303-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Wang Y, Fu Y, Liang Z, Ma Y, Leventhal AG. Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys. Eur. J. Neurosci. 2008;28:201–207. doi: 10.1111/j.1460-9568.2008.06300.x. [DOI] [PubMed] [Google Scholar]