Abstract
Class I cardiac antiarrhythmic drugs, e.g. lidocaine, mexiletine, flecainide, quinidine, and procainamide, continue to play an important role in the therapy of cardiac arrhythmias because of the presence of use-dependent block. Lidocaine, as well as related drugs like mepivacaine, bupivacaine, and cocaine, also belong to the class of medications referred to as local anesthetics. In this review we will consider lidocaine as the prototypical antiarrhythmic drug because it continues to be widely used both as an antiarrhythmic drug (first used as an antiarrhythmic drug in 1950) as well as a local anesthetic agent. Both of these clinical uses depend upon block of sodium current (INa), but it is the presence of use-dependent INa block, i.e. an increasing amount of block at faster heart rates, that allows a local anesthetic agent to be a useful antiarrhythmic drug. Although many early studies investigated the action of antiarrhythmic drugs on Na currents, the availability of site-directed mutant Na channels has allowed for major advances in understanding their mechanisms of action based upon molecular conformations of the Na channel.
Keywords: antiarrhythmic drug, voltage clamp, gating currents, lidocaine, local anethestic
The Voltage-Gated Na Channel Protein
The ~2000 amino acid Na channel α-subunit contains the channel's pore and gating machinery and all of the drug and toxin interaction sites identified to date (Catterall 2000). It has four similar domains (DI-DIV), each with six transmembrane segments (S1-S6), analogous to the typical K channel's four separate subunits. Although there are no currently available crystal structures for voltage-gated Na channels, they are thought to have an overall structural organization similar to the K channels that have been crystallized. The general pore organization with S6 segments forming the walls of the inner pore (Jiang et al. 2002, Uysal et al. 2009) is likely highly conserved, although available mutagenesis data suggest a more superficial outer vestibule with side chains rather than backbone carbonyls forming the selectivity filter (Lipkind and Fozzard 2000). The location of the voltage sensors (S4 segments) towards the periphery of the K channel interacting with lipid (Long et al. 2005) is likely to apply to a wide range of channels, including Na channels, although differences in the sequences between the four domains in Na channels raise the possibility of specialization of channel function between domains.
Even though the actual voltage-dependent conformational change in the S4 voltage sensor helical structure remains controversial, the crystal structures of voltage-gated K channels (e.g. Long et al. 2005), suggest that depolarization causes the S4's to move outward, thereby lifting the S4-S5 amphipathic helical linkers which permit the four intracellular ends of the S6 helices to hinge outward to open the pore. It is very likely that the S4-S5 linkers in Na channels also couple the voltage-sensor domain containing the S4's to the pore domain containing the S6's. After movement of the S4's K channels inactivate in a voltage-independent fashion by either N-type (Hoshi et al. 1990) or C-type (by rearrangement of the pore, Cordero-Morales et al. 2006), whereas fast inactivation of the Na channel involves the DIII-DIV intracellular interdomain linker, which forms the putative inactivation lid with its IFM motif (West et al. 1992). The receptor for this inactivation lid is not well defined, suggesting that it represents several different parts of the channel such as S6's and the S4-S5 linkers.
Although many details of the coupling of the voltage-sensor S4 segments to pore opening and inactivation for Na channels remain unresolved, the S4 in domain IV (DIVS4) has been shown to have a specialized role in coupling fast inactivation to voltage-dependent channel opening (Hanck and Sheets 1995, Yang and Horn 1995, Sheets et al. 1999, Chanda and Bezanilla 2002). Inhibition of the DIVS4 movement by site-3 toxins has determined that about 30% of maximal gating charge is associated with the open-to-inactivated state transition (Sheets et al. 1999, Campos et al. 2008), and the movement of the DIVS4 is slow, so that it largely moves after channels open (Sheets and Hanck 1995, Chanda and Bezanilla 2002). This contrasts with Shaker K channels, where most, if not all, of the gating charge is associated with channel activation, with little or no gating charge movement after channel opening.
The inner vestibule of the Na channel pore, formed by the four S6 segments, has been shown to contain the high affinity use-dependent binding site for local anesthetics and antiarrhythmic drugs (LA's). In particular, mutational studies have identified a cluster of residues in the DIII and DIV S6's that are important for block, including DIVS6-Phe1759 and DIVS6-Try1766, and DIIIS6-Leu1461 (numbering based on NaV1.5a) (Ragsdale et al. 1994, Li et al. 1999, Nau et al. 2003). Phe1759 is clearly the most important, because its mutation to a non-aromatic residue abolishes use-dependent block (Ragsdale et al. 1994, McNulty et al. 2007, Ahern et al. 2008, Hanck et al. 2009). Across the three most commonly studied Na channel isoforms, NaV1.2 (neuronal), NaV1.4 (skeletal), and NaV1.5 (cardiac), mutational effects on LA block have been shown to be, in general, remarkably similar or identical.
Factors That Affect Block of Sodium Current by Antiarrhythmic Drugs
One important component in the action of antiarrhythmic drugs that has long been appreciated is a voltage-dependent change in the affinity of the drug binding site, i.e. the channel is a modulated receptor (Hille 1977, Hondeghem and Katzung 1977). In addition, restricted access/egress to binding site(s) can also contribute to drug action, a phenomenon that has been called the guarded receptor model (Starmer and Hollett 1985). This is best illustrated by the action of the permanently-charged quaternary amines such as QX-222 or QX-314, which, in general, must be applied to the intracellular space of Na channels in order to block the channel (Butterworth and Strichartz 1990), and consequently they are not useful as antiarrhythmic drugs. They access the binding site while the channel is open, which contributes to their apparent use-dependent block. One example of a clinically-used drug with significant restricted access/egress is flecainide, with its very high pKa of 9.3, so that only about 1% is uncharged at pH 7.4 (Liu et al. 2003). These examples stand in contrast to the prototypical antiarrhythmic drug, lidocaine, which has a pKa of 7.7-7.9, so that about 30% of the drug is uncharged at a pH of 7.4. It, therefore, can more readily access/egress the channel when it is closed.
In addition to considerations of access/egress, LA drugs have been shown to have three mechanisms of block: (1) block of the open/inactivated state (voltage-sensor block), (2) block of the closed state (lipophilic block), and (3) fast-flicker block of the open channel. Of these, only the first has a high enough affinity to be potentially useful as an antiarrhythmic drug (and probably for antiepileptic/anticonvulsant drugs as well) owing to its use-dependent action. Block of the closed state has an EC50 in the mM range, and so, while useful for producing local anesthesia, is unlikely to be useful to control arrhythmias. Fast-flicker block is unlikely to be clinically relevant for either antiarrhythmic or local anesthetic therapy because of its very large EC50 ( ~5 mM at a membrane potential of 0 mV for lidocaine, Zamponi et al. 1993). Flicker-block results from positively charged drug interacting with the intracellular side of the selectivity filter in a voltage-dependent manner to block single channel conductance. Consequently, neutral local anesthetic drugs such as benzocaine are unable to cause flicker block.
High Affinity Block of the Open/Inactivated State Requires Voltage-Sensor Involvement (Voltage-Sensor Block)
Use-dependent block, which is the clinically relevant block for treatment of tachyarrhythmias, is a high affinity block that requires either repetitive or prolonged depolarizations. It results from voltage-dependent changes in channel conformation, and so it obligatorily implies involvement of the S4 segments. This type of block, voltage-sensor block, is clinically important in the treatment of arrhythmias and for some forms of antiepileptic treatment, because of its ability to distinguish between rapid rhythms (i.e. rapid depolarizations) from normal depolarization rates. Two conditions are required for use-dependent block: 1) an increased on-rate when channels gate, i.e. the voltage-sensor effect, and 2) a sufficiently slow off-rate so that block of Na channels accumulates. Benzocaine, which is not use-dependent, illustrates the need for both conditions; it has a voltage-dependent change in on-rate, but it has a fast off-rate that does not allow for accumulation of block, (e.g. Schwarz et al. 1977, Hanck et al. 2009).
Direct evidence for voltage-sensor participation in high affinity block by antiarrhythmic drugs such as lidocaine comes from studies measuring gating currents, the small non-linear electrical signals arising from the transmembrane movement of the four positively charged S4 segments. High affinity block of sodium current (INa) by lidocaine has an EC50 of 5-20 μM (Grant et al. 1989), and it is associated with alteration of the gating charge-voltage relationship characterized by a 38% reduction of maximal gating charge with the appearance of additional gating charge at negative test potentials (e.g. Cahalan and Almers 1979, Hanck et al. 1994, Hanck et al. 2000). These changes have been shown to result from stabilization of the DIIIS4 in a depolarized position and partial inhibition of movement of DIVS4 with an alteration of its voltage dependence (Sheets and Hanck 2003, Muroi and Chanda 2009). It is important to note that use-dependent block of INa by lidocaine (Hanck et al. 2000) or by the quaternary amine, QX-222 (Hanck et al. 1994), is directly proportional to the reduction in maximal gating charge as expected if the two effects occurred simultaneously, resulting from the same event. Furthermore, the EC50's for lidocaine block of INa and for reduction in gating charge were both in the 20 μM range, and comparable to lidocaine's clinically therapeutic serum levels. Additional evidence for S4 participation in high-affinity, use-dependent block by lidocaine comes from studies in which the S4's in DIII and DIV were artificially stabilized in an outward conformation (Sheets and Hanck 2007). The EC50 for lidocaine block decreased to 28 μM after stabilization of both S4's, which is comparable to the value previously reported for high-affinity use-dependent block by lidocaine during a fast pulse train (10 Hz) in native cardiac Na channels recorded under similar experimental conditions (Hanck et al. 2000).
If the crystal structure of the voltage-gated Kv1.2 channel (Long et al. 2005) is applicable to the Na channel, it is the S4-S5 linker that couples the voltage-sensor domain to the pore domain. Depolarization would be expected to result in outward translocation of the S4's, thereby permitting the S6 segments to hinge open leading to channel activation and current flow. Recent Na channel studies suggest that it is the hinge regions in the S6's that play an important role in coupling the rigid S4 helices to the S6 segments (Muroi et al. 2010). Movement of the Na channel's S4 segments then creates a channel conformation within the S6 segments, in particular those in DIII and DIV, which make the most important contributions to the high-affinity LA binding site. Consequently, the movement of the S4's in DIII and DIV during depolarization would be expected to enhance the binding of LA's by conformational changes in the S6 segments. During repolarization the voltage sensors in DIII and DIV have the opposite effect; their movement back to an inward, rested position biases the S6 segments toward a closed position, thereby decreasing lidocaine affinity.
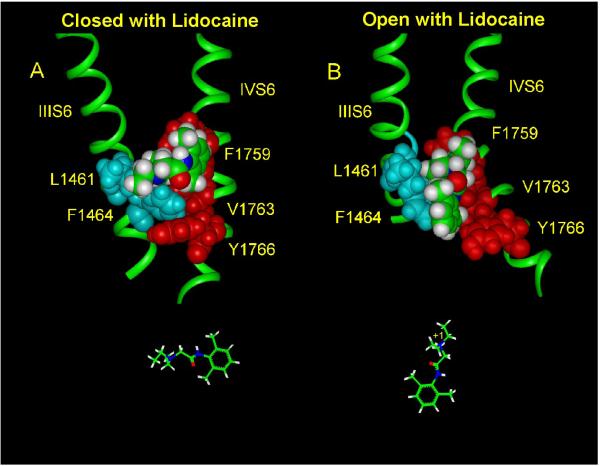
Based on the crystal structure of the open K channel MthK (Jiang et al. 2002) a homology model of the open Na channel pore with lidocaine docked into the binding site formed by the S6 segments has been developed (Lipkind and Fozzard 2005, Hanck et al. 2009). Bulk water is predicted to fill the large inner vestibule of the open Na channel pore, thereby favoring protonation of the alkylamino head of lidocaine, and allowing the charged amino group to make an electrostatic π-electron interaction with the aromatic ring of Phe1759 in the DIVS6 (Figure 1). Simultaneously, the alkylamino head can also dock with the Leu1461 in the DIIIS6 because these two residues are predicted to be separated only by a narrow cavity (about 6 Å in width) even though they are in different S6 segments. Such a conformation would allow coupling of lidocaine to both domains III and IV. Tikhonov and Zhorov (2007), using a slightly different model, have found similar orientations. The non-polar tail of lidocaine, i.e. its alkyl chains and aromatic ring, then interacts with Tyr1766 in the DIVS6, thereby achieving dense packing of lidocaine vertically with the open channel between DIIIS6 and DIVS6.
Figure 1.
Proposed location for lidocaine in the closed (A) and open/inactivated (B) Na channel pore. Lidocaine is shown as space-filled (green represents C atoms, blue represents N atoms, and red represents O atoms) docking in the interface of DIIIS6 and DIVS6 (both green ribbons). Amino acid residues making contacts with lidocaine are shown by blue (DIIIS6) and red (DIVS6) colors. Lidocaine is predicted to be oriented horizontally with its aromatic ring aligned with Phe1759 in the closed channel (shown below panel A). In contrast, the charged, tertiary amine of lidocaine is predicted to be oriented vertically in the open/inactivated channel (shown below panel B). (Adapted from Hanck et al. 2009).
Closed-State Block Represents Neutral Interactions with Channel Walls (Lipophilic Block)
The final type of block, which is experimentally associated with the closed state, might better be called lipophilic block because it most likely represents a neutral drug interaction with the closed configuration of the channel (Figure 1). This lower affinity block is experimentally determined as the block of INa when the membrane potential is held at very negative potentials that bias Na channels to a closed conformation that is fully available for opening, i.e. channels occupy rested/closed states presumably represented by a channel conformation with the S6's in a closed bundle conformation with the voltage-sensors “retracted” in a hyperpolarized position. In contrast to the strong association between Phe1759 and voltage-sensor block of Na channels, it is likely that the site for low-affinity lipophilic block is more diffuse and involves multiple residues in the inner pore segments, because point substitutions with an Ala or Cys in the S6 helices show only small (2-fold) changes in affinity of closed-state block (Ragsdale et al. 1994, Nau et al. 2003). A homology model of the closed Na channel pore (Hanck et al. 2009) can accommodate neutral LA's, and our current belief is that the neutral forms of antiarrhythmic drugs interact with non-polar residues that form the inner vestibule to produce lipophilic block. The exact mechanism of decreased INa by lipophilic block remains unclear. It is possible that antiarrhythmic and local anesthetic drugs may produce lipophilic block by stabilizing the bundle crossing of the S6 segments similar to a “hydrophobic seal” mechanism found in voltage-gated Shaker K channels, where substitution with Trp for a Val at the bundle crossing inhibits channel opening (Kitaguchi et al. 2004).
Tonic Block Contains Contributions From Both Lipophilic and Voltage Sensor Bock
Experimentally, closed-state block is often approximated by tonic block, the measurement of INa in a single depolarization after holding the membrane potential very negatively for a relatively long time. It is important to note that measurement of tonic block typically represents a combination of both lipophilic and voltage-sensor block, and it may have a great variability depending upon the Na channel isoform, the specific drug's affinity (on- and off-rates) and the membrane potential's effects on intrinsic channel kinetics.
Because cell preparations do not tolerate very negative holding membrane potentials, it is better to isolate lipophilic block from voltage-sensor block by using a mutant channel like F1759K, which has no high-affinity voltage-sensor block (Hanck et al. 2009). In this mutant Na channel, lipophilic block is found to have a EC50 of ~2 mM. Such a large EC50 would not expected to be beneficial in the therapy of arrhythmias, although it could contribute to local anesthetic action, where locally high concentrations may be achieved.
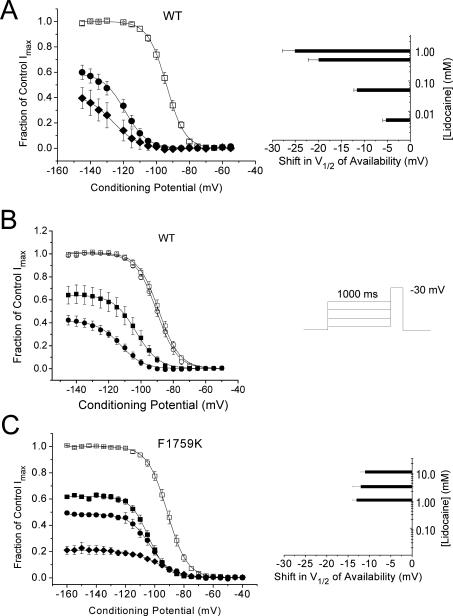
The dual contributions of voltage-sensor and lipophilic block can be appreciated by the classic effects of antiarrhythmic drugs on steady-state voltage-dependent Na channel availability curves (Figure 2). One of the hallmarks of antiarrhythmic drugs is the concentration-dependent shift in the V½ of the Na channel availability curve as the conditioning potential becomes more positive. At any given concentration of lidocaine, there is a progressively higher drug affinity as the magnitude of voltage-sensor block increases with more positive conditioning potentials producing a leftward (negative) shift in V½. As the lidocaine concentration increases, the change in V½ becomes more prominent (Figure 2A, right), because block of INa reflects a combination of lipophilic and an increasing amount of voltage-sensor block. The effect of changing the membrane holding potential (Vhp) on lidocaine block is illustrated in Figure 2B, where steady state voltage-dependent availability was determined from two different, but quite negative, holding potentials. In the absence of drug there is no difference between voltage-dependent availability measured from these two different holding potentials. However, in the presence of lidocaine, there is a greater reduction in INa accompanied by a larger shift in V½ when the holding potential is more positive. In the case in which tonic block consists only of lipophilic block, as in the mutant channel F1759K, there is no concentration-dependent change in the V½ (i.e. no voltage-sensor block), and the reduction in magnitude is proportional across the voltage range with a much higher EC50 (2.2 mM).
Figure 2.
Steady-state voltage-dependent Na channel availability curves for cells expressing NaV1.5a (WT) (A) and (B), and for F1759K mutant channels (C). The voltage protocol is shown as an insert to (B). Summary data for Na channel availability in WT (A) channels in control (□) and after exposure to lidocaine (0.5 mM (•), 1 mM (◆) from a holding potential (Vhp) of −150 mV. The right panel of (A) shows the shifts in V½ of availability for cells exposed to four concentrations of lidocaine including cells exposed to 5 μM and 50 μM, concentrations at which little reduction in maximal INa was evident. (B) Data showing the effect of holding potential on tonic block in WT cells held at −135 mV (•) or -155 mV (■) exposed to 0.5 mM lidocaine. In control solutions steady-state voltage-dependent availability (○ and □) were not different. However, in the presence of lidocaine the amount of tonic block from a Vhp of −155 mV (■) was 0.36 while from a Vhp of −135 mV (•) it was 0.58, an amount almost twice as large. Furthermore, the leftward shift in V½ was 10 mV greater with Vhp of −135 mV compared to −155 mV. (C) Data for cells expressing F1759K exposed to 1 mM (■), 3 mM (•), and 10 mM (◆). The right panel shows that the shift in V½ is independent of lidocaine concentration. The ED50's calculated from the fitted asymptotes of the Na channel availability relationships were 0.78 mM for WT (A), and 2.2 mM for F7159K(C). (Adapted, in part, from (Hanck et al. 2009).
Future Directions
Although the general basis of basis of use-dependent block of sodium channels by antiarrhythmic drugs may be understood, important questions remain. The role of fast inactivation in antiarrhythmic drug affinity remains poorly understood. It has long been known that removal of fast inactivation markedly decreases drug affinity (Cahalan 1978). When voltage sensors involved in voltage sensor block were artificially held outward, the highest affinity binding could only be achieved when the inactivation lid was intact (Sheets and Hanck 2007). Similarly, the structural basis and the role of slow inactivation in antiarrhythmic drug binding requires better understanding. Additional studies to quantify the contributions of the specific residues in both the high-affinity binding site responsible for voltage-sensor block and the lower-affinity lipophilic binding site are needed to better understand how to design drugs to target the high-affinity site while minimizing lipophilic block. Mathematical models of arrhythmias using multicellular and/or whole heart models that incorporate specific etiologies of arrhythmias (such as chronic scarring due to myocardial infarction, long QT syndrome or Brugada syndrome) should lead to the generation of an ideal “virtual antiarrhythmic drug” that would not have proarrhythmic properties. Recent progress has been made in the construction of such mathematical models, (e.g. Clancy et al. 2007, Rudy et al. 2008). Understanding the energies involved in the binding of current antiarrhythmic drugs at the molecular level in combination with the binding parameters from an “ideal” drug should help lead to the smart design of new antiarrhythmic drugs. It is likely that many of the findings of antiarrhythmic drug binding to cardiac Na channels can also be applied to understanding the action of anticonvulsant medications targeted to brain Na channels in the therapy of seizures and, perhaps, in control of pain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ahern CA, Eastwood AL, Dougherty DA, Horn R. Electrostatic contributions of aromatic residues in the local anesthetic receptor of voltage-gated sodium channels. Circ Res. 2008;102:86–94. doi: 10.1161/CIRCRESAHA.107.160663. [DOI] [PubMed] [Google Scholar]
- Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia - a Review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- Cahalan MD. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophysical Journal. 1978;23:285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979;27:39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FV, Chanda B, Beirao PS, Bezanilla F. Alpha-scorpion toxin impairs a conformational change that leads to fast inactivation of muscle sodium channels. J Gen Physiol. 2008;132:251–263. doi: 10.1085/jgp.200809995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Chanda B, Bezanilla F. Tracking Voltage-dependent Conformational Changes in Skeletal Muscle Sodium Channel during Activation. J Gen Physiol. 2002;120:629–645. doi: 10.1085/jgp.20028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol. 2007;292:H66–75. doi: 10.1152/ajpheart.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales JF, Cuello LG, Perozo E. Voltage-dependent gating at the KcsA selectivity filter. Nat Struct Mol Biol. 2006;13:319–322. doi: 10.1038/nsmb1070. [DOI] [PubMed] [Google Scholar]
- Grant AO, Dietz MA, Gilliam FR, Starmer CF. Blockade of cardiac sodium channels by lidocaine. Single-channel analysis. Circulation Research. 1989;65:1247–1262. doi: 10.1161/01.res.65.5.1247. [DOI] [PubMed] [Google Scholar]
- Hanck DA, Makielski JC, Sheets MF. Kinetic effects of quarternary lidocaine block of cardiac sodium channels: A gating current study. Journal of General Physiology. 1994;103:19–43. doi: 10.1085/jgp.103.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanck DA, Makielski JC, Sheets MF. Lidocaine alters activation gating of cardiac Na channels. Pflugers Arch. 2000;439:814–821. doi: 10.1007/s004249900217. [DOI] [PubMed] [Google Scholar]
- Hanck DA, Nikitina E, McNulty MM, Fozzard HA, Lipkind GM, Sheets MF. Using lidocaine and benzocaine to link sodium channel molecular conformations to state-dependent antiarrhythmic drug affinity. Circ Res. 2009;105:492–499. doi: 10.1161/CIRCRESAHA.109.198572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanck DA, Sheets MF. Modification of inactivation in cardiac sodium channels: Ionic current studies with Anthopleurin-A toxin. J Gen Physiol. 1995;106:601–616. doi: 10.1085/jgp.106.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. Journal of General Physiology. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem LM, Katzung BG. Time- and voltage- dependent interaction of antiarrhythmic drugs with cardiac sodium channels. Biochimica et Biophysica Acta. 1977;472:373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Kitaguchi T, Sukhareva M, Swartz KJ. Stabilizing the closed S6 gate in the Shaker Kv channel through modification of a hydrophobic seal. J Gen Physiol. 2004;124:319–332. doi: 10.1085/jgp.200409098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Galue A, Meadows L, Ragsdale DS. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Mol Pharmacol. 1999;55:134–141. doi: 10.1124/mol.55.1.134. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. KcsA crystal structure as framework for a molecular model of the Na(+) channel pore. Biochemistry. 2000;39:8161–8170. doi: 10.1021/bi000486w. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68:1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na+ channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- McNulty MM, Edgerton GB, Shah RD, Hanck DA, Fozzard HA, Lipkind GM. Charge at the lidocaine binding site residue Phe-1759 affects permeation in human cardiac voltage-gated sodium channels. J Physiol. 2007;581:741–755. doi: 10.1113/jphysiol.2007.130161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Chanda B. Local anesthetics disrupt energetic coupling between the voltage-sensing segments of a sodium channel. J Gen Physiol. 2009;133:1–15. doi: 10.1085/jgp.200810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Arcisio-Miranda M, Chowdhury S, Chanda B. Local anesthetics disrupt energetic coupling between the voltage-sensing segments of a sodium channel. Nat Struct Mol Biol. 2010;17:230–237. doi: 10.1038/nsmb.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau C, Wang SY, Wang GK. Point mutations at L1280 in Nav1.4 channel D3-S6 modulate binding affinity and stereoselectivity of bupivacaine enantiomers. Mol Pharmacol. 2003;63:1398–1406. doi: 10.1124/mol.63.6.1398. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Rudy Y, Ackerman MJ, Bers DM, Clancy CE, Houser SR, London B, et al. Systems approach to understanding electromechanical activity in the human heart: a national heart, lung, and blood institute workshop summary. Circulation. 2008;118:202–1211. doi: 10.1161/CIRCULATIONAHA.108.772715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz W, Palade PT, Hille B. Local anesthetics: effect of pH on use-dependent block of sodium channels in frog muscle. Biophysical Journal. 1977;20:343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Voltage-dependent open-state inactivation of cardiac sodium channels: Gating currents studies with Anthopleurin-A toxin. J Gen Physiol. 1995;106:617–640. doi: 10.1085/jgp.106.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Molecular action of lidocaine on the voltage sensors of sodium channels. J Gen Physiol. 2003;121:163–175. doi: 10.1085/jgp.20028651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Hanck DA. Outward stabilization of the S4 segments in domains III and IV enhances lidocaine block of sodium channels. J Physiol. 2007;582:317–334. doi: 10.1113/jphysiol.2007.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MF, Kyle JW, Kallen RG, Hanck DA. The Na channel voltage sensor associated with inactivation is localized to the external charged residues of domain IV, S4. Biophys J. 1999;77:747–757. doi: 10.1016/S0006-3495(99)76929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer CF, Hollett MD. Mechanisms of apparent affinity variation of guarded receptors. J Theor Biol. 1985;115:337–349. doi: 10.1016/s0022-5193(85)80196-x. [DOI] [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophys J. 2007;93:1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal S, Vasquez V, Tereshko V, Esaki K, Fellouse FA, Sidhu SS, et al. Crystal structure of full-length KcsA in its closed conformation. Proc Natl Acad Sci U S A. 2009;106:6644–6649. doi: 10.1073/pnas.0810663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+ -channel inactivation. Proc Natl Acad Sci U S A. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Horn R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Doyle DD, French RJ. Fast lidocaine block of cardiac and skeletal muscle sodium channels: One site with two routes of access. Biophysical Journal. 1993;65:80–90. doi: 10.1016/S0006-3495(93)81042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]