Abstract
The metal-exporting systems CusCFBA of Escherichia coli and GesABC of Salmonella are RND-type multi-protein systems responsible for detoxification during metal stress. In this study the substrate range was determined for each metal transport system and possible amino acid residues important in substrate specificity identified. The Ges system, previously identified as a gold-efflux system, conferred resistance to the greatest number and variety of organic chemicals including chloramphenicol, not previously recognized as a substrate. Phylogenetic analysis showed that GesB is most closely related to a class of RND transporters including MexF that have been shown to be responsible for exporting fluoroquinolones, chloramphenicol, and biocides. However, many of the closest homologs of GesABC appear to have a role in metal resistance judging from the genetic context. In contrast, CusCFBA belongs to a distinct family of RND-type monovalent metal exporter systems containing a number of essential metal-binding methionines resulting in a much narrower substrate range.
Keywords: Escherichia coli, RND transporters, metal-resistance, copper efflux
Introduction
Efflux is the most common widespread mechanism to regulate the concentration of a myriad of substances in all organisms. Substrate specificities of transporters vary widely and the mechanisms governing substrate recognition and subsequent transport are not well understood. Multi-protein complexes of the RND (Resistance-Nodulation-Division) family in Gram-negative bacteria are both of medical and environmental importance. Within the genome of E. coli, there are seven genes belonging to the RND family, acrB, acrD, acrF, cusA, mdtB, mdtC, and mdtF. Together with a membrane fusion protein (MFP) and outer membrane factor (OMF) these inner membrane proteins form a complex responsible for the extrusion of a large variety of substrates mainly from the periplasm in a proton-gradient dependant manner. The best characterized member in E. coli, AcrB, forms a complex with the membrane fusion protein AcrA and the outer membrane protein TolC. Aptly termed periplasmic “vacuum cleaners” (Lomovskaya et al., 2007), the broad specificity for AcrAB-TolC varies from hydrophilic to hydrophobic, and includes bile salts, antibiotics, ethidium bromide, SDS, and crystal violet (Pos, 2009). The substrates of AcrEF-TolC are similar to that of AcrAB-TolC, while AcrDA-TolC confers resistance to more hydrophilic substances such as SDS and aminoglycoside antibiotics (Elkins and Nikaido, 2002). MdtF substrates include fluoroquinolones, macrolides, oxacillin, novobiocin, and ethidium bromide (Bohnert et al., 2007). Complexed with MFP protein MdtA and OMF protein MdtB, the RND pair MdtBC (YegNO) can shuttle out bile salts, norfloxacin, and kanamycin, among others (Baranavo and Nikaido, 2002).
Other RND-type transporters are involved in conferring resistance to metals such as copper, zinc, cadmium and gold (Nies, 2003; Pontel et al., 2007). E. coli possesses the cusCFBA determinant, which is proposed to extrude copper and silver from the periplasm to the extracellular environment (Franke et al., 2003). The inner membrane RND protein CusA interacts with both the membrane fusion protein CusB and outer membrane factor CusC. Additionally, the small periplasmic protein CusF binds copper and silver (Kittleson et al. 2006), and subsequently transfers it to CusB (Bagai et al. 2008). Several essential, conserved methionine residues have been identified both in CusB and CusA (Bagai et al., 2008; Franke et al., 2003). The recently discovered gold-efflux determinant gesABC in Salmonella encodes the inner-membrane RND transporter GesB, the membrane-fusion protein GesA, and the outer membrane factor GesC. GesABC is able to pump organic molecules including methylene blue and crystal violet, after induction by gold ions (Pontel et al., 2007). The outer membrane factor GesC can be substituted by TolC so gesAB alone can be functionally expressed in E. coli (Nishino et. al, 2006).
Here, three strains of E. coli with different gene deletions encoding RND transporters were transformed with plasmids containing cusCFBA and gesAB and tested for sensitivity to approximately 240 chemicals. Following initial screening, select compounds were tested further on liquid and solid media. While GesAB was shown to have broad substrate specificity typical for other RND-type systems, the CusCFBA was found to have limited substrate specificity.
Materials and Methods
Bacterial strains and plasmids
The strains and plasmids used in this study are listed in Table 1. E. coli strains were grown in Luria-Bertani (LB) broth at 37°C. To determine substrates of the efflux pumps, strains were grown overnight from a single colony, diluted, and tested for growth as described below. All experiments were performed at least three times. Antibiotic concentrations for ampicillin were 100 μg mL−1.
Table 1.
Strains and plasmids used in this study.
| Strains | Relevant Genotype | Source or Ref. | |
|---|---|---|---|
| W3110 | wild-type | Bachmann et al., 1972 | |
| W4680AD | W3110 ΔacrA/B ΔacrD | Krishnamoorthy et al., 2007 | |
| W4680AE | W3110 ΔacrA/B ΔacrE/F | Krishnamoorthy et al., 2007 | |
| 5X RND | W3110 ΔacrB::kan, ΔacrD, ΔacrF, ΔyegNO, ΔmdtF | Personal gift from D. Nies | |
| Plasmids | |||
| pGEM-T | Empty vector control, ApR | Promega | |
| pUH21 | Empty vector control, ApR | Soncini et al., 1995 | |
| pCusCFBA | pGEM-T-cusCFBA, ApR | Franke et al., 2001 | |
| pGesAB | pUH21-gesAB, ApR | Pontel et al. 2007 | |
Biolog Assay
Biolog (Biolog, Inc., Hayward, CA) has developed a rapid screen to determine the phenotypic classifications of bacteria and fungi. Simply, the growth rates of tested strains are compared after exposure to essential nutrients, carbon and nitrogen sources, or metals and complex organics (Bochner et al., 2001; Bochner, 2003). Detection and analysis is done colorimetrically, which represents bacterial growth. A tetrazolium dye is introduced into the medium and acts as the terminal electron acceptor during growth. Once reduced, the colorless dye turns violet, with a λmax of 590 nm. The intensity of dye is directly proportional to the amount of bacteria in the wells.
To verify results from the rapid screening method, positive compounds (i.e. chemicals conferring resistance) were tested using both solid and liquid media. All stock solutions were stored at −20° C in the dark.
Additional strains containing their respective plasmids were tested simultaneously (Table 2). These included wild-type E. coli W3110, 5X RND and W4680AE carrying pCusCFBA, pGesAB, pUH21, or pGEM-T. For liquid tests, all strains were pre-cultured in LB (containing 100 μg mL−1 ampicillin when necessary) to an OD600 = 0.6 – 1.0. Bacteria were then diluted to a final concentration of 5 × 105 cell mL−1 in LB and exposed to different levels of the test chemical. Dose-response curves were created by recording OD600 versus concentration after 16 hours of exposure. In solid media tests, compounds were diluted into cooling agar at different concentrations reflective of levels present in liquid media tests. E. coli strains W3110, W4680AD, W4680AE, or 5X RND carrying no plasmid, vector control, pCusCFBA or pGesAB were streaked onto an agar plate, and minimum inhibitory concentrations (MICs) were determined.
Table 2.
Substrates to which E. coli W4680AD expressing pGesAB shows resistance, as determined by Biolog assay.
| Resistance Level | Chemical | Class/Function |
|---|---|---|
| Strong | Chlorquinaldol | Antiseptic |
| Acriflavine | Antiseptic | |
| Alexidine | Disinfectant (found in mouthwash) | |
| Chelerythrine | Protein kinase C Inhibitor | |
| Chloramphenicol | Bacteriostatic antibiotic | |
| Cloxacillin | β-lactam antibiotic | |
| Nafcillin | β-lactam antibiotic | |
| Niaproof | Anionic detergent | |
| Plumbagin | Antimicrobial | |
| Proflavine | Flavone, antibacterial | |
| Sanguinarine | Antimicrobial | |
| Thiamphenicol | Bacteriostatic antibiotic | |
| Moderate | 1-Chloro-2,4-dinitrobenzene | Oxidation, glutathione |
| 5,7-Dichloro-8-hydroxyquinoline | Antibacterial; antifungal | |
| Amikacin | Aminoglycoside antibiotic | |
| Bleomycin | Glycopeptide antibiotic | |
| Captan | Fungicide | |
| Cefoxitin | β-lactam antibiotic | |
| Cephalothin | β-lactam antibiotic | |
| Chlortetracycline | Tetracycline antibiotic | |
| Dichlofluanid | Fungicide | |
| Dodine | Fungicide | |
| Domiphen bromide | Antimicrobial (cosmetics) | |
| Hydroxylamine | DNA mutagen | |
| Oxacillin | β-lactam antibiotic | |
| Weak | Sulfamethazine | Veterinary antibacterial |
The responses to different classes of chemicals varied in the Biolog assay. Certain levels and/or chemicals were toxic to both strains (empty vector vs. vector containing) creating no response in the growth curves. For chemicals that had no effect on growth, the empty vector control and metal exporter growth curves were identical, indicating no resistance exhibited by expression of the respective RND-type metal export system. Growth rates of the expression of the RND-type metal export system exceeded that of the empty vector strain were recorded as conferring resistance. It was possible to approximate the MICs of an individual chemical using the Biolog assay based on the level of response. No metals were added to overexpress pCusCFBA and pGesAB in these experiments, and consequently expression levels are likely to be low. Thus, it is possible that some potential substrates may not have been identified.
E. coli strain W4680AD (ΔacrA/B, ΔacrD) containing the control vectors (pGEM-T, pUH21) or metal exporters (pCusCFBA and pGesAB) were grown in LB medium supplemented with ampicillin, 100 μg mL−1 overnight at 37°C. The inoculum was then diluted in IF-10 Base (Biolog part number 72264) to a concentration of 5 × 106 cells mL−1 (Bochner et al., 2001). A solution containing the cell suspension (1.2 mL), sterile water (18.8 mL, IF-10 Base (98.8 mL), and Dye Mix D (Biolog part number 74224, 1.2 mL) was mixed and dispensed (100 mL/well) to each of the ten 96-well assay plates (Biolog panels PM11-PM20, part nos. 12211 – 12220). Each plate contained twenty-four chemicals of varying structures and functions at concentrations spanning orders of magnitude (Table S1). The plates were incubated at 37°C and the absorbance of the reduced tetrazollium dye, an indicator of cell growth, was recorded at A590 periodically over forty-eight hours. Absorbance versus time was plotted for each chemical at four concentrations, comparing the strain containing the metal exporter to the strain containing an empty vector (control). The Biolog assay was repeated in triplicate on three different occasions.
Phylogenetic Analysis
Protein sequences for the two metal-exporting pumps described thus far were aligned with sixty other RND proteins with known function and substrates using ClustalW (Higgins et al., 1994). RND pumps were first identified through a search of the NCBI and SwissProt databases using CusA and GesB as the queries.
Genomic sequence resources and analysis
We examined fully sequenced bacterial genomes in the γ-proteobacteria class (195 unique genomes were available as of September, 22, 2009). Sequenced genomes that were used in this study can be found on the NCBI website.
CusF (gi:16128556) and CusB (gi:16128557) were queried against all 195 γ-proteobacteria sequenced genomes using blastp with default parameters (Altschul et al., 1990). Sequence alignment hits with E-values less than 0.001 and sequence percent identity higher than 25 percent were further analyzed. Subsequently, these sequences were scanned for metal binding motifs, M21M36M38 for CusB and W/M36H44M47M49 for CusF.
Results and Discussion
Novel substrates for two metal-transporting RND-type systems could be identified via Biolog assay
Our aim in this study was to determine additional potential substrates of two RND-type transport systems, the gold transporter GesAB and the copper and silver transporter CusCFBA. Biolog assay plates were used for the initial screening of approximately 240 organic and inorganic compounds (Table S1). The level of resistance due to expression of metal exporter was then classified as weak, moderate, or strong. Resistance was classified as strong when the strain expressing an RND-type exporter attained log growth, while the empty vector strain failed to grow, or grew only slightly, over 48 hours. When the growth rate of the empty vector strain was within 50% of the metal exporting strain, the resistance was classified as moderate. Resistance was classified as weak when the growth rate of the metal exporting strain was only slightly greater than the control. Compounds to which resistance was observed for strains expressing pGes or pCusCFBA were identified (Tables 2 and 3). Chemicals to which moderate or strong resistance was exhibited were selected for further testing with liquid and solid media.
Table 3.
Substrates to which E. coli W4680AD expressing pCusCFBA shows resistance, as determined by Biolog assay.
| Resistance Level | Chemical | Class/Function |
|---|---|---|
| Strong | 2,4-Dintrophenol | Oxidative phosphorylation uncoupler |
| 1,3-Dinitrobenzene | Proton ionophore, H+ | |
| Ethionamide | Used to treat tuberculosis | |
| Moderate | 2, 2′-Dipyridyl | Iron chelator |
| 4-Hydroxycoumarin | Rodenticide | |
| Benserazide | Used to treat Parkinson’s disease | |
| Boric Acid | Insecticide | |
| Chloroxylenol | Pesticide | |
| Cytosine-1-beta-D-arabinofuranoside | Inhibits DNA polymerase | |
| FCCP | Disrupts mitochondrial membrane potential | |
| Fusaric acid | Mycotoxin | |
| Sodium arsenate | Metal | |
| Sulfamethoxazole | Sulfonamide antibiotic | |
| Weak | 3-Amino-1,2,4-triazole | Herbicide |
| Cefmetazole | β-lactam antibiotic | |
| Cobalt chloride | Cetal | |
| Guanidine hydrochloride | Denatures proteins | |
| Lincomycin | Lincosamide antibiotic | |
| Oxolinic acid | Quinolone antibiotic | |
| Sodium metaborate | Insecticide |
Potential substrates were identified for E. coli W4680AD (ΔacrA/B, ΔacrD) expressing pCusCFBA or pGesAB, suggesting that the RND transporter is responsible for increased resistance (data not shown). Subsequently, a few promising substrates were further tested in other E. coli strains. The strains E. coli W4680AE (ΔacrA, ΔacrB, ΔacrE, and ΔacrF) and E. coli strain 5X RND (ΔacrB, ΔacrD, ΔacrF, ΔmdtF, and ΔmdtBC) were employed for further analysis in addition to E. coli W4680AD.
GesAB has a broad substrate range
E. coli W4680AD expressing the gold-efflux-system from pGesAB exhibited strong resistance towards 12 chemicals (Table 2). The classes of compounds included β-lactams, the bacteriostatics chloramphenicol and thiamphenicol, several other antimicrobials, a surfactant, and a protein kinase C inhibitor. Although the cloning vector contained coding sequences for β-lactamase and chloramphenicol acetyltransferase (Soncini et. al, 1995), the resistance observed for β-lactams, chloramphenicol, and thiamphenicol was much greater than the empty vector control. Moderate resistance was detected in approximately the same number of chemicals and consisted mostly of antibiotics, fungicides, a cationic surfactant, and a DNA mutagen. Of the chemicals initially identified from the Biolog screen, chloramphenicol, chlorquinaldol, and dichlofluanid were chosen for further analysis. Methylene blue and crystal violet were previously reported to be substrates of GesABC in Salmonella typhimurium (Nishino et. al, 2006; Pontel et al., 2007), and were also tested here.
All three E. coli strains expressing GesAB showed chloramphenicol resistance in the liquid media tests (Figure S3). MIC analysis showed the level of resistance increased 4-fold in E. coli strain 5X RND and 8-fold in strains W4680AD and W4680AE (Table 4). Chloramphenicol had not been identified as a substrate of the Ges system previously. Moreover, chlorquinaldol resistance was detected in all tested strains expressing GesAB in liquid media tests (data not shown). E. coli strains W4680AD and W4680AE carrying pGesAB were resistant to chlorquinaldol with a 2-fold increase MIC value, via the agar results. The discrepancy between growth in the Biolog assay and MIC assays in LB medium could be attributed to differing growth conditions (media, incubation time, detection method). Crystal violet and methylene blue, which was not present in the Biolog panels, were tested because GesABC conferred resistance to both compounds in Salmonella (Nishino et. al, 2006; Pontel et al., 2007). Previous studies have shown the MIC values for crystal violet and methylene blue are 8- and 16- fold greater when the gold efflux system is over-expressed in a ΔgesABC, ΔacrB knock-out (Nishino et. al, 2006). Here, only the MIC value of E. coli W4680AD containing pGesAB exposed to crystal violet was greater than the control, but gesAB expressed in E. coli strains W4680AE and 5X RND did not show any difference from the vector control. It is possible that the level of expression of gesAB in these backgrounds is not sufficient to detect a difference in MIC values when compared to the vector controls. Crystal violet is a polyaromatic compound and is the largest of the chemicals studied with the Ges system. Liquid media results show that E. coli strain W4680AD containing pGesAB conferred resistance to crystal violet, while E. coli strains W4680AE and 5X RND containing pGesAB did not (Figure S4). In liquid media tests, the E. coli strains containing pGesAB did not display a significant difference in resistance to methylene blue (data not shown). Agar results showed that low level resistance was conferred by pGesAB in E. coli strains W4680AD (> 1X) and W4680AE (1.3X) when exposed to methylene blue (Table 4).
Table 4.
Minimum Inhibitory Concentrations (MIC, mg/L) for five compounds in different E. coli strains expressing pGesAB or pUH21.
| E. coli strain | MIC (mg/L) | ||||
|---|---|---|---|---|---|
| Chloramphenicol | Chlorquinaldol | Crystal Violet | Dichlofluanid | Methylene Blue | |
| W4680AD pUH21 | 2.5 | 0.25 | 0.5 | >100 | 4 |
| W4680AD pGes | 20 | 0.5 | 0.6 | >100 | >4 |
| W4680AE pUH21 | 2.5 | 0.25 | 0.5 | >100 | 3 |
| W4680AE pGes | 20 | 0.5 | 0.5 | >100 | 4 |
| 5XRND pUH21 | 2.5 | 0.5 | 0.5 | >100 | 4 |
| 5XRND pGes | 10 | 0.5 | 0.5 | >100 | 4 |
CusCFBA has a narrow substrate range
To determine whether cusCFBA is functionally expressed in pCusCFBA, growth of copper sensitive strain GR10 (ΔcueO ΔcusCFBA; Grass and Rensing, 2001) containing either pGEM-T or pCusCFBA was monitored for growth on LB medium containing different concentrations of copper. Only pCusCFBA but not pGEM-T was able to confer copper resistance in strain GR10 confirming that cusCFBA was functionally expressed (data not shown). During initial Biolog screening, pCusCFBA conferred strong resistance to dinitrophenol, dinitrobenzene, and ethionamide in W4680AD (Table 3 and Figure S1). Both dinitrophenol and dinitrobenzene are similar in structure with a single aromatic ring. Ethionamide contains a heterocycle and two uncommon side chains. All three compounds are relatively small. The chemicals classified as moderate (10 in total) and weakly resistant (7 in total) covered a wide range of functionalities and structures and included antibiotics, metals, a metal chelator, and other biologically active compounds (Table 3).
Additional testing in liquid media revealed that the presence of pCusCFBA in E. coli W4680AD conferred resistance to dinitrobenzene and dinitrophenol but results obtained from exposure to ethionamide were inconclusive. For dinitrobenzene, a 1.2 to 1.4 fold-increase in the MIC value was observed for the three mutant strains expressing cusCFBA (Table 5). Liquid tests verified the results for strain W4680AD, but increased sensitivity was not observed between the control and metal-exporting strains in W4680AE and 5X RND (Figure S2). These results infer that dinitrobenzene may be exported by AcrE/F, which is present in W4680AD and not W4680AE or 5X RND. For dinitrophenol, MIC levels varied depending on the strain (Table 5) (3-fold for W4680AD, 1.5-fold for W4680AE, and 0.63-fold for 5X RND in metal-exporter versus control). Liquid results were similar for dinitrobenzene in that differences were observed between W4680AD pCusCFBA and control, but not for W4680AE and 5X RND. Dinitrophenol may be exported by AcrE/F. Finally, no difference was observed in any mutants exposed to ethionamide. The three strains and controls responded similarly to different concentrations of ethionamide in both liquid and agar tests (Table 5). Concentrations beyond 200 μg mL−1 ethionamide were not evaluated due to solubility issues. In conclusion, only a limited number of potential substrates could be identified in initial screenings. However, in an extended analysis using different strains and growth conditions, the ability of CusCFBA to confer resistance to these substances could not be verified. These results strongly suggest narrow substrate specificity for the CusCFBA system.
Table 5.
Minimum Inhibitory Concentrations (MIC, mg/L) for three compounds in different E. coli strains expressing pCusCFBA or pGEM-T.
| E. coli strain | MIC (mg/L) | ||
|---|---|---|---|
| Dinitrobenzene | Dinitrophenol | Ethionamide | |
| W4680AD pGEM-T | 60 | 50 | >200 |
| W4680AD pCusCFBA | 80 | 150 | >200 |
| W4680AE pGEM-T | 60 | 100 | >200 |
| W4680AE pCusCFBA | 70 | 150 | >200 |
| 5XRND pGEM-T | 70 | 200 | >200 |
| 5XRND pCusCFBA | 100 | 125 | >200 |
Differing results between the Biolog and MIC assays may be due to differences in preparation of the tested compounds. The native Biolog multi-well plate contained dry deposited chemicals and the concentration range of the chemicals covered orders of magnitude. It is possible that in the Biolog assay certain hydrophobic analytes were not fully soluble, such that the bacteria were not exposed to the intended concentration. In the MIC assays, organic solvents or ionic mixtures were used to solubilize the compounds to a particular concentration. Thus, some differences may be seen if the end concentrations are different between the two assays.
Thioethers constitute signature sequence in putative monovalent RND-type efflux pumps
Narrow substrate specificity can be attributed to the metal binding sites of CusB and CusF. X-ray absorption spectroscopy data shows that Cu(I) is bound to CusB in a 3-coordinate environment, indicative of Cu-S interactions (Bagai et al., 2007). CusB does not contain any cysteine residues, consequently the sulfur-containing species in CusB that coordinate Cu(I) are methionine residues. Through site-directed mutagenesis and subsequent isothermal titration calorimetry data, Bagai et al., showed that three methionine residues, M21M36M38 are important in metal binding and subsequent copper efflux. Moreover, CusF, a metallochaperone of the Cus complex has been shown to bind metal via a primarily three-coordinate metal binding site (Loftin et al., 2007) and directly transfers the metal ion to the periplasmic component, CusB (Bagai et al., 2008). Here, Cu(I) is coordinated with two sulfurs from M47 and M49 and a nitrogen from H36, with W44 capping the metal site. These methionine residues in CusB and CusF are essential in the extrusion of copper and silver from the periplasm to the extracellular space.
To determine the prevalence of these metal binding motifs, BLAST analysis was performed against all sequenced γ-proteobacterial genomes (Altschul et al., 1990). The number of sequences that contained these specific metal binding motifs is shown in Figure 1. All orders within the γ-proteobacteria class, except one, Pasteurellaceae, contain genes encoding CusB and CusF-like proteins with the metal binding motifs. Interestingly, when performing BLAST analysis on the membrane fusion protein GesA, no highly conserved residues were found. Consequently, the narrow substrate specificity for the CusCFBA complex may be attributed to the conserved residues for metal binding in CusB and CusF.
Figure 1.
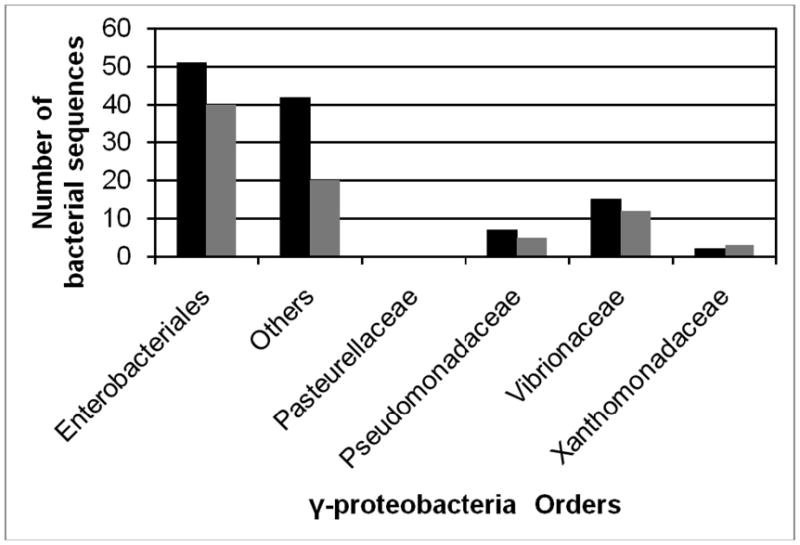
Prevalence of CusB and CusF-like proteins containing metal-binding motifs among γ-proteobacteria sequences.
Black bars represent CusB-like proteins with metal binding motif, M21M36M38. Grey bars represent CusF-like proteins with metal binding motif, W36H44M47M49.
Analysis of CusA showed that it belongs specifically to a group of efflux pumps responsible for the extrusion of heavy metals. CusA shares high sequence identity to SilA (Salmonella typhimurium, 86% identity), S0480 (Photobacterium profundum, 56% identity), and CebA (Legionella pneumophila, 46% identity). All four proteins possess three methionines that may be responsible for copper/silver binding and export. Interestingly, the three essential methionines present in CusA (Franke et al., 2003) are located in a periplasmic cleft shown to be important for substrate binding and function in AcrB (Takatsuka & Nikaido, 2007).
ClustalW alignments showed that GesB belongs to the class of RND proteins containing MexQ (Pseudomonas aeruginosa, 69% identity), MexF (Pseudomonas aeruginosa, 62% identity), BpeF (Burkholderia mallei, 59% identity), SdeB (Serratia marcescens, 55% identity), and LmxF (Legionella pneumophila, 41% identity). Both MexQ and MexF export macrolides, biocides, fluoroquinolones, tetracycline, and chloramphenicol (Mima et al, 2007). SdeB is known to pump fluoroquinolones (Begic and Worobec, 2008). Substrates of BpeF are chloramphenicol and trimethoprim (Kumar et. al, 2006).
Further analysis of GesB showed that it may possess methionine residues capable of coordinating with metals. Like MexB of Pseudomonas aeruginosa (Guan et. al, 1999), GesB (42% identity) has two periplasmic loops which interact with substrates. Within loop 2 of GesB (residues 567–881) resides three Met residues, M636, M639, and M864, and a potential metal ligand H826. Both H826 and M864 are conserved in proteins with high sequence identity to GesB, MexQ, and MexF, while M636 and M639 are conserved only in proteins with high sequence identity to GesB and MexQ. GesB, MexQ, and MexF have greater than 62% sequence identity to each other, which is higher than the CusA homologues stated above. As gold lies within the same transition metal group as copper and silver (Group IB), it is expected that efflux will occur through interaction with metal-coordinating residues such as methionine and histidine, although the exact pathway is yet to be determined. In Salmonella gesABC is adjacent to an operon encoding a Cu(I)-translocating P-type ATPase and a CueR-like regulator. Similarly, a GesB homolog (RPD_2310) in Rhodopseudomonas palustris is encoded adjacent to a GesA homolog (RPD_2311) and a CueR-regulated Cu(I)-translocating P-type ATPase and a putative Cu(I) chaperone (RPD_2307, RPD_2308 and RPD_2309). In contrast, GesB-like proteins are encoded adjacent to genes encoding putative Cd(II), Zn(II), and Pb(II)-translocating P-type ATPases in Pseudomonas aeruginosa LESB58 (CadA is PLES_26261; GesB is PLES_26281), Diaphorobacter sp. TPSY (CadA is Dtpsy_1151; GesB is Dtpsy_1153) and Shewanella sp. W3-18-1 (CadA is Sputw3181_1126; GesB is Sputw3181_1130). These examples illustrate that the GesABC system is possibly not the only RND-type complex related to the broader MexQ family involved in efflux of metals. However, at this time the substrate range of these related transporters is not known and awaits further studies.
Conclusions
The extended substrate spectrum of two metal-exporting RND systems was determined. The gold-transporting GesAB system was confirmed to have a broad substrate spectrum with chloramphenicol identified as an additional substrate. In contrast, CusCFBA had a narrow substrate spectrum transporting Cu(I) and Ag(I) almost exclusively. Three conserved residues in these metal exporters might be responsible for substrate recognition and specificity.
Supplementary Material
Figure S1. Biolog plots for dinitrobenzene (top), dinitrophenol (middle), and ethionamide (bottom). The absorbance of the reduced tetrazolium dye was plotted versus time of exposure. E. coli strain W4680AD containing pCusCFBA (dashed) grew at a faster rate than the E. coli strain W4680AD containing the control vector pGEM-T (solid) for all three chemicals.
Figure S2. Growth of E. coli strains W4680AD (top), W4680AE (middle), and 5X RND (bottom) expressing pCusCFBA (dashed line) or the control vector pGem-T (solid line) in liquid media containing different concentrations of dinitrobenzene.
Figure S3. Biolog results for chlorquinaldol (top), chloramphenicol (middle), and dichlofluanid (bottom). E. coli strain W4680AD containing pGesAB (dashed) grew at a faster rate than the E. coli strain W4680AD containing the control vector pUH21(solid) for all three chemicals.
Figure S4. Growth of E. coli strains W4680AD (top), W4680AE (middle), and 5X RND (bottom) harboring pGesAB (dashed line) or pUH21 (solid line) in liquid media containing different concentrations of crystal violet.
Acknowledgments
We greatly appreciate the strains and plasmids supplied by our colleagues. Helen Zgurskaya provided the E. coli deletion strains W4680AD and W4680AE, as well as valuable correspondence. The deletion strain E. coli 5X RND and plasmid pCusCFBA were supplied by Dietrich Nies. Fernando Soncini provided plasmids pUH21 and pGesAB. E.K. is a scholar of the Alfred P. Sloan Foundation. This work was supported by National Institutes of Health Grant GM079192 to M.M.M. and C.R.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Blasic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bachmann BJ. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagai I, Liu W, Rensing C, Blackburn NJ, McEvoy MM. Substrate-linked Conformational Change in the Periplasmic Component of a Cu(I)/Ag(I) Efflux System. J Biol Chem. 2007;282(49):35695–35702. doi: 10.1074/jbc.M703937200. [DOI] [PubMed] [Google Scholar]
- Bagai I, Rensing C, Blackburn NJ, McEvoy MM. Direct Metal Transfer between Periplasmic Proteins Identifies a Bacterial Copper Chaperone. Biochemistry. 2008;47(44):11408–11414. doi: 10.1021/bi801638m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova N, Nikaido H. The BaeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J Bacteriol. 2002;184:4168–4176. doi: 10.1128/JB.184.15.4168-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begic S, Worobec EA. The role of the Serratia marcescens SdeAB multidrug efflux pump and TolC homologue in fluoroquinolone resistance studied via gene-knockout mutagenesis. Microbiology. 2008;154:454–61. doi: 10.1099/mic.0.2007/012427-0. [DOI] [PubMed] [Google Scholar]
- Bochner BR, Gadzinski P, Panomitros E. Phenotype MicroArrays for High-Throughput Phenotypic Testing and Assay of Gene Function. Genome Res. 2001;11:1246–1255. doi: 10.1101/gr.186501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR. New technologies to Assess Genotype-Phenotype Relationships. Nature Rev. 2003;4:309–314. doi: 10.1038/nrg1046. [DOI] [PubMed] [Google Scholar]
- Bohnert JA, Schuster S, Fähnrich E, Trittler R, Kern WV. Altered spectrum of multidrug resistance associated with a single point mutation in the Escherichia coli RND-type MDR efflux pump YhiV (MdtF) J of Antimicrobial Chemotherapy. 2007;59(6):1216–1222. doi: 10.1093/jac/dkl426. [DOI] [PubMed] [Google Scholar]
- Elkins CA, Nikaido H. Substrate Specificity of the RND-Type Multidrug Efflux Pumps AcrB and AcrD of Escherichia coli Is Determined Predominately by Two Large Periplasmic Loops. J Bacteriol. 2002;184(23):6490–6498. doi: 10.1128/JB.184.23.6490-6498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke S, Grass G, Nies DH. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology. 2001;147(4):965–972. doi: 10.1099/00221287-147-4-965. [DOI] [PubMed] [Google Scholar]
- Franke S, Grass G, Rensing C, Nies DH. Molecular Analysis of the Copper-Transporting Efflux System CusCFBA of Escherichia coli. J Bacteriol. 2003;185(13):3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Rensing C. Genes Involved in Copper Homeostasis in Escherichia coli. J Bacteriol. 2001;183(6):2145–2147. doi: 10.1128/JB.183.6.2145-2147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L, Ehrmann M, Yoneyama H, Nakae T. Membrane Topology of the Xenobiotic-exporting Subunit, MexB, of the MexA, B-OprM Extrusion Pump in Pseudomonas aeruginosa. J Biol Chem. 1999;274(15):10517–10522. doi: 10.1074/jbc.274.15.10517. [DOI] [PubMed] [Google Scholar]
- Higgins D, Thompson J, Gibson T, Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nuc Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittleson JT, I, Loftin R, Hausrath AC, Engelhardt KP, Rensing C, McEvoy MM. Periplasmic metal-resistance protein CusF exhibits high affinity and specificity for both CuI and AgI. Biochemistry. 2006;45(37):11096–102. doi: 10.1021/bi0612622. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G, Tikhonova EB, Zgurskaya HI. Fitting Periplasmic Membrane Fusion Proteins to Inner Membrane Transporters: Mutations That Enable Escherichia coli AcrA To Function with Pseudomonas aeruginosa MexB. J Bacteriology. 2007;190(2):691–698. doi: 10.1128/JB.01276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chua KL, Schweizer HP. Method for Regulated Expression of Single-Copy Efflux Pump Genes in a Surrogate Pseudomonas aeruginosa Strain: Identification of the BpeEF-OprC Chloramphenicol and Trimethoprim Efflux Pump of Burkholderia pseudomallei 1026b. Antimicrob Agents and Chemo. 2006;50(10):3460–3463. doi: 10.1128/AAC.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftin IR, Franke S, Blackburn NJ, McEvoy MM. Unusual Cu(I)/Ag(I) coordination of Escherichia coli CusF as revealed by atomic resolution crystallography and X-ray absorption spectroscopy. Protein Sci. 2007;16(10):2287–2293. doi: 10.1110/ps.073021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya O, Zgurskaya HI, Totrov M, Watkins WJ. Waltzing transporters and ‘the dance macabre’ between humans and bacteria. Nat Rev Drug Disc. 2007;6(1):56–65. doi: 10.1038/nrd2200. [DOI] [PubMed] [Google Scholar]
- Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol. 2007;189(21):7600–7609. doi: 10.1128/JB.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pos KM. Drug transport mechanism of the AcrB efflux pump. Biochim et Biophys Acta – Proteins & Proteomics. 2009;1794(5):782–793. doi: 10.1016/j.bbapap.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Micro Rev. 27(2–3):313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Nishino K, Latifi T, Groisman EA. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol Micro. 2006;59(1):126–141. doi: 10.1111/j.1365-2958.2005.04940.x. [DOI] [PubMed] [Google Scholar]
- Pontel LB, Pérez Audero ME, Espariz M, Checa SK, Soncini FC. GolS controls the response to gold by the hierarchical induction of Salmonella-specific genes that include a CBA efflux-coding operon. Mol Micro. 2007;66(3):814–825. doi: 10.1111/j.1365-2958.2007.05963.x. [DOI] [PubMed] [Google Scholar]
- Soncini FC, Vescovi EG, Groisman EA. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177(15):4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuka Y, Nikaido H. Site-directed disulfide cross-linking shows that cleft flexibility in the periplasmic domain is needed for the multidrug efflux pump AcrB of Escherichia coli. J Bacteriol. 2007;189(23):8677–8684. doi: 10.1128/JB.01127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Biolog plots for dinitrobenzene (top), dinitrophenol (middle), and ethionamide (bottom). The absorbance of the reduced tetrazolium dye was plotted versus time of exposure. E. coli strain W4680AD containing pCusCFBA (dashed) grew at a faster rate than the E. coli strain W4680AD containing the control vector pGEM-T (solid) for all three chemicals.
Figure S2. Growth of E. coli strains W4680AD (top), W4680AE (middle), and 5X RND (bottom) expressing pCusCFBA (dashed line) or the control vector pGem-T (solid line) in liquid media containing different concentrations of dinitrobenzene.
Figure S3. Biolog results for chlorquinaldol (top), chloramphenicol (middle), and dichlofluanid (bottom). E. coli strain W4680AD containing pGesAB (dashed) grew at a faster rate than the E. coli strain W4680AD containing the control vector pUH21(solid) for all three chemicals.
Figure S4. Growth of E. coli strains W4680AD (top), W4680AE (middle), and 5X RND (bottom) harboring pGesAB (dashed line) or pUH21 (solid line) in liquid media containing different concentrations of crystal violet.


