Abstract
Nucleotide Binding Domains (NBDs) of multidrug transporter of Candida albicans, CaCdr1p possess unique divergent amino acids in their conserved motifs. For example, NBD1 (N-terminal-NBD) possesses conserved Signature motifs while the same motif is divergent in NBD2 (C-terminal-NBD). In this study, we have evaluated the contribution of these conserved and divergent Signature motifs of CaCdr1p in ATP catalysis and drug transport. By employing site directed mutagenesis, we made three categories of mutant variants. These included mutants where all the Signature motif residues were either replaced with alanines or mutants with exchanged equipositional residues to mimic the conservancy and degeneracy in opposite domain. In addition, a set of mutants where Signature motifs were swapped to have variants with either both the conserved or degenerated entire Signature motif. We observed that conserved and equipositional residues of NBD1 and NBD2, and swapped Signature motif mutants showed high susceptibility to all the tested drugs with simultaneous abrogatation in ATPase and R6G efflux activities. However, some of the mutants displayed selective increase in susceptibility to the drugs. Notably, none of the mutant variants and WT-CaCdr1p showed any difference in drug and nucleotide binding. Our mutational analyses show that not only certain conserved residues of NBD1 Signature sequence (S304, G306 and E307) are important in ATP hydrolysis and R6G efflux but a few divergent residues (N1002 and E1004) of NBD2 Signature motif have also evolved to be functionally relevant and are not interchangeable. Taken together, our data suggest that the Signature motifs of CaCdr1p whether it is divergent or conserved, are non-exchangeable and functionally critical for ATP hydrolysis.
Keywords: ABC transporter, Nucleotide Binding Domain, Signature motif, Drug resistance, Drug transport, ATPase activity
1. Introduction
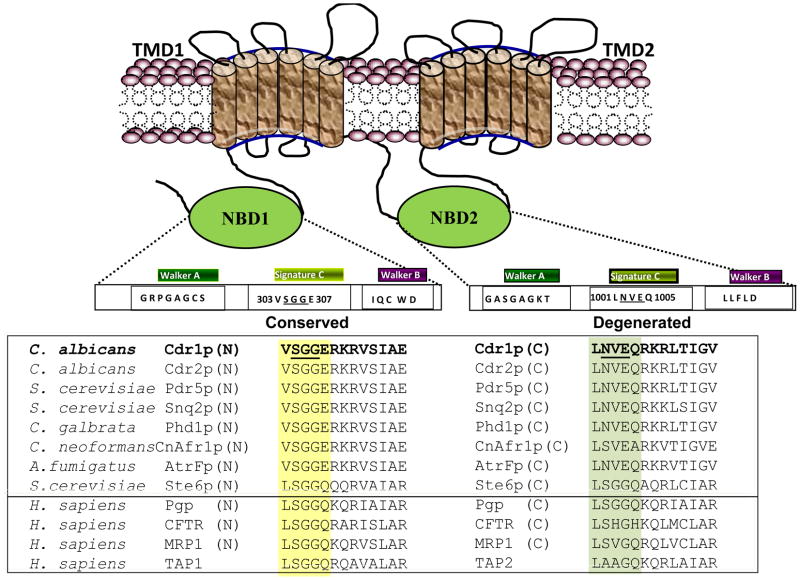
It is well established that an overexpression of multidrug transporter protein CaCdr1p, belonging to the ABC superfamily, represents one of the predominant attributes of multidrug resistance (MDR) in clinical isolates of Candida albicans [1–9]. The protein CaCdr1p is expressed as a single polypeptide of 1501 amino acids which is comprised of two Nucleotide Binding Domains (NBDs) and two Trans Membrane Domains (TMDs) [10]. Each TMD is made up of six transmembrane segments (TMS) which are involved in the formation of overlapping multiple substrates binding sites [11]. The substrates of CaCdr1p vary enormously and include structurally unrelated compounds such as azoles, lipids and steroids [4, 12]. Several lines of evidences from mammalian ABC proteins suggest that transport of substrates leads to conformation changes in the TMDs region of the protein that converts to a high affinity substrate binding site (inward facing) to low affinity binding site (outward facing) [13]. These conformation changes in the TMDs are triggered by the ATP binding and hydrolysis to the NBDs [14–15]. The NBDs are characterized primarily by the presence of several highly conserved motifs such as Walker A (GxxGxGKS/T, where “x” represents any amino acid), Walker B (hhhhD; where “h” represents any aliphatic residue) and Signature C (LSGGQQ/R/KQR) motif which couples the ATP hydrolysis to power drug extrusion [16].
We have shown that unlike other transporters of higher eukaryotes, CaCdr1p of C. albicans do not share all the consensus conserved motifs of the ABC transporters. Remarkably, in contrast to the Walker A (GRPGAGCS) and B (IQCWD) motifs of the NBD1 of CaCdr1p, which have substitution of typical critical residues that are unique to the fungal ABC transporters, it has conserved Signature motif (VSGGERKRVSIA). On the other hand, the Walker A (GASGAGKS) and B (LLFLD) motifs of the NBD2 are well conserved [10, 17–18]; however, its Signature motif (LNVEQRKRLTIGV) is degenerated (Fig. 1). The complexity and diversity of the fungal transporters have been analyzed and reveals that the evolutionary uniqueness present in PDR family is highly conserved in fungal ABC transporters [19–20]). Our group has extensively examined the functional significance of these unique substitutions present in the NBD domains of CaCdr1p. We have established that the substitution of a typical cysteine with lysine (C193K), present in equivalent position in other ABC transporters, lead to severe abrogation of ATP hydrolysis [21] with no affect on its binding. However, exchange of unique tryptophan of Walker B region of NBD1 with alanine (W326A) resulted in reduced ATP binding with no effect on its hydrolysis [18]. We also showed that substitution of well conserved aspartate with asparagine (D327N) strongly impaired the ATPase activity without affecting the ATP binding. Unlike the other non-fungal ABC transporters, aspartate (D327) in the Walker B motif of NBD1 of CaCdr1p is not involved in Mg2+ coordination and has a role in ATP catalytic cycle [22]. Thus the unique evolutionary replacements in CaCdr1p and other yeast ABC transporters are functionally indispensable [21–23].
Fig. 1.
Topology of CaCdr1p and sequence alignment of Signature motifs from various ABC transporters. The sequence alignment of Signature motif residues in NBDs with those from other nucleotide binding domains of some known ABC transporters is shown. Signature motifs of CaCdr1p are shown in bold and the conserved and divergent residues are underlined.
Similar to the ABC transporters associated with antigen processing (TAP) [24–25], cystic fibrosis transmembrane conductance regulator (CFTR) [26] and multidrug resistance protein 1 (MRP1) of humans [27], CaCdr1p presumably also forms two distinct cytosolic ATPase sites [22]. One non-canonical ATPase site comprises of three unique motifs viz degenerated Walker A and B of NBD1 and degenerated Signature motif of the NBD2 and other canonical site formed by the conserved Walker A and B of NBD2 and conserved Signature motif of NBD1. The biochemical studies of TAP revealed that the mutant with two degenerated ATPase sites show drastically reduced transport activity and suggested that the canonical ATP binding site is critical for its function [24]. Several structural and biochemical studies have shown that the Signature motifs are involved in the head to tail ATPase site formation with the Walker A and Walker B motifs of the opposite NBDs, sandwiched with two ATP molecules [26, 28–30]. The Signature motif physically contributes to the dimerization of NBDs forming two heterologous nucleotide binding pockets, interacting via hydrogen bonds with ribose and the γ-phosphate moiety of ATP [29]. A similar structure of NBDs was also deduced for a DNA repair ABC protein Rad50 [28]. Mutation in the Signature motif affects the protein function. For example, mutation of second conserved glycine residue (LSGGQ) of Signature motif abolished ATP hydrolysis in human CFTR [26]. Mutation at this position in human Pgp also leads to impaired protein function and abrogated inter-domain communication [31]. The analogous mutation made in the NBD1 Signature motif of both the a-factor transporter ScSte6p and the ScYdf1p transporter of Saccharomyces cerevisiae, drastically impaired the substrate transport [32–33].
Although, the role of Signature motifs in ATP catalysis, crosstalk between NBD and TMD and the ATP mediated drug transport has been analyzed for the mammalian ABC proteins, the role of evolutionary divergent Signature motifs of NBD of yeast ABC drug transporters remains unexplored. This is particularly important since not only yeast ABC drug transporters but also C family of human ABC transporters (ABCC) possess divergent residues in their conserved motifs [1].
This study evaluates the role of Signature motifs of the PDR subfamily of the yeast ABC transporter; CaCdr1p where NBD1 has a conserved Signature motif (VSGGE) while this motif is degenerated in NBD2 (LNVEQ). For this, we mutagenized Signature motif residues by either replacing them with alanines or replacing residues with equiposition residues of another Signature motif. We also swapped an entire motif with either a conserved or degenerated Signature motif. By employing drug susceptibility test, efflux of substrate and ATPase activity measurements, we show that the canonical or non-canonical Signature motifs in the NBDs of CaCdr1p are functionally asymmetric and critical.
2. Experimental Procedures
2.1 Materials
Ribonucleotides (ATP), protease inhibitors (PMSF, leupeptin, pepstatin A, aprotinin, TLCK, and TPCK) and drugs, cycloheximide (CYH), anisomycin (ANI), Rhodamine 6G (R6G), DTT, oligomycin, and other molecular grade chemicals, were obtained from Sigma Chemical Co. (St. Louis, MO). Oligonucleotides used in this study, as listed in Table. 1 (supplementary data), were commercially procured from Sigma Genosys, Inc. Fluconazole (FLC) was kindly provided by Ranbaxy Laboratories (New Delhi, India). Anti-GFP mono-clonal antibody and [α-32P] 8-azido ATP (15–20 Ci/mmol) were purchased from BD Biosciences Clontech (Palo Alto, CA) and Affinity Labeling Technologies, Inc. (Lexington, KY), respectively. The radio labeled [125I] IAAP (2300 Ci/mmol) was procured from Perkin-Elmer Life Sciences (Boston, MA).
2.1.1. Media Chemicals and Strains
Plasmids were maintained in Escherichia coli, DH5α. E. coli was cultured in Luria-Bertani medium (Difco, BD Biosciences, NJ) to which ampicillin was added (0.1 mg/ml). The yeast strains were cultured in YEPD broth (Bio101, Vista, CA) or SD-ura− (Bio101). Table 2 (supplementary data) lists all of the strains used in this study.
2.2 Methods
2.2.1. Site-Specific Mutagenesis
Site directed mutagenesis was performed using the quick-change mutagenesis system as described previously [34]. The mutations were introduced into plasmid pPSCDR1-GFP according to the manufacturer’s instructions, and the mutated plasmid pPSCDR1-GFP linearized with XbaI was used to transform S. cerevisiae strain, AD1-8u− cells by the lithium acetate transformation protocol exploiting uracil prototrophy [34–35].
2.2.2. Immunodetection of CaCdr1p
Plasma membrane (PM) was prepared from S. cerevisiae cells grown in YEPD to late exponential phase, as described previously [34]. The Western blot analysis was carried out using anti-GFP monoclonal antibody (1:5000 dilution) as described previously [34].
2.2.3. ATPase activity assay
The WT-CaCdr1p associated ATPase activity of the purified PM was measured as an oligomycin-sensitive release of inorganic phosphate as described previously [34]. Brie y, purified PM (10 μg) was incubated at 30°C in 0.1ml of reaction mixture containing 8 mM MgCl2 and 60 mM Tris-HCl, pH 7.5 (ATPase reaction buffer) and 20 μM oligomycin where indicated. To eliminate possible contributions from non-specific vacuolar and mitochondrial ATPases, 50 mM KNO3, and 10 mM NaN3, respectively were included in the reaction mixture. The reaction was started by addition of 5 mM ATP and was stopped by the addition of 0.1ml of 5% SDS solution. The amount of inorganic phosphate released was determined immediately as described previously [36].
2.2.4. Rhodamine 6G efflux assay
Efflux of Rhodamine 6G (R6G) was determined essentially using a previously described protocol [34, 37]. Brie y, approximately 4 × 10−6 yeast cells from an overnight grown culture were transferred into YEPD media and allowed to grow for 5 h. Cells were pelleted and washed and then resuspended in phosphate-buffered saline (PBS) as a 2% cell suspension to which R6G was added at a final concentration of 10 μM and incubated for 1 h at 30°C. The cells were then washed and resuspended in PBS and reaction was initiated by the addition of 2% glucose. Samples of 1 ml volume were withdrawn at indicated time (30 min) and centrifuged at 9000 g for 2 min. The supernatant was collected and absorption was measured at 527 nm.
2.2.5. Drug susceptibility
The susceptibilities of S. cerevisiae cells to different antifungal drugs were determined using spot assays. In this assay, 3 μl samples of five fold serial dilutions of each yeast culture (each with cells suspended in normal saline to an optical density at 600nm of 0.1) were spotted on to YEPD plates in the absence (control) or in the presence of the drugs [38]
2.2.6. Confocal Microscopy and Flow Cytometry
Confocal imaging and flow cytometric (FACS) analysis of CaCdr1p and its mutant variants carrying S. cerevisiae cells were performed with a Bio-Rad confocal microscope (MRC 1024) with a 100 μl oil immersion objective and FACS or flow cytometer (Becton-Dickson Immuno cytometry systems, San Jose, CA) as described previously [34].
2.2.7. Photo affinity labeling with [125I] IAAP
The PM proteins (30 μg) were incubated with the indicated drug for 10 min at 37°C in 50 mM Tris-HCl, pH 7.5 in the absence of ATP. The samples were brought to room temperature and 7.5 nM [125I] Iodoarylazidoprazosin (IAAP) (2300Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp assembly (PGC, Scientifics, Gaitherberg, MD) fitted with two black light (self filtering) UV-A long wavelength F15T8BLB tubes (365nm) for 10 min at room temperature. Following SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8%Tris-glycine gel at constant voltage, gels were dried and exposed to Bio- MaxMR film (Eastman Kodak, Rochester, NY) at −80°C for 12 to 24 h. The radioactivity incorporated into the WT-CaCdr1p and its mutant’s bands were quantified using a STORM 860 phosphorimager system (Molecular Dynamics, Sunnyvale, Calif.) and the software Image QuaNTas described previously [34].
2.2.8. Binding of [α-32P] 8-azido-ATP
The PM protein (30 μg) was incubated in the ATPase reaction buffer containing 10 μM [32P] 8-azido-ATP (10 μCi/nmol) in the dark at 4°C (on ice) for 5 min in the presence or absence of 10 mM ATP. The samples were then illuminated with a UV lamp assembly (365-nmwavelength) for 10 min on ice (4°C) as described previously [39]. Following SDS-PAGE on an 8% Tris-glycine gel at constant voltage, the gels were dried and exposed to Bio-Max MR filmat 80°C for 12 to 24 h. The radioactivity incorporated into the WT-CaCdr1p and its mutant variants bands were quantified as described above.
3. Results
3.1. Site directed mutagenesis of Signature motif residues
Earlier structural studies have revealed that the first five core residues of Signature motif (LSGGQ) are critical which hold the ATP molecule at the catalytic site [28–29]. To address the role of Signature motifs of NBDs of CaCdr1p (Fig. 1), in this study, we have mutagenized first five residues of both the conserved and degenerated Signature motifs of NBD1 (VSGGE) and NBD2 (LNVEQ), respectively. The first category of mutants included variants where all the residues of both the Signature motifs were replaced with alanines such as V303A, S304A, G305A, G306A and E307A of NBD1 and L1001A, N1002A, V1003A, E1004A and Q1005A of NBD2. In the second category of mutant variants, we substituted the position of an individual residue of a Signature motif into its equipositional residue present in another domain. For example, valine (V303) present in the NBD1 was replaced with leucine which is present at the equivalent position in NBD2. Thus, we constructed V303L, S304N, G305V, G306E and E307Q of NBD1 and L1001V, N1002S, V1003G, E1004G and Q1005E of NBD2. Finally, to further evaluate the functional contribution of each Signature motif, we swapped entire Signature motif by introducing mutations in a way to produce two consensuses (Signature 1-1), two degenerated (Signature 2-2) and swapped (Signature 2-1) motifs variants which were placed in the third category.
All the mutations were introduced into C-terminal GFP tagged CaCdr1p which was stably overexpressed at the PDR5 locus in a heterologous host S. cerevisiae mutant strain, AD1-8u− and expressed as GFP fused CaCdr1p (WT-CaCdr1p) localized in PM [34]. The host AD1-8u− is deleted in seven PM encoded major ABC transporters and is derived from a pdr1-3 mutant strain with a gain of function mutation in the transcription factor Pdr1p, resulting in a constitutive hyperinduction of the PDR5 promoter [34]. A single integration copy of CDR1 ORF at the PDR5 locus in the host genome of each mutant was confirmed by Southern hybridization (data not shown).
3.2. Substitution of Signature motifs residues with alanines abrogates function
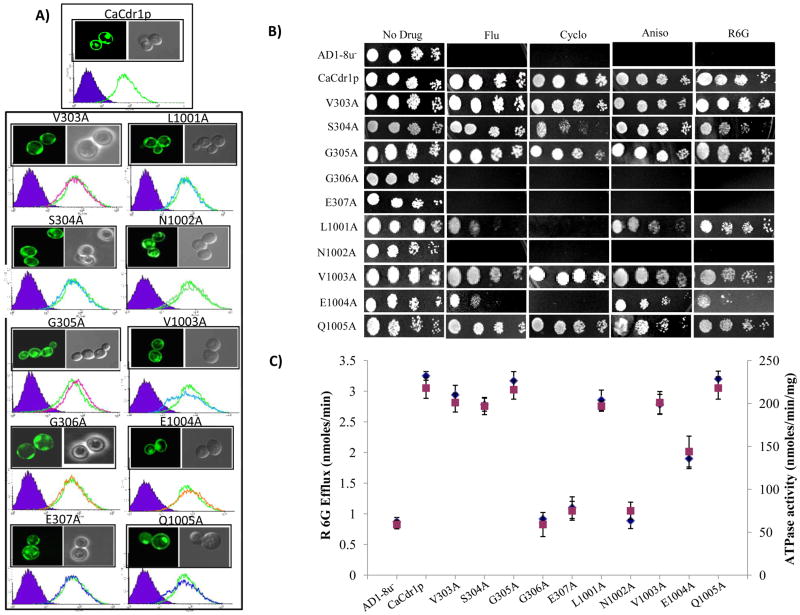
Similar to the WT-CaCdr1p, all the alanine scanning mutant variants of Signature motif were equally expressed as revealed by Western blot analysis of PM fraction of the cell (Fig. S1, supplementary data) and were properly surface localized as confirmed by confocal microscopy and FACS analysis (Fig. 2A). However, these mutant variants displayed variable drug susceptibilities. For example, cells expressing G306A and E307A of NBD1 and N1002A of NBD2 were hypersensitive to all the tested drugs (Fig. 2B). The enhanced drug sensitivity was corroborated by observed abrogated efflux of a fluorescent substrate R6G [34]. We used intact cells expressing different mutant variants and de-enerigized them and monitored energy dependent extrusion of R6G which was initiated by the addition of glucose [37]. As compared to host strain AD1-8u−, cells expressing the WT-CaCdr1p were able to extrude higher R6G which was evident from an increase in extracellular concentration of R6G (3±0.5 nmoles R6G/ml/30min). However, drug hypersensitive mutant variants, G306A and E307A of NBD1 and N1002A of NBD2 displayed severely impaired R6G efflux and behaved like the host strain AD1-8u−(Fig. 2C). The loss of R6G efflux activity in these mutants was associated with a simultaneous impaired ATPase activity (Fig. 2C). Notably, we had earlier observed that unlike mammalian ABC proteins, WT-CaCdr1p does not show dramatic drug stimulated ATPase activity [40]. For this reason, we routinely measure basal (un-stimulated) ATPase activity. Mutants such as S304A, L1001A and E1004A displayed increased sensitivity to only selected drugs. For example, the conserved serine residue of Signature motif of NBD1, when replaced with alanine (S304A), was only partially sensitive to cycloheximide (CYH) and R6G while similar to WT-CaCdr1p, remained resistant to other drugs. In contrast, L1001A of NBD2 displayed sensitivity to CYH while it was only partially sensitive to fluconazole (FLC) and anisomycin (ANI). All these mutant variants which showed partial abrogation in resistance towards selected drugs did not show any significant difference in their ability to efflux R6G (Fig. 2C). The basal ATPase activity which drives active extrusion of R6G was also comparable to the WT-CaCdr1p and was not changed. However, mutant variant E1004A was an exception, which was sensitive to CYH and only partly sensitive to FLC, and R6G, showed only about 42% decrease in the efflux of R6G (Fig. 2C). Recent studies showed that NBD residues indeed could selectively influence susceptibility towards drugs. H1068A mutation in H-loop of Pdr5p of S. cerevisiae selectively abrogated resistance towards tested drugs. For example, the mutant variant H1068A remained resistant to ketoconazole (KTC), FLC and CYH while showed dramatic loss of resistance to rhodamine 123 [23].
Fig. 2.
Alanine scanning of the Signature motifs of CaCdr1p. (A) Fluorescence imaging (upper panel) by confocal microscope showing membrane localization of WT-CaCdr1p and its mutant variant protein expressing cells. Flow cytometry (lower panel) of S. cerevisiae cells expressing WT-CaCdr1p and its mutant variants. The histogram derived from the cell quest program depicts fluorescence intensities for AD1-8u- (control) (purple filled area) and WT-CaCdr1p (solid orange line) for each panel, and the other extra line represents that for the respective CaCdr1p mutant variant-expressing cells. B) Drug resistance profile of WT-CaCdr1p and its Signature motifs mutant variants were determined by spot assay. It was done as per protocol described earlier [34]. In the spot assays, 5 μl of five-fold serial dilutions of each yeast culture (each with cells suspended in normal saline to an OD of 0.1 (A600) were spotted on YEPD plates in absence (control) and presence of the following drugs: FLC, 5 μg/mL; CYH, 0.15 μg/mL; ANI, 1.0 μg/mL; and R6G 5 μg/mL. C). R6G efflux (left Y-axis) indicated by blue diamond (◆) and oligomycin sensitive ATPase activity (right Y-axis) of WT-CaCdr1p with its mutant variants indicated by green square ( ) [34, 37].
) [34, 37].
This decrease in R6G transport was coupled with a simultaneous abrogation of ATPase activity. All the other mutant variants such as V303A, G305A of NBD1 and V1003A and Q1005A of NBD2 had phenotypes, similar to cells expressing WT-CaCdr1p (Fig. 2).
3.3. Restoration of conservancy in Signature motifs does not support functionality of NBDs
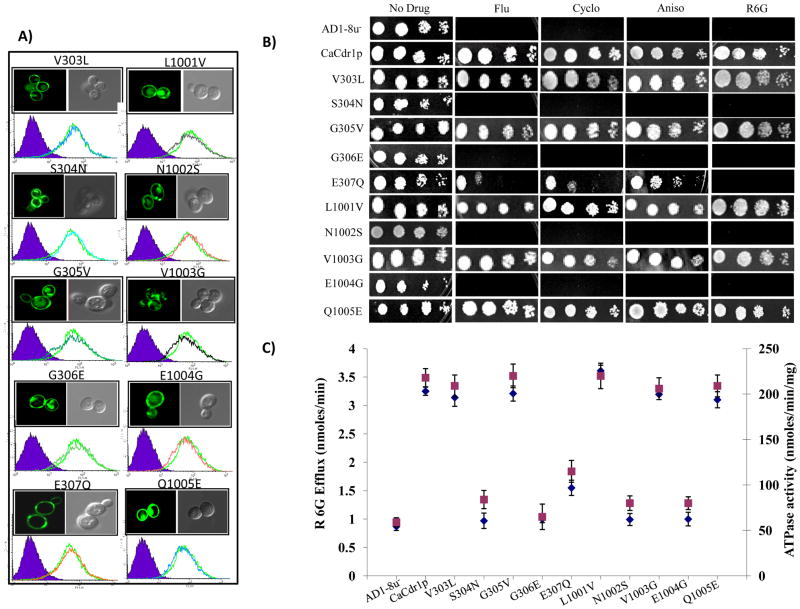
To further examine the functional compatibility and to underscore significance of the conserved and degenerated Signature residues, we introduced mutations in both the Signature motifs by replacing residue with their equivalent positional residue of the other domain. These substitutions did not affect surface expression and localization of WT-CaCdr1p as determined by Confocal and FACS analysis (Fig. 3A). Replacement of conserved serine with asparagine (S304N) to mimic degeneracy at this position and asparagine with serine (N1002S) to mimic conservancy in NBD2 resulted in hypersensitivity to all the tested drugs with severely diminished R6G efflux and ATPase activities (Fig. 3C). Similar results were also observed when highly conserved glycine of NBD1 changed into glutamate (G306E) and its equivalent residue glutamate of NBD2 exchanged with glycine (E1004G). Both these mutants were hypersensitive to all the drugs with abrogated R6G efflux and ATPase activity (Fig. 3C). The equipositional mutant E307Q displayed drug selective phenotype which was hypersensitive to R6G and was only partially sensitive to other drugs. This mutant variant showed 52% abrogation in R6G efflux with a simultaneous loss of ATPase activity. In contrast, the replacement of equipositional residues such as V303L, G305V of NBD1 and L1001V, V1003G and Q1005E of NBD2, had no effect on the drug susceptibility, R6G efflux and ATPase activity (Fig. 3).
Fig. 3.
Equipositional replacements of the Signature sequences of CaCdr1p. A). Fluorescence imaging (upper panel) by confocal microscope showing membrane localization of CaCdr1p and its mutant variant protein expressing cells. Flow cytometry (lower panel) of S. cerevisiae cells expressing WT-CaCdr1p and its mutant variants. The histogram derived from the cell quest program depicts fluorescence intensities for AD1-8u-(control) (purple filled area) and WT-CaCdr1p (solid orange line) for each panel, and the other extra line represents the respective CaCdr1p mutant variant-expressing cells. B). Drug resistance profile of WT-CaCdr1p and its Signature motif mutant variants was determined by spot assays as described for Fig 2. C). R6G efflux (left Y-axis) indicated by blue diamond (◆) and oligomycin sensitive ATPase activity (right Y-axis) of WT-CaCdr1p with its mutant variants indicated by green square ( ) [34, 37].
) [34, 37].
3.4. Swapping of the Signature motifs results in complete loss of function
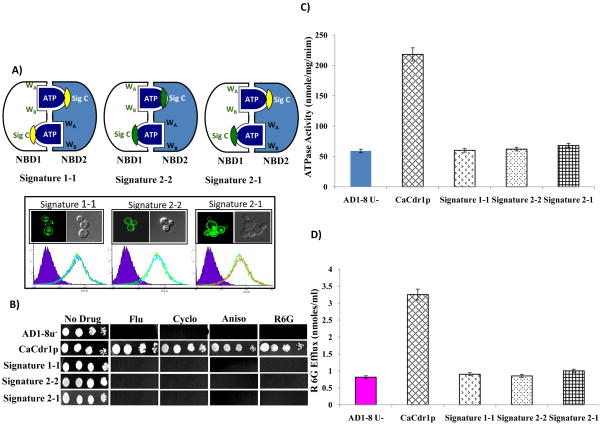
The cells expressing the Signature swapped mutants, Signature1-1, Signature 2-2 and Signature 2-1 were hypersensitive to all the tested drugs and displayed severely impaired R6G efflux and ATPase activity and behaved more like the host strain, AD1-8u− (Fig. 4). Nonetheless, all the Signature swapped variants were properly surface localized and equally well expressed.
Fig. 4.
Swapping of Signature motifs of CaCdr1p. A) Schematic diagrams of different CaCdr1p Signature swapped mutants variants. The entire Signature motif of NBD1 (white)/NBD2 (purple) was mutated to generate constructs with two conserved (Signature 1-1), two degenerated (Signature 2-2) and swapped (Signature 2-1) active site (upper panel). Localization and expression profile of WT-CaCdr1p and its swapped Signature mutant variants (Lower panel). B) Drug resistance profile of WT-CaCdr1p and its Signature motifs swapped mutants were determined by spot assays. C) Comparison of oligomycin sensitive ATPase activity of WT-CaCdr1p with its swapped mutant variants. ATPase activity of the PM fraction of cells expressing the WT-CaCdr1p and its mutant variants were assayed as described earlier [34]. D) R6G efflux by the WT-CaCdr1p and its mutant variant protein-expressing cells. The R6G efflux was measured as described previously [37]. The values are mean SD (± error bars) for three independent experiments.
3.5. [125I] IAAP and ATP binding remain unaffected in Signature motif mutants
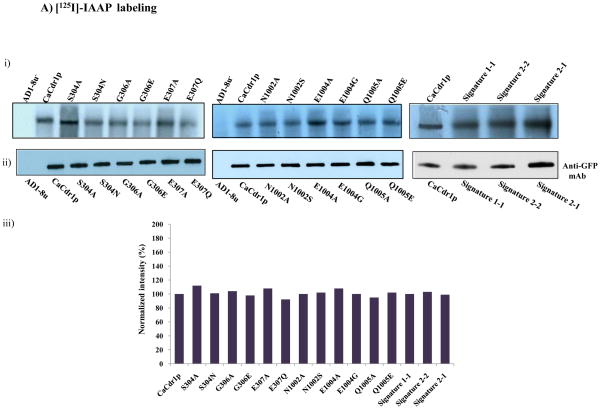
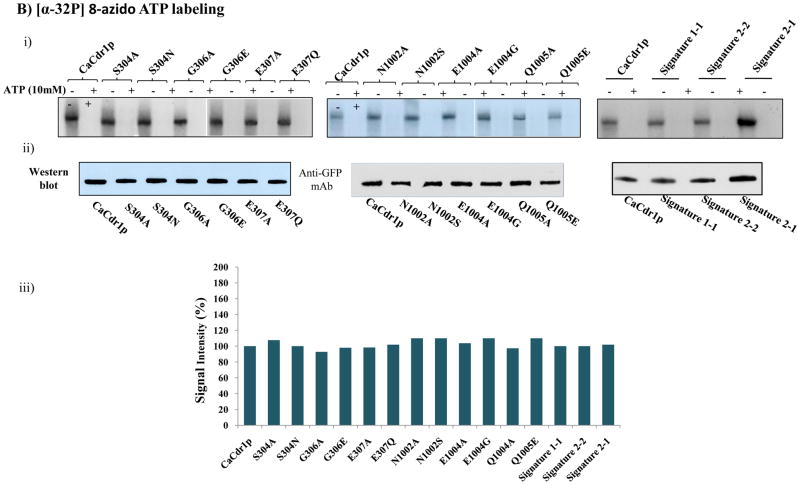
We have examined if the observed impairment of function i.e. R6G drug transport and ATP hydrolysis of Signature mutants was due to their inability to bind to CaCdr1p. We evaluated this aspect by using the [125I] iodoarylazidoprazosin (IAAP) and [α-P32] 8-azido ATP, which are photoaffinity analogues of substrate prazosin and ATP, respectively. We performed the binding experiments with both the Signature motifs of NBDs by selecting three Signature motif (VSGGE) residues S304, G306, and E307 of NBD1 and equivalent position residues of Signature motif (LNVEQ) of NBD2 viz: N1002, E1004 and Q1005. The selection of these residues was based on our data (this study) and from the crystal structure of MJ0796, an ABC protein of Methanocaldococcus jannaschii which showed that these residues are part of ATPase binding pocket and interact with the ATP moiety [29]. All the Signature motif mutant variants at a fixed concentration efficiently cross linked to [125I] IAAP comparable to that of WT-CaCdr1p. An equivalent amount of PM derived from the host cells (AD1-8u−) was used as a control, wherein no binding of [125I] IAAP was observed (Fig. 5A). We have earlier shown that [125I] IAAP efficiently crosslinked with WT-CaCdr1p and its binding can be competed out with molar excess of nystatin and the binding of [3H] aziodpine, a dihydropyridine photoaffinity analogue, is competed out with miconazole [34]. In this study, the competition experiments with drugs were not done since none of the drugs used in this study which showed selective susceptibilities compete with [125I] IAAP binding [34]. Thus, [125I] IAAP binding which did not change in the Signature mutant variants provides limited information and may not be a true indicator of drug binding sites of WT-CaCdr1p.
Fig. 5.
A). (i) Photoaffinity labeling of WT-CaCdr1p and its mutant variants with [125I]-IAAP. The PM fraction (30 μg protein) of cells expressing WT-CaCdr1p and its mutant variants were incubated with 7.5 nM [125I]-IAAP (2300 Ci/mmol). The samples were UV crosslinked and processed as described elsewhere [34]. (ii) Western blot analysis using anti-GFP antibody to ensure equal loading of WT-CaCdr1p and its Signature mutant variants (iii) Normalized incorporated [125I] IAAP labeling with Western blot intensity. The values are shown in percentage. B). Photoaffinity labeling of WT-CaCdr1p and its mutant variants with [α-32P] 8-azido ATP. The PM fraction (30 μg) of cells expressing the WT-CaCdr1p and its mutant variants were incubated with 10 μM [α-32P] 8-azido ATP 7.5 μCi/nmol at 4°C and competed with 10 mM cold ATP (+ATP lane) as described in [34]. (ii) Western blot analysis using anti-GFP antibody to ensure equal loading of WT-CaCdr1p and its Signature mutant variants (iii) Normalized incorporated [α-32P] 8-azido ATP labeling with Western blot intensity. The values are shown in percentage.
Additionally, all the Signature mutant variants were assessed for [α-P32] 8-azido ATP binding which was normalized by Western blot analysis. No cross linking was observed of this analogue with the host cell (AD1-8u−) membrane which served as negative control. Notably, binding of 8-azido-[α-32P] ATP to all Signature mutant variants were similar to WT-CaCdr1p, which was competed out with molar excess of the cold ATP (Fig. 5B).
4. Discussion
Signature motifs are the hallmark sequences of NBDs of ABC transporters which display highly conserved sequences across the evolutionary scale; however, there are instances of appearance of selective divergence within this motif. For example, human ABC transporters such as TAP [24] and CFTR [26] have degenerated Signature motifs (Fig 1). In contrast, all the family members of ABC transporters of fungi, particularly of PDR subfamily display divergence in their Signature motifs. Thus, the Signature motif of NBD1 of CaCdr1p is well conserved but has NBD2 with a degenerated Signature motif (Fig. 1). In the present study, we have explored the significance of degeneration in the Signature sequence of NBD2 versus conserved Signature motif of NBD1 of CaCdr1p. Our analysis revealed that the conserved and degenerated Signature sequences of the CaCdr1p are functionally indispensable and can not be exchanged. This emphasizes the uncompromised asymmetry that exists between the NBDs of CaCdr1p and in other yeast ABC transporters.
We show that similar to other ABC transporters, the well conserved serine (S304) and Glycine (G306) residues present in conserved Signature motif of NBD1 are also critical for the functioning of CaCdr1p. For example, even the substitution at the equivalent position residues of degenerated Signature motif of NBD2 with the conserved ones and vice-versa does not support the function of the transporter (Fig. 3C & D). In the ABC protein of Pyrococcus furiosus, Rad50, replacement of this serine (LSGGQ) to arginine prevents dimerization of NBDs [28] and in human CFTR, polymorphism at this residue with either asparagine, arginine or isoleucine result in cystic fibrosis [41–43]. Furthermore, if cysteine was introduced at this position in CFTR protein, it resulted in total loss of channel activity [44].
The well conserved glycine presents at fourth position of Signature motif (LSGGQ) is involved in the ATP catalysis (25–26, 31–32, and 45–46). Biochemical analysis revealed that a small change at this position (G→A) results in steric hindrance between methyl group of alanine and γ-phosphate of ATP. If this glycine is exchanged with bulky, charged aspartate or glutamate, it leads to a complete loss of ATPase and protein activity [46–47]. The critical nature of serine and glycine in WT-CaCdr1p can also be compared with similar residue of those proteins whose crystal structures are known. The existing structural information suggests that the Signature motifs of ABC proteins; Rad50 of Pyrococcus furiosus, MJ1096 of Methanocaldococcus jannaschii, GlcV of Sulfolobus solfataricus, Sav1866 of Staphylococcus aureus, mouse CFTR, HlyB and MalK of E. coli, are involved in the head to tail ATPase site formation with the Walker A and Walker B motifs of the opposite NBDs, sandwiched with ATP molecules wherein the Signature motif is a “sensor” for an ATP γ-phosphate in the opposing domain. [28–29, 48–52]. Based on the conserved nature of these motifs, it is reasonable to speculate that in CaCdr1p, the conserved S304 and G306 of NBD1 probably fall within close proximity of the ATP binding site. In addition, divergent residues present in NBD2 Signature region is also equally important and may be part of the ATPase site as well. However, it still requires experimental validation.
We also provide evidence that in addition to highly conserved and critical S304 and G306 residues, the equipositional residues N1002 and E1004 of degenerated Signature motif of NBD2 of WT-CaCdr1p have also evolved to be functionally essential. Notably, pairs of residue like V303, G305 of NBD1 and L1001, V1003, Q1005 of NBD2 Signature motif though part of otherwise conserved Signature sequences, has apparently no functional relevance. These residues when replaced with either alanines or with its equipositional substitute, continued to show phenotypes similar to cells expressing WT-CaCdr1p.
Functional nonequivalence in the NBDs of ABC proteins of yeast is the result of variations in the conserved motifs (Walker A, Walker B, H-loop and Signature motif). Our earlier results have shown that the equipositional exchange mutations such as C193K and K901C of Walker A of CaCdr1p yielded selectively impaired functional protein [21]. However, replacement of entire NBD of CaCdr1p to make a chimera with two NBD1 (NBD1/NBD1), and two NBD2 (NBD2/NBD2), yielded non-functional proteins and had severe cellular trafficking problem [53]. These results had established functional asymmetry nature of NBD’s in CaCdr1p. These variations in NBD1 may have evolved in response to degenerated Signature motif of NBD2. Thus in CaCdr1p, both canonical and non-canonical ATP binding sites are formed similar to TAP and CFTR proteins. Recently, Ernst et al. hypothesized that in Pdr5p of S. cerevisiae, a close homologue of CaCdr1p, one ATP molecule catalyzed at the canonical active site may be sufficient to reset the TMDs whereas the second non-canonical site (regulatory site) may be engaged to serve as platform for keeping domains in dimeric form (inward facing) [23, 54].
Our equipositional replacements data of Signature motifs demonstrate that the mutant variants, S304N and G306E of NBD1 and N1002S and E1004G of NBD2 severely impaired both transport (Fig. 3C) and ATPase activity (Fig. 3C). These results clearly show that both the conserved site and the degenerated site are equally important for ATP catalysis. The Signature sequence swapping data highlights the importance of the uniqueness of the Signature region of WT-CaCdr1p. Signature swapped mutants with either two conserved (Signature 1-1) or two degenerated (Signature 2-2) or swapped motif (Signature 2-1) rendered non-functional protein (Fig. 4) though their surface localization and expression was not affected (Fig. 4A). In this context, it is worth mentioning that TAP protein with two degenerate sites shows dramatically impaired activity and thus demonstrating that consensus site is critical. In contrast, only 10% loss in activity was seen with TAP protein having both consensus sites. Additionally, swapping between consensus and degenerative sequences retained more than 50 % activity. It would mean that TAP protein can remain partly functional if at least one of the Signature motifs retains the consensus site [24]. In contrast, NBDs of Pgp are symmetric in function and possess conserved Signature motifs at both positions. The replacement of either serine of both Signature motifs (S528/S1173) with alanine resulted in marginal loss in ATPase activity whereas double mutation at these positions in Signature motif (S528A/S1173A) alleviates strength of interaction in the transition state which implies that these serine residues of P-glycoprotein cooperatively accelerate ATP hydrolysis [55]. Taken together, our data clearly establish that the Signature motifs of NBDs are functionally non-identical and the evolutionary divergence of CaCdr1p and other members of PDR subfamily are critical and non interchangeable.
Supplementary Material
Acknowledgments
We thank R.D. Cannon for the gifts of the plasmid. We thank Ranbaxy Laboratories Ltd., New Delhi, India for providing fluconazole. AK acknowledges the University Grants Commission, India for the support in the form of Senior Research Fellowship.
Grant support
The work presented in this paper has been supported in part by grants to R. P. from Department of Biotechnology (DBT) [BT/PR9100/Med/29/03/2007], [BT/PR9563/BRB/10/567/2007], [BT/PR11158/BRB/10/640/2008]. SS and SVA were supported by the intramural research program National Institutes of Health, National Cancer Institute, Centre for Cancer research.
Abbreviations
- ABC
ATP binding cassette
- PDR
pleiotropic drug resistance
- NBD
nucleotide binding domain
- TMD
trans membrane domain
- TMS
trans membrane segment
- PM
plasma membrane
- FLC
fluconazole
- CYH
cycloheximide
- ANI
anisomycin
- R6G
rhodamine 6G
- [125I] IAAP
[125I] iodoarylazidoprazosin,
References
- 1.Holland I, Cole P, Kuchler K, Higgins C. ABC Proteins from Bacteria to Man. Academic Press; San Diego, CA: 2003. [Google Scholar]
- 2.Walmsley MB, Mckeegan KS, Walmsley AR. Structure and function in efflux pumps that confer resistance to drugs. Biochem J. 2003;376:313–338. doi: 10.1042/BJ20020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderone RA. Candida and Candidiasis. ASM Press; Washington, DC: 2002. [Google Scholar]
- 4.Prasad R, Panwar SL. Smriti, Drug resistance in yeasts—an emerging scenario. In: Poole RK, editor. Adv Microb Physiol. 1. Academic Press; London: pp. 155–201. [DOI] [PubMed] [Google Scholar]
- 5.White TC. Increased mRNA levels of ERG16, CDR1, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernaez ML, Gil C, Pla J, Nombela C. Induced expression of the Candida albicans multidrug resistance gene CDR1 in response to fluconazole and other antifungals. Yeast. 1998;14:517–526. doi: 10.1002/(SICI)1097-0061(19980430)14:6<517::AID-YEA250>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 8.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White TC. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azoleresistant lanosterol 14-R demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad R, Wergifosse PD, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;4:320–329. doi: 10.1007/BF00352101. [DOI] [PubMed] [Google Scholar]
- 11.Puri N, Gaur M, Sharma M, Shukla S, Ambudkar SV, Prasad R. The amino acid residues of transmembrane helix 5 of multidrug resistance proteinCaCdr1p of Candida albicans are involved in substrate specificity and drug transport. BBA. 2009;1788:1752–1761. doi: 10.1016/j.bbamem.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad R, Krishnamurthy S, Prasad R, Gupta V, Lata S. Multidrug resistance: an emerging threat. Curr Sci. 1996;71:205–213. [Google Scholar]
- 13.Ambudkar SV, Kim In-Wha, Sauna ZE. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1) Eur J Pharma Sci. 2006;5:392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Parcej D, Tampe R. Caught in the Act: an ABC Transporter on the Move. Structure. 2007;15:1028–1030. doi: 10.1016/j.str.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 16.Schneider E, Hunke S. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol Rev. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 17.Jha S, Karnani N, Dhar SK, Mukhopadhayay K, Shukla S, Saini P, Mukhopadhayay G, Prasad R. Purification and characterization of the N-terminal nucleotide binding domain of an ABC drug transporter of Candida albicans: uncommon cysteine 193 of Walker A is critical for ATP hydrolysis. Biochemistry. 2003;42:10822–10832. doi: 10.1021/bi0345900. [DOI] [PubMed] [Google Scholar]
- 18.Rai V, Shukla S, Jha S, Komath SS, Prasad R. Functional characterization of N-terminal nucleotide binding domain (NBD-1) of a major ABC drug transporter Cdr1p of Candida albicans: uncommon but conserved Trp326 of Walker B is important for ATP binding. Biochemistry. 2005;44:6650–6661. doi: 10.1021/bi0474160. [DOI] [PubMed] [Google Scholar]
- 19.Lamping E, Baret PV, Holmes AR, Monk BC, Goffeau A, Cannon RD. Fungal PDR transporters: Phylogeny, topology, motifs and function. Fungal Genet Biol. 2010 Feb;47(2):127–42. doi: 10.1016/j.fgb.2009.10.007. Epub 2009 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaur M, Devpriya C, Prasad R. The complete inventory of ABC proteins in human pathogenic yeast, Candida albicans. J Mol Microbiol Biotechnol. 2005;9:3–15. doi: 10.1159/000088141. [DOI] [PubMed] [Google Scholar]
- 21.Jha S, Dabas N, Karnani N, Saini P, Prasad R. ABC multidrug transporter Cdr1p of Candida albicans has divergent nucleotide-binding domains which display functional asymmetry. FEMS Yeast Res. 2004;5:63–72. doi: 10.1016/j.femsyr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Rai V, Gaur M, Shukla S, Shukla S, Ambudkar SV, Komath SS, Prasad R. Conserved Asp327 of walker B motif in the N-terminal nucleotide binding domain (NBD-1) of Cdr1p of Candida albicans has acquired a new role in ATP hydrolysis. Biochemistry. 2006;45:14726–14739. doi: 10.1021/bi061535t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst R, Kueppers P, Klein CM, Schwarzmueller T, Kuchler K, Schmitt L. A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. PNAS. 2008;105:5069–5074. doi: 10.1073/pnas.0800191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Procko E, Connell IF, Sze-Ling Ng, Gaudet R. Distinct Structural and Functional Properties of the ATPase Sites in an Asymmetric ABC Transporter. Mol Cell. 2006;24:51–62. doi: 10.1016/j.molcel.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Chen M, Abele R, Tampe R. Functional non-equivalence of ABC signature motifs in the transporter associated with antigen processing (TAP) J Biol Chem. 2004;279:46073–46081. doi: 10.1074/jbc.M404042200. [DOI] [PubMed] [Google Scholar]
- 26.Melin P, Thoreau V, Norez C, Bilan F, Kitzis A, Becq F. The cystic fibrosis mutation G1349D within the signature motif LSHGH of NBD2 abolishes the activation of CFTR chloride channels by genistein. Biochem Pharmcol. 2004;67:2187–2196. doi: 10.1016/j.bcp.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Ren X, Furukawa T, Haraguchi M, Sumizawa T, Aoki S, Kobayashi M, Akiyama S. Function of the ABC Signature Sequences in the Human Multidrug Resistance Protein 1. Mol Pharmacol. 2004;65:1536–1542. doi: 10.1124/mol.65.6.1536. [DOI] [PubMed] [Google Scholar]
- 28.Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 29.Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas JP, Hunt JF. ATP Binding to the Motor Domain from an ABC Transporter Drives Formation of a Nucleotide Sandwich Dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson AL, Chen J. ATP-Binding cassette transporters in Bacteria. Annu Rev Biochem. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 31.Szakacs G, Ozvegy C, Bakos E, Sarkadi B, Varadi A. Role of glycine-534 and glycine-1179 of human multidrug resistance protein (MDR1) in drug-mediated control of ATP hydrolysis. Biochem J. 2001;356:71–75. doi: 10.1042/0264-6021:3560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browne BL, McClendon V, Bedwell DM. Mutations within the first LSGGQ motif of Ste6p cause defects in a-factor transport and mating in Saccharomyces cerevisiae. J Bacteriol. 1996;178:1712–1719. doi: 10.1128/jb.178.6.1712-1719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falcon-Perez JM, Mazon MJ, Molano J, Eraso P. Functional domain analysis of the yeast ABC transporter Ycf1p by site-directed mutagenesis. J Biol Chem. 1999;274:23584–23590. doi: 10.1074/jbc.274.33.23584. [DOI] [PubMed] [Google Scholar]
- 34.Shukla S, Saini P, Smriti, Jha S, Ambudkar SV, Prasad R. Functional characterization of Candida albicans ABC transporter Cdr1p. Eukaryotic Cell. 2003;2:1361–1375. doi: 10.1128/EC.2.6.1361-1375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Niimi M, Niimi K, Holmes AR, Yates JE, Decottignies A, Monk BC, Goffeau A, Cannon RD. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob Agents Chemother. 2002;45:3366–3374. doi: 10.1128/AAC.45.12.3366-3374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA, Niederweis M. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–4858. [PubMed] [Google Scholar]
- 37.Maesaki S, Marichal P, Bossche HV, Sanglard D, Kohno S. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J Antimicrob Chemother. 1999;44:27–31. doi: 10.1093/jac/44.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay K, Kohli A, Prasad R. Drug susceptibilities of yeast cells are affected by membrane lipid composition. Antimicrob Agents Chemother. 2002;46:3695–3705. doi: 10.1128/AAC.46.12.3695-3705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauna ZE, Ambudkar SV. Characterization of the catalytic cycle of ATP hydrolysis by human P-glycoprotein. J Biol Chem. 2001;276:11653–11661. doi: 10.1074/jbc.M011294200. [DOI] [PubMed] [Google Scholar]
- 40.Shukla S, Rai V, Banerjee D, Prasad R. Characterization of Cdr1p, A Major Multidrug Efflux Protein of Candida albicans: Purified Protein Is Amenable to Intrinsic Fluorescence Analysis. Biochemistry. 2006;45:2425–2435. doi: 10.1021/bi0519147. [DOI] [PubMed] [Google Scholar]
- 41.Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genetics. 1995;29:777–807. doi: 10.1146/annurev.ge.29.120195.004021. [DOI] [PubMed] [Google Scholar]
- 42.Zielenski J, Patrizio P, Corey M, Hanhelin B, Markiewicz D, Asch R, Tsui LC. CFTR gene variant for patients with congenital absence of vas deferens. Am J Hum Genet. 1995;57:958–960. [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson MP, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- 44.Cotton JF, Welsh MJ. Covalent modification of the nucleotide binding domains of cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1998;273:31873–31879. doi: 10.1074/jbc.273.48.31873. [DOI] [PubMed] [Google Scholar]
- 45.Szentpetery Z, Kern A, Liliom K, Sarkadi B, Varadi A, Bakos E. The role of the conserved Glycines of ATP-binding cassette signature motifs of MRP1 in the communication between the Substrate-binding site and the Catalytic centers. J Biol Chem. 2004;279:41670–41678. doi: 10.1074/jbc.M406484200. [DOI] [PubMed] [Google Scholar]
- 46.Ramaen O, Sizun C, Pamlard O, Jacquet E, Lallemand JY. Attempts to characterize the NBD heterodimer of MRP1: transient complex formation involves Gly771 of the ABC signature sequence but does not enhance the intrinsic ATPase activity. Biochem J. 2005;391:481–490. doi: 10.1042/BJ20050897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmees G, Stein A, Hunke S, Landmesser H, Schneider E. Functional consequences of mutations in the conserved ‘signature sequence’ of the ATP binding- cassette protein MalK. Eur J Biochem. 1999;266:420–430. doi: 10.1046/j.1432-1327.1999.00871.x. [DOI] [PubMed] [Google Scholar]
- 48.Verdon G, Albers SV, van Oosterwijk N, Dijkstra BW, Driessen AJ, Thunnissen AM. Formation of the productive ATP-Mg2+-bound dimer of GlcV, an ABC-ATPase from Sulfolobus solfataricus. J Mol Biol. 2003;334:255–267. doi: 10.1016/j.jmb.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 49.Dawson RJP, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 50.Zaitseva J, Oswald C, Jumpertz T, Jenewein S, Wiedenmann A, Holland IB, Schmitt L. A structural analysis of asymmetry required for catalytic activity of an ABC-ATPase domain dimer. EMBO. 2006;25:3432–3443. doi: 10.1038/sj.emboj.7601208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–522. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 52.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saini P, Gaur NA, Prasad R. Chimeras of the ABC drug transporter Cdr1p reveal functional indispensability of transmembrane domains and nucleotide-binding domains, but transmembrane segment 12 is replaceable with the corresponding homologous region of the non-drug transporter Cdr3p. Microbiol. 2006;152:1559–1573. doi: 10.1099/mic.0.28471-0. [DOI] [PubMed] [Google Scholar]
- 54.Ernst R, Kueppers P, Stindt J, Kuchler K, Schmitt L. Multidrug efflux pumps: Substrate selection in ATP-binding cassette multidrug efflux pumps – first come, first served. FEBS J. 2010;277:540–549. doi: 10.1111/j.1742-4658.2009.07485.x. [DOI] [PubMed] [Google Scholar]
- 55.Tombline G, Bartholomew L, Gimi K, Tyndall Grace A, Alan E. Senior, Synergy between Conserved ABC Signature Ser Residues in P-glycoprotein Catalysis. J Biol Chem. 2004;279:5363–5373. doi: 10.1074/jbc.M311964200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.