Summary
Brief monocular deprivation (MD) shifts ocular dominance (OD) in primary visual cortex by causing depression of responses to the deprived eye. Here we address the extent to which the shift is expressed by a modification of excitatory synaptic transmission. An OD shift was first induced with 3 days of MD, and then the influences of intracortical polysynaptic inhibitory and excitatory synapses were pharmacologically removed, leaving only “feed-forward” thalamocortical synaptic currents. The results show that the rapid OD shift following MD is strongly expressed at the level of thalamocortical synaptic transmission.
Introduction
A robust example of experience-dependent brain plasticity first described by Hubel and Wiesel is the ocular dominance (OD) shift in the primary visual cortex following monocular deprivation (MD). Temporary closure of one eyelid results in a selective and persistent decrease in cortical responsiveness to stimulation of the deprived eye (Mioche and Singer, 1989; Wiesel and Hubel, 1963). The OD shift following brief MD has now been documented in a number of species with various methods, including single unit recordings, intrinsic signal imaging, visually evoked potentials (VEPs), two-photon calcium imaging and immediate-early gene induction (Antonini et al., 1999; Frenkel and Bear, 2004; Gordon and Stryker, 1996; Hofer et al., 2006; Mioche and Singer, 1989; Mower, 1991; Mrsic-Flogel et al., 2007; Tagawa et al., 2005). With long periods of MD, there are clear and well-documented structural changes in the excitatory thalamocortical inputs to cortex (LeVay et al., 1980; Shatz and Stryker, 1978). However, the OD shift measured electrophysiologically saturates with just a few days of MD, well before structural plasticity has reached an asymptote. Thus, rapid changes in synaptic transmission are among the first consequences of deprivation, but the precise nature and locus of these early changes remain unclear and have been vigorously debated for many years.
The simplest explanation for the selective loss of responsiveness to deprived-eye stimulation is the depression of excitatory synaptic strength by afferents conveying information from this eye (reviewed by (Smith et al., 2009). However, an alternative hypothesis—first proposed over 30 years ago (Burchfiel and Duffy, 1981; Duffy et al., 1976)—is that adjustments in the strength of cortical inhibition are induced by MD and these lead to selective suppression of responses evoked by deprived eye stimulation. The notion of inhibitory plasticity has recently been supported by several studies in rodent visual cortex. By recording from synaptically coupled pairs of neurons in cortical layer 4, it was shown that brief visual deprivation increases the strength of inhibitory, GABAA receptor-mediated synaptic responses (Maffei et al., 2006). In addition, by measuring the early consequences of MD in different classes of cortical neurons in vivo, it has been shown that responses of inhibitory cells are also modified by deprivation (Gandhi et al., 2008; Yazaki-Sugiyama et al., 2009; Kameyama et al., 2010).
The precise contribution of inhibitory plasticity to the OD shift following MD is unclear, however. One view is that adjustments in inhibition following MD are ancillary, serving mainly to facilitate Hebbian plasticity of excitatory connections (Gandhi et al., 2008). According to this model, the OD shift is expressed by changes in the strength of excitatory synaptic connections, but at a rate that is determined in part by inhibitory tone. The alternate view is that the early OD shift is actually a consequence of inhibitory suppression of deprived-eye responses in visual cortex (Burchfiel and Duffy, 1981; Duffy et al., 1976; Maffei et al., 2006; Yazaki-Sugiyama et al., 2009). The latter model predicts that acute relief from intracortical inhibition would preferentially restore responsiveness to the deprived eye and at least partially reverse the OD shift.
In this study, we attempt to parse the relative contribution of excitation and inhibition to expression of the OD shift in mice. Our approach was to first induce an OD shift with 3 days of MD, and then acutely probe for its persistence under conditions of either reduced cortical inhibition, produced by the GABAA receptor antagonist bicuculline methiodide (BMI), or silenced polysynaptic intracortical transmission, produced by the GABAA receptor agonist muscimol (Liu et al., 2007). We found that the OD shift induced by brief MD persists in the absence of cortical inhibition and is supported fully by the decrease in excitatory synaptic transmission at the thalamocortical synapse.
Results
Effect of reduced cortical inhibition on expression of the OD shift following MD
In rodents, it has been shown that brief MD causes changes in the strength of inhibitory synaptic transmission in cortical layer 4 (Maffei et al., 2006). Our objective was to test the hypothesis that these changes contribute to expression of the OD shift. To assess the strength of responses evoked by contralateral (C) and ipsilateral (I) eye stimulation, we monitored visually evoked potentials (VEPs). Because VEPs can be recorded through chronically implanted electrodes, it is possible to obtain measurements of absolute and relative visual responsiveness to each eye before and after MD in the same animals, without use of anesthesia (Frenkel and Bear, 2004; Sawtell et al., 2003). Changes in VEPs following MD agree both qualitatively and quantitatively with other measurements of visual responsiveness. Before MD, the C/I VEP ratio is approximately 2; when the contralateral eyelid is closed for 3 days, the ratio shifts to ~1 due to a selective loss of cortical responsiveness to the stimulation of the contralateral eye (Frenkel and Bear, 2004).
We initially used BMI to remove inhibition following MD, a traditional approach first introduced in the cat by Duffy, et al. (1976). If selective inhibitory suppression of excitatory responses occurs after MD in the mouse, we should observe a recovery of deprived eye responses relative to non-deprived eye responses during GABAA receptor blockade—i.e., the C/I ratio should increase towards the pre-MD value when inhibition is removed. A limitation of this approach is that it is impossible to completely eliminate inhibition without causing epileptiform activity in the cortex. This limitation is not specific to BMI; it applies to any approach that attempts to remove all inhibition while leaving polysynaptic excitatory transmission intact. Thus, the best outcome that could be achieved using this approach is a partial reversal of the OD shift caused by a partial reduction in inhibition.
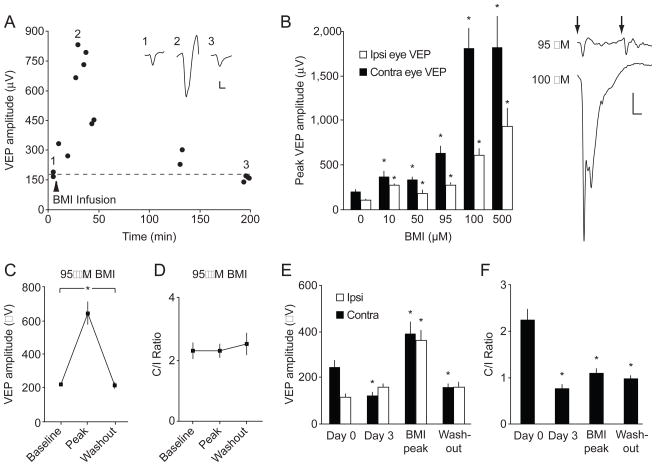
With these caveats in mind, we set out to produce a maximal reduction in cortical inhibition. The experimental design appears in Figure 1. A guide cannula was chronically implanted in close proximity to the tip of the VEP recording electrode (separated by 640 ± 40 μm, n = 29). Drug infusion (0.5 μl of 10–500 μM BMI over 5 min) invariably increased the magnitude of the VEPs (Figure 2A), which ultimately recovered to baseline levels. In control (non-deprived) mice, we investigated the effects of various doses of BMI on visual responses and the C/I ratio (Figure 2B–D). These experiments revealed that the maximum tolerated dose of BMI was 95 μM. Above that concentration we saw the abrupt emergence of epileptiform activity in visual cortex that obscured visual responses (see Figure 2B). Infusion of 95 μM BMI still produced significant disinhibition as evidenced by an approximately 3-fold increase in VEP amplitude (Figure 2C; baseline = 207.7 ± 9.22 μV, peak = 639 ± 73.8 μV, washout = 201 ± 19.4 μV, n = 4, post hoc Fisher test, p = 0.001). Although the amplitude changes were substantial, BMI infusion in control mice did not alter the C/I ratio (Figure 2D; baseline = 2.22 ± 0.25, peak = 2.21 ± 0.21, washout = 2.44 ± 0.34, n = 4).
Figure 1.
Experimental design. (A) Mice were chronically implanted with an electrode and a cannula in binocular visual cortex at P24. Following habituation to the recording apparatus (P25–27), baseline VEPs were recorded at P28 and mice were monocularly deprived for 3 days. At P31, the eye was reopened and VEPs were recorded. After baseline recordings, 0.5 μL of BMI or muscimol+SCH50911 (hereafter referred to as muscimol+) was infused at a rate of 6 μL/hr for 5 min and VEPs were continuously recorded for 1 hour. Final recordings (for BMI only) were made approximately 4 hrs post drug infusion in order to allow for a complete washout of the drug. (B) Diagram of recording electrode and cannula placement in the visual cortex. The recording electrode was implanted at a depth of 450 μm and the cannula was implanted at a depth of 150 μm at a 45° angle to the electrode.
Figure 2.
Reducing cortical inhibition does not alter the OD shift that accompanies brief MD. (A) A representative example of the change in VEP amplitude in response to visual stimulation of the contralateral eye after infusion of 95 μM BMI. Traces represent baseline contralateral VEP prior to BMI application (1), peak VEP following BMI (2) and VEP after drug washout (3). Scale bar: 0.1 mV, 25 ms. (B) Peak VEP amplitudes before and after infusion of increasing concentrations of BMI. “0 μM” represents the average pre-infusion VEP amplitude for all BMI concentrations. The increase in the contralateral and ipsilateral eye VEP amplitudes is significant across all drug concentrations (n = 4–8 for all groups, p < 0.05, one-way ANOVA, stars indicate a significant difference of the contralateral and the ipsilateral VEP from baseline respectively). Right panel shows representative VEP traces evoked by 1Hz visual stimulation (arrows indicate time of stimulus reversal). 95 μM BMI produces an increased, but faithful VEP response to each stimulus reversal, whereas 100 μM BMI produces an epileptiform discharge. Scale: 0.5 mV, 100 ms. (C) Application of BMI in non-deprived mice increases VEP amplitude, which return to baseline levels following washout of the drug. Peak amplitude of VEPs during BMI application was significantly different from both baseline and washout (n = 4, post hoc Fisher test, p < 0.05). (D) Application of BMI in non-deprived mice has no effect on the C/I ratio (n = 4, repeated measures ANOVA). (E) MD produces a depression of contralateral-eye responses (black bars), but no change in ipsilateral-eye responses (white bars; day 0 vs. day 3, n = 7). BMI infusion caused an increase in both contralateral- and ipsilateral-eye responses, which recovered following washout. * indicates a level of significance of p < 0.05 as compared to day 0 (post hoc Fisher test). (F) There is a decrease in the C/I ratio after MD that remains constant throughout BMI infusion and after washout (day 3 baseline is not significantly different from the BMI peak or washout values and BMI peak is not significantly different from washout; all comparisons were made using post hoc Fisher test).
We then proceeded to infuse 95 μM BMI into visual cortex of mice that had been monocularly deprived for 3 days to probe the persistence of the OD shift with reduced cortical inhibition. Three-day MD was chosen specifically because this is when the OD shift is asymptotic and is caused solely by loss of responsiveness to the deprived eye (Frenkel and Bear, 2004; Liu et al., 2008; Mrsic-Flogel et al., 2007; Sawtell et al., 2003).
As expected, before BMI treatment we observed depression of the contralateral deprived-eye VEP (246 ± 29.1 μV pre-MD vs. 120 ± 13.8 μV post-MD baseline, n = 7 post hoc Fisher test, p = 0.04) and a shift in the C/I ratio following MD (Figure 2E, F). BMI infusion caused an increase in the amplitude of the contralateral deprived-eye VEP (to 392 ± 47.4 μV) and the ipsilateral non-deprived eye VEP (to 361 ± 43.5 μV); and both responses recovered following washout (to 155 ± 19.8 μV and 161 ± 21.5 μV, respectively). Again, however, the C/I ratio appeared to remain stable before, during, and after BMI (pre-MD C/I = 2.3 ± 0.23; post MD = 0.77 ± 0.08; peak BMI = 1.1 ± 0.1; washout = 0.98 ± 0.06; n = 7, pre-MD value vs. all other values, p = 0.02, but post-MD baseline not significantly different from the BMI peak or the washout; Wilcoxon Signed Rank test).
Pharmacological isolation of a monosynaptic field potential in visual cortex in vitro
The possibility remained that residual inhibition in the presence of BMI might be sufficient to support selective suppression of the deprived eye response relative to the non-deprived eye response. We therefore decided to take a different approach: we asked if the full OD shift was already manifest by changes in “feed-forward” excitatory thalamocortical (TC) synaptic transmission after 3 days of MD.
To address this question, we adopted a clever method introduced recently by Liu et al. (2007) in mouse auditory cortex. Because sensory evoked potentials reflect excitatory synaptic currents, blockade of spiking in cortical neurons should leave a residual field response that reflects the sum of TC synaptic input to cortex. Liu et al. (2007) showed that infusion in vivo of a cocktail of muscimol and SCH5091 (we term muscimol+) eliminates postsynaptic cortical spiking, but leaves intact the isolated TC synaptic response. Muscimol, a GABAA receptor agonist, silences spiking of all cortical neurons by strongly inhibiting them. SCH5091, a GABAB receptor antagonist, preserves TC synaptic transmission by preventing the non-specific activation by muscimol of presynaptic GABAB receptors.
To ensure that polysynaptic responses could be blocked while leaving intact monosynaptic responses, we examined the action of the muscimol+ cocktail in a cortical slice preparation. We performed field potential (FP) and whole-cell recordings in layer 4 in response to white matter stimulation, with bath application of SCH50911 (70 μM), followed by muscimol (50 μM), and finally by CNQX (10 μM), an AMPA receptor antagonist, to eliminate all synaptic activity. In current clamp recordings, stimulation of the white matter evoked a rapid EPSP followed by a disynaptic IPSP (Figure 3A,B). As expected, application of SCH50911 eliminated the slow GABAB receptor-mediated IPSP, but did not affect the EPSP or the early GABAA receptor mediated IPSP. Addition of muscimol to the bath eliminated all polysynaptic responses as evidenced by the complete loss of the disynaptic IPSP (peak IPSP in muscimol + SCH50911 = 1.65 ± 1.37 % of baseline, n = 11, p < 0.0001). However, a smooth EPSP remained, albeit at a moderately reduced amplitude (EPSP in muscimol + SCH50911: 71.42 ± 8.88 % of baseline, n = 11, p = 0.002). The remaining EPSP was virtually eliminated by addition of CNQX (EPSP in CNQX = 10.9 ± 1.84 % of baseline, n = 11, p < 0.0001). These experiments indicate that the EPSP in the muscimol+ cocktail is monosynaptic—i.e., it is evoked by direct electrical stimulation of axons making glutamatergic synapses on the recorded layer 4 neurons.
Figure 3.
Application of muscimol+ cocktail blocks polysynaptic transmission but preserves monosynaptic excitation in visual cortex in vitro. (A–C) Current-clamp recordings of postsynaptic potentials evoked by WM stimulation. Neurons were maintained at −40 mV, just below spiking threshold, to clearly resolve EPSPs and IPSPs. (A) Overlay of averaged PSP waveforms before (black) and after bath application of SCH50911 (blue), muscimol+SCH cocktail (green), and CNQX (orange). (B) Normalized PSP amplitudes as a percentage of baseline (± SEM) before and after drug cocktail application. EPSP and IPSP amplitudes are not changed by SCH50911 application; EPSP amplitude is significantly reduced in the presence of muscimol+SCH (n = 11, p = 0.002), while IPSPs are completely eliminated (n = 11, p < 0.0001). Further application of CNQX abolished the remaining EPSP (n = 11, p < 0.0001). (C) Averaged time to peak (± SEM) latencies of the EPSPs remain unchanged by the application of SCH50911 alone or muscimol+SCH (n = 11). (D) Averaged FP waveforms before (black) and after bath application of SCH50911 (70 μM) (blue), muscimol (50 μM) +SCH50911 (green), and CNQX (10 μM) (orange). Scale bar: 25 μV, 25 ms. (E) Normalized FP amplitude as a percentage of baseline (± SEM) before and after drug cocktail application. FP amplitude is not changed by SCH50911 application, but is significantly reduced in the presence of muscimol+SCH (n = 9, p < 0.0001). CNQX completely abolishes the remaining synaptic FP (n = 5, p < 0.0001). The short latency FP (< 3 msec) observed in CNQX is not synaptic, as it persists in 0 Ca2+ and under total pharmacological blockade of excitatory synaptic transmission (Bear et al., 1992; Kimura et al., 1989). This response is excluded from analysis. (F) Averaged time to peak (± SEM) latencies of the synaptic FP remain unchanged by the application of SCH50911 alone or muscimol+SCH. (B,E) The grey line, indicating the limit of detection in our measurements, is the mean ± SEM fluctuation in voltage measured 5 ms prior to electrical stimulation (3.47% ± 0.62 for B and 18.5% ± 1.67 for E) taken across 5 ms prior to electrical stimulation.
Stimulation of the white matter also evoked a negative-going FP in layer 4, believed to reflect summed excitatory synaptic currents. Bath application of SCH50911 alone did not alter FP responses (Figure 3D, E; 100.22 ± 3.4 % of baseline, n = 9) and, although reduced in amplitude, a clear FP response also remained following addition of muscimol to the bath (Figure 3E; 47.1 ± 5.7 % of baseline, n = 7, p < 0.0001). Bath application of CNQX eliminated this remaining FP, showing that it is indeed synaptic (Figure 3E; 11.5 ± 11.5 % of baseline, n = 5, p < 0.0001). These experiments strongly support the claim by Liu et al. (2007) that it is possible to isolate a monosynaptic excitatory FP by suppressing spiking of cortical neurons with a muscimol+ cocktail.
It is interesting to note that the latency to the peak negative FP did not change after isolating the monosynaptic component (Figure 3F; baseline: 5.48 ± 0.29 ms, n = 9; SCH50911: 5.30 ± 0.25 ms, n = 9, p = 0.67; muscimol + SCH50911: 5.31 ± 0.36 ms, n = 7). This observation suggests that even under control conditions, the peak negative FP reflects monosynaptic activation. Much of the reduction in FP amplitude during muscimol+ can be explained by shunting of depolarizing currents caused by tonic activation of GABAA receptors.
Contribution of modified thalamocortical input to the OD shift following 3 days of monocular deprivation
Next, we tested whether the application of the muscimol+ cocktail in vivo also eliminates intracortical activity, while preserving a measurable TC response. The cocktail was infused into the binocular segment of primary visual cortex according to the exact protocol established by Liu et al. (2007), and we recorded single-unit activity at multiple cortical depths using a multichannel array (“stagger dagger”) electrode (Liu et al., 2008). The approximate distance between the infusion cannula and the electrode was 500 μm, and the electrode sampled units in all layers. Treatment eliminated all spiking activity in the large majority of units (Figure 4A, B). On rare occasions a unit continued to fire post drug applicaton. Analysis of the spike waveforms revealed that these non-responding units had extremely narrow spike widths (Figure 4B, inset) and were largely monophasic; histological reconstruction showed they were located in layer 4. These findings indicate that these are afferent axons (Chapman et al., 1991; Hubel and Wiesel, 1968) which, indeed, should not be affected by the drug cocktail.
Figure 4.
The OD shift following brief MD is expressed in the isolated thalamocortical component of the VEP. (A) Raster plot of a representative unit recorded before and after muscimol+ infusion demonstrating that cortical spiking was completely eliminated. (B) Single unit firing rate is dramatically reduced following muscimol+ infusion (n = 29, black bars represent medians (4.4 and 0 for baseline and drug respectively); p < 0.0001, Mann-Whitney U test). Inset shows waveform width analysis of the units: filled gray circles correspond to the gray bar and the narrowest spike width. Data are plotted in 0.25 ms bins. Units were sampled in all layers: 11 in superficial layers, 8 in layer 4, and 9 in deep layers. Units unresponsive to muscimol+ were axons in layer 4. (C) Experimental design for induction of c-fos expression after muscimol+ infusion. 24 hour dark exposure of mice with cannulated mice was followed by infusion of 0.5 μl of muscimol+ and an hour of subsequent light exposure. Mice were then perfused and stained for c-fos. Black boxes on the diagram represent the regions that were analyzed for c-fos expression. (D) c-fos expression is normal in the uninfused hemisphere (left) and completely abolished in the infused hemisphere (right). Scale: 100 μm. Insets represent enlarged areas from the control and infused cortices to clearly illustrate the absence of stained nuclei in the hemisphere treated with muscimol+. Scale: 15 μM. (E) A representative example of the change in VEP amplitude to binocular stimulation before and after infusion of muscimol+. Grey circles represent baseline VEP amplitude and black circles represent VEP amplitude following muscimol+ infusion. (F) Representative VEP traces from (E) evoked by 1 Hz visual stimulation (arrows indicate time of stimulus reversal). Grey rectangle highlights the baseline VEP trace. Scale bar: 0.1 mV, 0.1 s. (G) Traces indicate representative FPs evoked by contrast reversing grating stimuli during baseline (left), after infusion of muscimol+ (middle), and after infusion of CNQX (right). Scale: 50 μV, 50 ms. The baseline VEP is significantly reduced by infusion of muscimol+ and is completely eliminated by the infusion of CNQX. The grey line represents the limit of detection (see Figure 3). (H) Traces indicate representative FPs evoked by contrast reversing grating stimuli during baseline (left), after infusion of muscimol+ into the cortex (middle), and after injection of muscimol into the ipsilateral LGN (right). Scale: 50 μV, 50ms. The baseline VEP is significantly reduced by infusion of muscimol+ and is completely eliminated by the injection of muscimol into LGN. The grey line represents the limit of detection. (I) Application of muscimol+ significantly reduces VEP amplitude (n = 7, paired t-test, p = 0.0004), but has no significant effect on the C/I ratio. (J) The decrease in the C/I ratio following MD persists following acute muscimol+ infusion (day 3 baseline is not significantly different from the muscimol+ values (Wilcoxon Signed Rank test). * indicates a level of significance of p < 0.05 as compared to day 0.
We also took advantage of the cellular activity reporter protein c-fos to determine the spatial extent of the cortical activity blockade following muscimol+ infusion. Animals were placed in the dark for 24 hours to reduce c-fos expression. Visual cortex of one hemisphere was then infused with the muscimol+ cocktail (the other hemisphere serving as control) and the animals were exposed to light to induce c-fos in active neurons. Following one hour of light exposure, animals were euthanasized and perfused transcardially with fixative (Figure 4C). After histological processing, c-fos expression in both hemispheres was examined by immunofluorescence. As shown in Figure 4D, we found that muscimol+ treatment blocked c-fos induction in all cortical layers over a wide spatial extent (essentially all of V1). Therefore any synaptic response remaining in visual cortex following mucimol+ treatment is unlikely to derive from intracortical connections.
Infusion of the muscimol+ cocktail reduced the trough-to-peak amplitude of the VEP but did not change the latency to the peak negativity, similar to what was observed in the slice preparation (Figure 3D, E). The negative-going VEP had a simple morphology in the presence of the drug, similar to what was reported by Liu et al. (2007) and consistent with the interpretation that this is a monosynaptic field EPSP evoked by TC inputs (Figure 4E,F). The residual VEP in muscimol+ was completely eliminated by subsequent infusion of CNQX (Figure 4G; baseline: 217 ± 22.4 μV; muscimol+: 74.0 ± 8.53 μV; CNQX: 16.4 ± 1.30 μV; n = 5; post-CNQX value was significantly reduced from both baseline (p < 0.001) and muscimol+ (p = 0.003), post hoc Fisher test), confirming that this response reflects excitatory postsynaptic currents. Furthermore, the residual VEP in muscimol+ was also eliminated by silencing the ipsilateral lateral geniculate nucleus (LGN) with a stereotaxic injection of muscimol (Figure 4H; baseline: 207 ± 13.2 μV; cortical muscimol+: 77.0 ± 3.5 μV; LGN muscimol: 16.0 ± 4.9 μV; n = 3; post-LGN injection value was significantly reduced from both baseline (p = 0.006) and muscimol+ (p = 0.01), post hoc Fisher test). This finding supports the interpretation that the VEPs are of thalamocortical origin and rules out any contribution of interhemispheric input via the corpus callosum (Restani et al., 2009). As an additional confirmation that the residual VEP reflects direct TC input, we performed an analysis of response latency before and after muscimol+ infusion. If muscimol+ treatment were to unmask long-tract, cortico-cortical inputs, we should observe a shift to longer latencies due to the requirement for polysynaptic transmission and impulse conduction over greater distances. However, there was no shift to longer latencies after muscimol+ (Figure S1), supporting the interpretation that the VEPs in muscimol+ cocktail are solely of thalamocortical origin.
Although the VEP amplitude is reduced by muscimol+, in control (non-deprived) mice there was no significant effect on the C/I ratio (Figure 4I; baseline C/I = 2.2 ± 0.13; muscimol+ = 2.0 ± 0.21 (peak), n = 7, Wilcoxon Signed Rank test). This finding is consistent with the conclusion that the baseline C/I ratio in mouse visual cortex reflects mainly the differential innervation by thalamic (lateral geniculate nucleus) axons serving the two eyes(Coleman et al., 2009).
To test whether the expression of the full OD shift is apparent in the TC component of the VEP, we induced the OD shift with 3 days of MD and then infused the muscimol+ cocktail to probe for the persistence of the shift in the absence of all polysynaptic cortical activity (Figure 4J). Before drug infusion, we observed deprived-eye depression of the VEP (232 ± 13.3 μV pre-MD vs. 160 ± 12.7 μV post-MD baseline, n = 5 post hoc Fisher test, p < 0.001) and a shifted C/I ratio. Following muscimol+ application, the deprived eye response decreased to 58.6 ± 2.42 μV; however, the shifted C/I ratio was not significantly altered by muscimol+ infusion (C/I = 2.8 ± 0.24 pre-MD vs. 1.1 ± 0.14 post-MD baseline, and 1.4 ± 0.14 during muscimol+, n = 5, pre-MD value was significantly different from all other values, p = 0.04, but post-MD baseline was not significantly different from the muscimol+ value, Wilcoxon Signed Rank test). Thus, the effect of MD on OD appears to manifest fully at the level of thalamocortical excitatory transmission.
Discussion
To our knowledge, the current work represents the first demonstration that the rapid OD shift caused by brief MD is expressed by a modification of TC synaptic transmission. Elimination of all polysynaptic, local circuit influences—both excitatory and inhibitory—had no significant effect on the magnitude of the OD shift as reflected by the C/I ratio of the VEP. These data argue strongly that a major locus of ocular dominance plasticity is the excitatory synapses formed by lateral geniculate axons onto cortical neurons. Understanding how these synapses modify likely holds the key to understanding how deprivation leads to permanent visual disability.
When considering the meaning of the current findings, it is appropriate to draw a distinction between induction and expression of the OD shift. A host of mechanisms have been implicated in induction (e.g., NMDA receptors), which may determine whether OD plasticity can occur and may influence the qualities of the OD shift (Smith et al., 2009). Expression mechanisms, on the other hand, are the molecular and synaptic changes that allow the shift to be measured and maintained. The mechanisms mediating the expression of the rapid OD shift have been debated for decades.
Early studies in young kittens that were monocularly deprived for weeks found a significant rearrangement of thalamocortical afferents where the area of cortex serving the deprived eye shrinks, while the cortical area serving the open eye expands (Shatz and Stryker, 1978). Furthermore, electrophysiological recordings provided strong evidence for considerable weakening of the deprived-eye inputs following MD (Mitzdorf and Singer, 1980; Singer, 1977; Tsumoto and Suda, 1978). However, an alternative hypothesis of interocular inhibition was put forth based on an observation that following a period of MD, enucleation of the non-deprived eye leads to a substantial recovery of cortical responses driven by the deprived eye (Kratz and Spear, 1976). This idea was further investigated by Duffy et al who intravenously infused BMI into visually deprived kittens and observed that the drug restored deprived-eye responsiveness to as many as 50% of cortical neurons (Duffy et al., 1976). The finding was additionally strengthened by demonstrating that local iontophoretic administration of BMI in the cortex also restored deprived-eye responsiveness (Burchfiel and Duffy, 1981).
It was not clear, however, if these observations reflect plasticity of inhibitory connections. For example, even in cats with normal vision, visually unresponsive cells become responsive following application of intracortical BMI (Sillito, 1975). Indeed, a follow-up study in monocularly deprived kittens by Sillito et al. (1981) concluded that although subliminal excitatory influences could be revealed by reducing inhibition, this effect was not specific to the deprived eye. Considered together with contemporaneous studies which failed to detect sprouting of inhibitory neurons or to detect any changes in strength of inhibitory synaptic transmission following MD, scientific consensus grew that the intracortical inhibition has a passive, rather than active role in the OD shift resulting from MD (Bear et al., 1985; Sillito et al., 1981; Singer, 1977).
With the advent of rodent models, much more sophisticated mechanistic studies of OD plasticity became feasible. One such study revisited the hypothesis that intracortical inhibition may be responsible for maintaining the OD shift by focusing on changes in intracortical inhibition in layer 4 following MD (Maffei et al., 2006). Maffei et al studied synaptically coupled pairs of neurons in visual cortex ex vivo and found that following deprivation there is an increase in the inhibitory tone via strengthening of excitatory cell connections onto inhibitory interneurons and vice versa, but no change in the strength of excitatory connections onto other excitatory cells. The strengthening of inhibitory transmission occurs through a novel form of potentiation (LTPi), which was occluded by prior MD.
While the Maffei et al (2006) study provides important validation for the concept of experience-dependent plasticity of intracortical inhibition, the relevance to OD plasticity remains to be determined. The experiments were performed in the monocular segment of visual cortex, receiving input only from the contralateral eye. It is not known if monocular and binocular segments of cortex modify according to the same rules and mechanisms. Moreover, even if they do, the observations by Maffei et al may have more relevance to the effect of total visual deprivation than to the OD shift caused in binocular cortex by MD.
Several very recent reports have addressed the question of how inhibitory interneurons respond to MD in binocular visual cortex of the mouse in vivo. Although there is consensus that the visual responsiveness of interneurons is modified by deprivation, these studies disagree on both the qualities and the rate of the OD shift in these cells (Ghandi et al., 2008; Yazaki-Sugiyama et al., 2009; Kameyama et al., 2010). Of greatest relevance to the current findings, Yazaki-Sugiyama et al. (2009) examined the effect of intracellular infusion of picrotoxin on the OD shift expressed by cortical neurons after 3 days of MD. This treatment was designed to block inhibition by suppressing membrane chloride conductance. Although the picrotoxin often changed the eye dominance of responses in individual neurons, on a population level there was no effect on the OD shift.
Thus, it continues to be an open question of whether modifications of inhibitory synaptic transmission play a role in the selective depression of deprived-eye responses. Our findings certainly do not rule out the possibility of inhibitory plasticity in binocular cortex. However, it seems unlikely that inhibitory plasticity plays a major role in expression of the OD shift, which appears to be fully manifest by a change in TC excitatory synaptic transmission, at least after 3 days of MD.
Our findings do not contradict abundant evidence for a role for inhibition in modulating the induction of ocular dominance plasticity. For instance, it was previously demonstrated that a level of inhibitory tone is required for the ocular dominance shift to occur (Hensch, 2005; Ramoa et al., 1988). Perhaps strengthening of inhibition precedes and is necessary for the subsequent modification of excitatory synapses that express the OD shift. Results of a recent study by Gandhi et al (2008) are consistent with this possibility. These investigators report that brief MD first produces a shift in the responses of excitatory cells well before a shift in the responses of inhibitory cells. Although contradictory reports have appeared recently (Kameyama et al., 2010; Yazaki-Sugiyama et al., 2009), the findings of Ghandi et al. agree nicely with the early observation that disynaptic inhibition in the cortex is less affected by MD than monosynaptic excitation (Singer, 1977). Gandhi et al argue that the slow modification of inhibition could actually accelerate Hebbian long-term depression (LTD) of excitatory synapses by accentuating the decorrelation of pre- and postsynaptic responses to deprived-eye input activity.
The current findings support the conclusion that LTD at TC synapses is a primary cause of the OD shift after brief MD. It is noteworthy that the magnitude of the C/I ratio shift observed in the TC field EPSPs is as great as that observed in various measures of cortical neuronal responses, including single units and calcium spikes (Gordon and Stryker, 1996; Hofer et al., 2006; Mrsic-Flogel et al., 2007). Thus, although modifications of intracortical connections clearly occur following deprivation, our data suggest that the OD shift is already expressed fully by modification of feed-forward TC inputs.
Conclusion
To conclude, our study attempts to identify the mechanism which supports deprived-eye depression following a brief MD. We first induce an OD shift in the mouse visual cortex and then acutely block cortical inhibition or eliminate cortical activity altogether. We find that both of these manipulations do not alter the OD shift and do not restore visual responsiveness through the formerly deprived eye. Our data suggest that the enhancement of cortical inhibition is not the primary mechanism by which the deprived-eye depression is maintained. Furthermore, we directly demonstrate a change in the strength of thalamocortical synaptic transmission following MD and show that this change can fully account for the magnitude of the observed OD shift.
Experimental Procedures
Animal Preparation
C57/BL6 mice were anesthetized with 50 mg/kg ketamine and 10 mg/kg xylazine i.p. A local anesthetic, 0.1% lidocaine, was injected under the scalp. A head post was attached just anterior to bregma with cyanoacrylate glue (Small parts Inc., Miami Lakes, FL). Reference electrodes were placed bilaterally in prefrontal cortex. A small craniotomy (~1 mm) was made over binocular visual cortex (3 mm lateral to lambda), and tungsten microelectrodes (FHC, Bowdoinham, ME) were inserted 450 μm below the cortical surface. A second small craniotomy was made 0.5 mm lateral and 0.5 mm posterior to the electrode placement. A guide cannula (Plastics One, Roanoke, VA) was inserted 150 μm below the cortical surface at ~45° angle to the plane of electrode placement, thereby minimizing the distance between the tip of the electrode and the tip of the cannula. Electrodes and guide cannulae were secured in place by cyanoacrylate glue and dental cement (Lang Dental Inc., Nashua, NH). Animals were monitored postoperatively and were allowed at least 24 hr recovery period before habituation to the restraint apparatus. For single unit recordings, a custom-made multichannel linear array (“stagger dagger”) consisting of eight tungsten microwires (Calilfornia Fine Wire, Grover Beach, CA) attached by instant adhesive were implanted and fixed in place with dental acrylic. Details of the stagger dagger electrode construction are described in Liu et al., 2008(Liu et al., 2008).
Monocular Deprivation
Mice were anesthetized by inhalation of isoflurane (IsoFlo 2%–3%) and placed under a surgical microscope. Lid margins were trimmed and antibiotic ophthalmic ointment (Vetropolycin, Pharmaderm) was applied to the eye. Eyelids were sutured with mattress stitches opposing the full extent of the trimmed lids using 6-0 vicryl. Mice were recovered by breathing room air and were monitored daily to ensure that the sutured eye remained shut and uninfected. Animals whose eyelids were not fully shut for the entirety of the deprivation period were excluded from the study. At the end of the deprivation period, mice were reanesthetized, stitches were removed, and lid margins were separated. Eyes were then flushed with sterile saline and checked for clarity under a microscope. Mice with corneal opacities or signs of infection were excluded from the study.
Visual Stimuli
Stimuli consisted of full-field sine-wave gratings of 100% contrast, square-reversing at 1Hz, and presented at 0.05 cycles/degree. Stimuli were generated by a VSG2/2 card (Cambridge Research System, Cheshire, UK) and presented on a CRT computer monitor. VEPs were elicited by either horizontal or vertically oriented gratings. Orientation of stimuli during the first recording was randomized, but the stimuli presented to the same animal before and after deprivation were always orthogonal to each other to avoid the confound of stimulus-selective response potentiation(Frenkel et al., 2006). The display was positioned 20 cm in front of the mouse and centered at the vertical meridian, occupying 92° × 66° of the visual field. Mean luminance was 27 cd/m2.
In vivo Electrophysiology
All recordings were conducted in awake mice. The animals were alert and head-restrained during recording. Following postoperative recovery, the animals were habituated to the restraint apparatus for 1–2 hrs. For recording sessions visual stimuli were presented to left and right eyes randomly. A total of 100 to 200 stimuli were presented per each condition. VEP amplitude was routinely quantified by measuring peak negativity to peak positivity of the response amplitude. For single-unit analysis, FPs and spike activity were evoked by sinusoidal gratings and collected simultaneously from all channels of the implanted multichannel array (Liu et al., 2008).
Infusion
On the day of the infusion, the dummy cannula was removed and replaced with a 33 GA infusion cannula, attached with tubing to a 100 μL Hamilton syringe (VWR, West Chester, PA). 0.5 μL of bicuculline methiodide (BMI; 10–500 μM; Sigma, St. Louis, MO), or a cocktail of muscimol (4 mM; Sigma, St. Louis, MO) and SCH50911 (6 mM; Sigma, St. Louis, MO), or cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX, 3 mM; Sigma, St. Louis, MO)was infused with an infusion pump (VWR, West Chester, PA) over a 5 min period at a rate of 6 μl/hr. VEPs were recorded throughout the infusion and for at least an additional hour or, in the case of BMI infusions, until the drug washout was observed.
Cortical Slice Preparation
Following an overdose of barbiturates (i.p.), mice were decapitated upon disappearance of corneal reflexes in compliance with the U.S. Department of Health and Human Services. The brain was rapidly removed and immersed in ice-cold dissection buffer (composition: 87 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 75 mM sucrose, 10 mM dextrose, 1.3 mM ascorbic acid, 7 mM MgCl2, 0.5 mM and CaCl2) bubbled with 95% O2 and 5% CO2. The visual cortex was rapidly removed and 350 μm coronal slices were cut using a vibrating microtome (Leica VT100S). Slices recovered for 15 min in a submersion chamber at 32 °C filled with warmed artificial cerebral spinal fluid (ACSF; 124 mM NaCl, 5 mM KCl, 1.25 mM Na2PO4, 26 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 10 mM dextrose, saturated with 95% O2 and 5% CO2) and then cooled gradually to room temperature until use.
Extracellular Electrophysiology
Slices were transferred to an interface recording chamber maintained at 30 °C and perfused with ACSF at a rate of 2.5 ml/min. A stimulation electrode (concentric bipolar tungsten) was positioned in white matter, and a glass recording electrode (~1 MΩ) filled with ACSF was positioned in layer IV. The magnitude of responses evoked by a 200 μsec pulse was monitored by the amplitude of the FP. Stimulation intensity was adjusted to elicit half the maximal response, and stable baseline responses were elicited every 30 sec. Objective criteria (baseline drifts no greater than 5% and proper waveform alignment) were applied as inclusion criteria for further analysis. The data were normalized, averaged, and reported as means ± SEM. For the drug cocktail experiments, responses were recorded every 20 sec. The resulting signals were filtered between 0.1 Hz and 3 kHz, amplified 1000 times, and captured at 10 kHz on an IBM-compatible computer using pCLAMP 9.2 software (Molecular Devices).
Current-Clamp Recordings
The internal solution consisted of: 130 mM K-gluconate, 4 mM KCl, 2 mM NaCl, 10 mM HEPES, 0.2 mM EGTA, 4 mM Mg–ATP, 0.3 mM Na-GTP, 14 mM phosphocreatine, 0.2% biocytin, with pH adjusted to 7.26, and osmolarity adjusted to 296 mOsm using ddH2O. Pipette resistances were ≈6 MΩ when filled with internal solution. For current-clamp recordings, stimuli (0.2 msec) were delivered at 0.05 Hz. Recordings were considered acceptable if membrane potentials were maintained between −55 and −70 mV. To clearly resolve EPSPs and IPSPs, depolarizing current was passed through the pipette to maintain the membrane potential at −40 mV. At least 5 stable responses were collected before and after infusion of SCH50911, muscimol, and CNQX (Sigma, St. Louis, MO). EPSPs and IPSPs were acquired and analyzed via pClamp and Clampfit software. The reversal potential of chloride of these solutions is −82 mV. All neurons studied fell within the borders of layer 4, as identified by trans-illumination. In addition, neurons were routinely filled with biocytin and confirmed to be in layer 4 and to have spiny dendrites.
Data analysis
The peak BMI-evoked response was the maximum observed response (a summed mean response over 100 stimulus presentations) after infusion of the drug and usually occurred within a window of 20–30 min after the start of infusion. In the muscimol+SCH50911 experiments, all responses over an hour of recording were summed and the mean was reported. All statistical analyses were performed using StatView 5.0.1 (Abacus Concepts, Berkeley, CA). A Student’s paired t-test or a global ANOVA was always performed where appropriate, and relevant post hoc comparisons were made using Fisher’s protected least square difference analysis. In all cases, significance was set at p < 0.05. For single-unit analysis, spikes were sorted using offline discrimination of single unit activity that was based on waveform shape, while multiunit activity was excluded. Firing rate was calculated as the (total number of spikes)/(total recording time). Single-unit firing rates and C/I ratios were not normally distributed (Lillie test for normality of distribution), therefore statistics were performed using Wilcoxon Signed Rank test, Kruskal-Wallis test and Mann-Whitney U test.
Supplementary Material
Acknowledgments
We thank Rahmat Muhammad, Arnold Heynen, Gordon Smith, Jason Coleman, Kathleen Oram, Erik Sklar and Suzanne Meagher for assistance. This work was partly supported by grants from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonini A, Fagiolini M, Stryker MP. Anatomical correlates of functional plasticity in mouse visual cortex. J Neurosci. 1999;19:4388–4406. doi: 10.1523/JNEUROSCI.19-11-04388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Press WA, Connors BW. Long-term potentiation in slices of kitten visual cortex and the effects of NMDA receptor blockade. J Neurophysiol. 1992;67:841–851. doi: 10.1152/jn.1992.67.4.841. [DOI] [PubMed] [Google Scholar]
- Bear MF, Schmechel DE, Ebner FF. Glutamic acid decarboxylase in the striate cortex of normal and monocularly deprived kittens. J Neurosci. 1985;5:1262–1275. doi: 10.1523/JNEUROSCI.05-05-01262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfiel JL, Duffy FH. Role of intracortical inhibition in deprivation amblyopia: reversal by microiontophoretic bicuculline. Brain Res. 1981;206:479–484. doi: 10.1016/0006-8993(81)90551-5. [DOI] [PubMed] [Google Scholar]
- Chapman B, Zahs KR, Stryker MP. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J Neurosci. 1991;11:1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Law K, Bear MF. Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience. 2009;161:561–571. doi: 10.1016/j.neuroscience.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, Burchfiel JL, Conway JL. Bicuculline reversal of deprivation amblyopia in the cat. Nature. 1976;260:256–257. doi: 10.1038/260256a0. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Yanagawa Y, Stryker MP. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc Natl Acad Sci U S A. 2008;105:16797–16802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y, Tsumoto T. Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. J Neurosci. 2010;30:1551–1559. doi: 10.1523/JNEUROSCI.5025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Nishigori A, Shirokawa T, Tsumoto T. Long-term potentiation and N-methyl-D-aspartate receptors in the visual cortex of young rats. J Physiol. 1989;414:125–144. doi: 10.1113/jphysiol.1989.sp017680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz KE, Spear PD. Postcritical-period reversal of effects of monocular deprivation on striate cortex cells in the cat. J Neurophysiol. 1976;39:501–511. doi: 10.1152/jn.1976.39.3.501. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–345. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U, Singer W. Monocular activation of visual cortex in normal and monocularly deprived cats: an analysis of evoked potentials. J Physiol. 1980;304:203–220. doi: 10.1113/jphysiol.1980.sp013320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Paradiso MA, Freeman RD. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Exp Brain Res. 1988;73:285–296. doi: 10.1007/BF00248220. [DOI] [PubMed] [Google Scholar]
- Restani L, Cerri C, Pietrasanta M, Gianfranceschi L, Maffei L, Caleo M. Functional masking of deprived eye responses by callosal input during ocular dominance plasticity. Neuron. 2009;64:707–718. doi: 10.1016/j.neuron.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Blakemore C. The role of GABAergic inhibition in the cortical effects of monocular deprivation. Nature. 1981;291:318–320. doi: 10.1038/291318a0. [DOI] [PubMed] [Google Scholar]
- Singer W. Effects of monocular deprivation on excitatory and inhibitory pathways in cat striate cortex. Exp Brain Res. 1977;30:25–41. doi: 10.1007/BF00237856. [DOI] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Evidence for excitatory connections from the deprived eye to the visual cortex in monocularly deprived kittens. Brain Res. 1978;153:150–156. doi: 10.1016/0006-8993(78)91137-x. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Cateau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462:218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.