Abstract
Biomaterials that present multiple stimuli are attractive for a number of biomedical applications. In particular, electrical and biological cues are important factors to include in interfaces with neurons for applications such as nerve conduits and neural probes. Here, we report the combination of these two stimuli, by immobilizing nerve growth factor (NGF) on the surface of the electrically conducting polymer polypyrrole (PPy). NGF was immobilized using an intermediate linker provided by a layer of polyallylamine conjugated to an arylazido functional group. Upon exposure to UV light and activation of the azido groups, NGF was fixed to the substrate. Three different surface concentrations were obtained (0.21–0.98 ng/mm2) and similar levels of neurite extension were observed on immobilized NGF as with soluble NGF. Additionally, electrical stimulation experiments were conducted with the modified polymer and revealed a 50% increase in neurite outgrowth in PC12 cells compared to experiments without electrical stimulation. This novel modification of PPy provides both electrical and biological stimulation, by presenting tethered growth factors and only producing a small decrease in the material's properties (conductivity ~10 S cm−1) when compared to other modification techniques (conductivity ~10−3–10−6 S cm−1.
Keywords: nerve growth factor, neural cell, surface grafting, electrical stimulation, protein immobilization
INTRODUCTION
Neurons are highly influenced by electrical stimuli because of their inherent nature in transmitting electro-chemical signals throughout the nervous system. As a consequence, engineered materials and devices capable of recording or stimulating nerve cells with electrical mechanisms have been investigated extensively. In addition to electrical stimulation, it is desirable to create materials that are biomimetic and thus integrate other cues that could enhance neuronal interfacing by inducing cell adhesion, recognition, neurite extension, and reducing inflammation and scar tissue. For example, microfabricated neural probes have been modified to present or deliver biomacromolecules that promote a more intimate contact with the surrounding tissue, improve recording performance, or chemically stimulate neurons.1-3 Silicon probes have been fabricated with hollow microchannels that can locally deliver compounds such as the neuromodulator γ-aminobutyric acid1 and therapeutic drugs to reduce inflammatory signals.2 Polyimide electrodes have also been modified to release nerve growth factor (NGF) via dextran hydrogels.3 These electrodes were reported to produce functional interfaces by supporting neurite growth toward the recording sites.
Polypyrrole (PPy), a biocompatible electrically-conductive polymer, has been used for neural prosthetics,4-9 in addition to in vitro and in vivo studies for nerve regeneration10-14 and other tissue engineering applications.15-20 PPy is an attractive material for neural probes because of its biocompatibility, good conductivity, ease of synthesis, and the potential to have high surface areas.9 Similarly, PPy has been investigated as a nerve conduit material because of its electrical characteristics and biocompatibility.12,14 However, it is still desired for these and other applications to provide additional cues such as biomolecules in conjunction with electroactivity provided by the conducting polymer. The ideal properties of nerve conduits for nerve regeneration are to include electrical stimulation with growth factors, biodegradability, and topographical features.21 Likewise, PPy coatings on electrodes and other devices have been combined with biomolecules to enhance cell interfacing. Adhesive peptides such as RGD and YIGSR,5,8,17 polysaccharides such as heparin, hyaluronic acid, and dextran15,19,20,22 and proteins such as human serum albumin and NGF22 have been incorporated into PPy as dopants. However, doping of biomolecules has some limitations such as low loading, decrease in conductivity (~3–4 orders of magnitude difference),20 and in the case of induced release upon reduction of the polymer, the supply is limited and the release is rather fast.22 As an alternative, surface immobilization of macromolecules has been explored to overcome these limitations. For example, PPy has been functionalized with hyaluronic acid by grafting amino-silanes on the surface,16,23 and other biomolecules such as heparin and enzymes have also been immobilized.24,25 RGD sequences have been grafted via either cystine residues,17,26 or peptides that selectively bind to PPy.27 More recently, immobilization has been performed via reactions with dopants that introduce reactive functional groups (e.g., glutamate),28 but could decrease conductivity. Although these techniques do not drastically affect PPy properties, some of them are more complex and have not been used yet for growth factor tethering.
Growth factors are potent modulators of cell responses. Neurotrophins, a family of growth factors, have been investigated extensively because of trophic and chemotactic effects, cell survival, and differentiation.29-31 NGF is the most studied and characterized neurotrophin, known for inducing several neuron responses, including neurite outgrowth. Although some of NGF's effects require endocytosis of the ligand-receptor complex, neurite outgrowth is not necessarily mediated by retrograde transport32-34 and is thought to be controlled by localized actin polymerization.35 As a consequence, NGF immobilized to different surfaces has proven to be effective in inducing neurite extension, turning, and sprouting.33,34,36-40 Other growth factors including insulin, epidermal growth factor, and vascular endothelial growth factor, have also been immobilized on a variety of substrates for multiple applications.41-43 A common approach for this protein immobilization is the use of arylazido-containing compounds, which can react nonspecifically with UV light by creating singlet nitrenes that undergo insertion into C—H, N—H, other bonds.44,45 Using this method, and proteins have been fixed to many substrates such as poly(vinyl alcohol), polystyrene, poly(ethylene terephthalate), and chitosan.41,42,46-49 Here we extended this approach for the surface decoration of PPy with NGF. This method is rather simple and in addition to the immobilization of the protein, the cell adhesive polymer polyallylamine (PAA) is also incorporated onto the surface of PPy, which further improves cell interfacing.
In an effort to modify PPy with a growth factor for chemical stimulation, we report the surface tethering of NGF mediated by a nonspecific arylazido-containing photolinker. This process rendered a conducting polymer capable of also stimulating neurons with specific ligands that trigger intracellular cascades to promote neurite extension. In addition, the conductivity of NGF-immobilized PPy (PPy-NGF) was not significantly affected, which was comparable to other surface modification methods16,23,26 and improved over the dopant method, and indicated it as a viable option to effectively combine multiple stimuli into a single material. Neurite outgrowth in PC12 cells cultured on this modified biomaterial (NGF surface concentration 1 ng/mm2) was possible without NGF added to the culture medium. Furthermore, electrical stimulation of cells using PPy-NGF revealed an increase in neurite length of 50% with respect to unstimulated controls. This modification of PPy allows combining electrical stimulation from the polymer with biochemical ligands on the surface, which could synergistically stimulate cells. As a consequence, this approach represents an alternative for simultaneous chemical and electrical interfacing with cells, and with only small alterations of the polymer properties.
MATERIALS AND METHODS
Polypyrrole synthesis
Polypyrrole (PPy) films were synthesized electrochemically as previously described.10,13 Briefly, microscope glass slides were coated with thin layers of chromium (3 nm) and gold (30 nm) deposited with a thermal evaporator (Denton). These slides were used for the galvanostatic deposition of the conducting polymer. Specifically, a three-electrode setup was used, which consisted of the gold-coated slide as the working electrode, a platinum gauze counter electrode, and a saturated calomel reference electrode. The films were deposited from an aqueous solution of 0.1M pyrrole monomer (Aldrich) and 0.1M sodium salt of poly(styrene sulfonate) (PSS) (Aldrich), using an offset voltage of 720 mV and a constant current of 7.2 mA. A Pine Instruments AFRDE5 bipotentiostat was used as the DC voltage source. Film thickness was determined by integrating current over time, and controlled by the passage of charge. Optically transparent films of 200 nm thickness were synthesized for cell experiments, dried, and desiccated. Thick films of 1.3 mm thickness were only synthesized for conductivity measurements. For nerve growth factor (NGF) immobilization and neurite outgrowth (without electrical stimulation), PPy slides were cut into smaller pieces (1 cm2) with a diamond cutter. Complete slides were used for electrical stimulation of cells. Plexiglas chambers (1 cm 1.5 cm) were attached to the slides and NGF immobilization and cell seeding were performed on the area enclosed by the chambers.
Conductivity measurements
Conductivity measurements of PPy and PPy-NGF films were performed using a four-point probe technique.50 PPy films were electrochemically synthesized to a thickness of 1.3 mm. The films were peeled off the gold-coated slides and fixed to unmodified glass slides with vacuum grease, and NGF immobilization was performed subsequently. A constant current was applied between two adjacent corners, and the voltage across the remaining two corners was measured to determine resistance. The conductivities of the films were calculated from these resistance measurements using the equations derived by Van der Pauw.50 Experiments were repeated two times on separate days.
NGF-FITC conjugation
NGF was conjugated to fluorescein for detection and characterization of the immobilization procedure. Forty microliters of sodium bicarbonate buffer (0.1M, pH 9) was mixed with 100 μL of NGF 2.5S (Invitrogen, 100 μg/mL) and 10 μL of fluorescein isothiocyanate in dimethyl sulfoxide (FITC, 12 mg/mL) (Molecular Probes). The reaction was performed at 48°C for ~10 h. The unreacted FITC was separated by centrifugation with size exclusion chromatography columns (Biorad, exclusion limit 6000 Da). Conjugation efficiency and degree of labeling were evaluated with a UV-vis Beckman DU500 spectrophotometer by measuring absorbance at 280 and 494 nm. NGF-FITC was only used for quantification and visualization purposes, but not for cell culture because some loss of activity was detected in PC12 cell neurite extension assays (results not shown).
A calibration curve for NGF characterization was obtained by casting known quantities of the fluorescent protein on defined areas without washing, calculating surface concentrations (ng/mm2) and capturing fluorescence images of the dry samples with a fluorescence microscope (IX-70, Olympus) using a constant exposure time. Before analysis, standards were exposed to UV light for 15 s (this was the exposure time for immobilizing NGF in the experiments), to take into account any loss of fluorescence as a result of photobleaching. For all cell culture, regular NGF was used in the same quantities as used as for NGF-FITC experiments.
NGF immobilization
NGF photochemical fixation was performed using a phenyl-azido group, a method developed by Matsuda and Sugawara46 and modified by Ito and coworkers for immobilization of growth factors in particular.41,42,47 The procedure consisted of three main steps (Fig. 1): (1) preparation of N-4-(azidobenzoyloxy)succinimide according to a previously published procedure46; (2) polyallylamine (PAA) conjugation to N-4-(azidobenzoyloxy)succinimide; and (3) fixation of NGF using the modified PAA. Briefly, N-4-(azidobenzoyloxy)succinimide was obtained by adding a solution of dicyclohexylcarbodiimide (Aldrich) (6.7 g) in tetrahydrofuran (25 mL) to a solution of N-hydroxysuccinimide (Aldrich) (3.7 g), and 4-azidobenzoic acid (TCI America) (4.8 g) in tetrahydrofuran (75 mL), followed by filtration and crystallization with isopropyl alcohol/diisopropyl ether (Aldrich). Subsequently, a solution of 15 mg of PAA (Aldrich) in 10 mL of phosphate buffered solution (PBS, pH = 7.4) was added to a solution of 13 mg of N-4-(azidobenzoyloxy)succinimide in 5 mL of N,N-dimethylformamide and stirred for 24 h at 4°C. The solution was ultrafiltered (Millipore, 10,000 Da NMWL) and washed three more times by adding 10 mL of distilled-deionized water and ultrafiltered again to finally obtain a volume of ~300 μL of photosensitive PAA-azido.
Figure 1.
Scheme of the NGF immobilization process. PAA was conjugated to an azido compound (PAA-azido). This conjugate was cast twice on PPy, followed by casting of NGF. UV light exposure promoted the formation of covalent bonds via the azido groups, immobilizing NGF to PPy. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The conjugation was confirmed by measuring UV-vis absorbance of the filtration retentate at 280 nm. Fifty microliters of this solution were cast on a PPy substrate (1 cm2), air dried, and exposed with a UV lamp (Blak-Ray, 22 mW/cm2, λmax =365 nm) for 15 s followed by three washes with 0.05M HCl and two washes with PBS. This step was followed by casting a second layer of the photosensitive PAA (50 μL) and a superimposed final layer of NGF (for cell culture) or NGF-FITC (for quantification) (0.5–2 μg in 50 μL of PBS). For controls, PBS only was added instead of NGF solution, which produced a PPy substrate only immobilized with PAA-azido (PPy-PAAazido). Finally, the substrate was exposed to UV light for 15 s and washed six times with PBS to remove unreacted protein. For neurite growth analysis, the treated substrates were subsequently glued with silicone to the bottom of sterile 3 cm tissue-culture dishes. For electrical stimulation experiments, NGF was immobilized on a PPy area (1 cm2) enclosed by a Plexiglas chamber attached to the PPy slide.
NGF ELISA assay
An ELISA assay was performed to check the concentration of any released NGF from the surface into the culture medium. PPy-NGF substrates (n = 3) were placed in 3 cm tissue-culture dishes and incubated in PBS for 3 days at 37°C. Similarly, PPy-NGF slides with chambers for electrical stimulation were incubated in the same conditions. Volumes of 200 μL were collected from the dishes at 6, 24, 48, and 72 h and analyzed with a commercially-available sandwich ELISA kit (NGF Emax kit, Promega). Briefly, 96-well ELISA plates were coated with a primary goat anti-NGF antibody overnight at 4°C, followed by blocking and incubations of samples and standards for 6 h at room temperature. Finally, a second rat anti-NGF antibody was incubated overnight, followed by incubation with anti-rat antibody conjugated to horseradish peroxidase for 2.5 h, and development with 3,3′,5,5′-tetramethylbenzidine. HCl 1M was added to all wells, and absorbance at 450 nm was recorded using a plate reader.
X-ray photoelectron spectroscopy
To confirm NGF immobilization, samples were characterized by X-ray photoelectron spectroscopy (XPS). Samples with and without immobilized NGF were analyzed. Spectra were taken on a PHI 5700 ESCA system using a monochromatic Al X-ray source operated at pass energies of 117.4 eV for surveys and 11.7 eV for high-resolution scans. The binding energy was calibrated using Au 4f, Cu 2p, and Ag 3d. Deconvolution peaks were obtained using an XPS peak fitting program (XPSPEAK 4, The Chinese University of Hong Kong) maintaining approximately constant the full width at half maximum for all peaks. A quantitative analysis of the N 1s/C 1s ratio was performed by calculating the ratio of the corresponding peak areas corrected by sensitivity factors, which results in the measurement of atomic ratios.
Cell culture
PC12 cells (ATCC) were cultured in Ham's F12K medium (Sigma) supplemented with 15% heat-inactivated horse serum (Hyclone), 2.5% fetal bovine serum (Hyclone), and 1% penicillin-streptomycin (Sigma). For experiments with serum-free conditions, cells were cultured in Ham's F12K medium with N-2 supplement (Invitrogen). Cells were cultured in standard tissue culture dishes and passaged 1:8 every 7 days. For neurite outgrowth experiments, 50 ng/mL 2.5S NGF was added to the culture medium when feeding cells for 1 week prior to the experiment (priming). NGF priming allows cells to grow neurites faster after plating. Cells were detached from the substrate using 0.25% Trypsin-EDTA (Sigma) and plated in 3 cm-tissue-culture dishes containing PPy substrates (1.25 × 104 cells/ cm2) and analyzed for neurite extension after 2 or 10 days in culture. This last time-point was found to be appropriate for demonstrating the overall ability of tethered NGF to produce long neurites after several days in culture. Control samples were cultured with NGF in solution (50 ng/mL), whereas experimental PPy-NGF samples were cultured without NGF in the medium. Experiments were repeated four times on separate days.
After 2 or 10 days in culture at 37°C and 5% CO2, PC12 cells were fixed with 4% paraformaldehyde (Sigma) and 4% sucrose (Fisher) in PBS for 20 min, followed by permeabilization for 5 min with 0.1% Triton-X 100 (Sigma) in 2% bovine serum albumin (BSA) (Jackson ImmunoResearch) in PBS, and blocking for 1 h with 2% BSA-PBS. Samples were incubated with phalloidin-TRITC (Sigma) in PBS for 30 min, washed, and imaged.
Dorsal root ganglion (DRG) neurons were obtained from 2-week-old Sprague–Dawley rat pups. The animals were acquired from a closed colony at the University of Texas Animal Resource Center and treated in accordance with the regulations established by the National Research Council in the Guide for the Care and Use of Laboratory Animals. Pups were euthanized with IsoFlo (Abbott Laboratories). The spinal column was then removed and the spinal cord exposed. The DRGs were then explanted into RPMI 1640 medium. Approximately 30 DRGs were collected from each animal, followed by incubation in Trypsin-EDTA for 30 min at 37°C, and incubation in collagenase in neurobasal medium (Sigma, 200 ng/mL) for 30 min at 37°C. DRGs were triturated extensively with a fire-polished pipette for dispersing individual cells, followed by centrifugation and plating. Neurons were cultured with neurobasal medium (Invitrogen) supplemented with B-27 (Invitrogen), l-glutamine (Fisher, 0.5 mM), and 1% antibiotic–antimycotic (Sigma, 10,000 units/mL of penicillin, 10 mg/mL of streptomycin, and 25 μg/mL of amphotericin), at a density of ~3 × 103 culture, cells/cm2 (~half DRG per substrate). After 2 days in neurons were fixed, permeabilized, and incubated with anti-GAP-43 (Sigma, 1:200) overnight, followed by incubation with a fluorescently-labeled secondary antibody (Alexa 488-conjugated, Molecular Probes). Experiments were repeated three times for two different animals.
Electrical stimulation
Plexiglas chambers were attached to PPy slides for electrical stimulation and NGF was immobilized in the area enclosed by the chamber. Electrical stimulation conditions and priming time were exactly followed as previously reported10 to compare neurite lengths with results in the literature. PC12 cells were primed for 24 h with soluble NGF (50 ng/mL) and subsequently seeded inside the Plexiglas wells (1.25–2 × 104 cells/cm2). Cells were allowed to incubate for an additional 24 h before being electrically stimulated. The PPy film served as the anode, a gold wire attached to the Plexiglas chamber served as the cathode, and a silver wire served as a quasireference electrode. A steady potential of 100 mV was applied for a total time of 2 h per sample. Cells were maintained in a CO2 incubator during the stimulation, and neurite lengths were analyzed 24 h after the stimulation. Experiments were run in duplicate and repeated three times on separate days.
Fluorescence microscopy
NGF-FITC immobilization and neurite extension were analyzed using an inverted phase contrast and fluorescence microscope. Images from the microscope were acquired using a color CCD video camera (Optronics MagnaFire, model S60800) and analyzed using Adobe Photoshop and Image J (NIH). Fluorescence images of NGF-FITC on PPy were captured with a constant exposure time and analyzed for intensity with the imaging software. For the different surface concentrations, experiments were repeated at least four separate times, and a total of 10–20 images were analyzed for fluorescence intensity per condition, averaged, and the standard error of the mean (SEM) calculated. The average was compared to an established standard curve, as explained in the NGF-FITC conjugation section, to determine the surface concentration. Statistical differences were analyzed with a Student's t-test.
Neurite extension analysis
The lengths of individual neurites were measured as previously described.10,13 Length was defined as the distance from the tip of the neurite to the junction between the cell body and neurite base. In the case of branched neurites, the length of the longest branch was measured from the tip of the neurite to the cell body, and then each branch was measured from the tip of the neurite to the neurite branch point. Neurite length was not normally distributed; therefore the complete distribution and median length are reported. Statistical differences between medians were calculated with the nonparametric Kruskal–Wallis test51 using the χ2-distribution for the level of significance. Percentage of cells with neurites was calculated by only counting cells with neurites longer than one cell body. Three samples were analyzed per condition, averaged, and the SEM calculated. Statistical differences were analyzed with a Student's t-test.
Scanning electron microscopy
PPy and PPy-NGF were analyzed with scanning electron microscopy (SEM) for surface morphology. Substrates were covered with a gold layer of a few angstroms thick. A LEO 1530 scanning electron microscope was used with an acceleration voltage of 10 kV. Cells on PPy substrates were fixed with 4% paraformaldehyde (Aldrich) and 4% sucrose in PBS for 20 min, and dehydrated with increasing concentrations of ethanol (30–100%) for a total time of 2 h, followed by 5 min with hexamethyl-disilazane (Sigma). After drying, samples were covered with a gold layer of a few angstroms thick and imaged with an acceleration voltage of 1 kV.
Atomic force microscopy
Atomic force microscopy (AFM) images were acquired using an Asylum Research MFP-3D Atomic Force Microscope for measuring surface roughness. Standard silicon cantilevers, AC240TS (Olympus Optical), were used in alternating current mode at a frequency of 72 kHz and a scan rate of 0.75 Hz. Root mean square (RMS) surface roughness was measured using MFP-3D software written in Igor Pro 5 (WaveMetrics).
RESULTS AND DISCUSSION
NGF immobilization
PPy has been proposed as an attractive material for neuron interfacing because of its electrical properties. Neurons not only respond to an electrical stimulus, but to a great variety of other factors, including chemical signals mediated by ligand-receptor complexes. As a consequence, the ability to modify biomaterials, including PPy, to present more than one stimulus simultaneously could represent a significant advantage over simpler materials. Following this idea, we report our efforts to immobilize NGF on PPy. The conjugation approach we have applied consists of using phenylazido-containing compounds. This technique has been used in numerous studies to nonspecifically create covalent bonds between a compound containing the azido functional group and a substrate, including NGF.36,37,41,43,46-49 In this type of photochemistry, aryl azides create highly reactive singlet or triplet nitrenes after irradiation, which can subsequently insert into a variety of bonds, including carbon–hydrogen and nitrogen–hydrogen bonds.44,45 Taking advantage of this high nonspecific reactivity, we successfully used this approach to immobilize NGF on PPy.
The NGF immobilization process is depicted in Figure 1. An initial conjugation step included the acylation of the azido-photolinker with PAA, an amine-containing polymer capable of reacting with the succinimide groups of the photolinker, and also known to enhance cell adhesion. The product of this conjugation was a photosensitive polymer (PAA-azido) containing phenyl-azido functional groups, confirmed by an absorbance peak at 280 nm. By casting an aqueous solution of PAA-azido and a superimposed layer of NGF, the protein could be immobilized by UV light activating the azido groups to create covalent bonds with both the protein and the substrate. Because of the multiple insertion mechanisms that singlet nitrenes can undergo, the covalent bonds with PPy and NGF could form in a variety of ways, such as via C—H or N—H insertion.
The efficiency of this reaction was analyzed using fluorescence microscopy. NGF was conjugated to FITC to render a fluorescent protein (NGF-FITC), which was only used for detection purposes and not for cell culture because of reduced bioactivity. Using this fluorescent conjugate it was possible not only to determine the presence of the growth factor after the immobilization process, but also to estimate the surface concentration. For this semiquantitative analysis, known concentrations of NGF-FITC were cast on PPy, fluorescence images were captured and their intensity determined. A calibration curve relating fluorescence intensity to surface concentration (ng/mm2) [Fig. 2(D)] was established and subsequently used to analyze the experimental samples. This method only provided an overall estimation of the surface concentration due to the nonuniform distribution of the protein on both the standards and the samples.
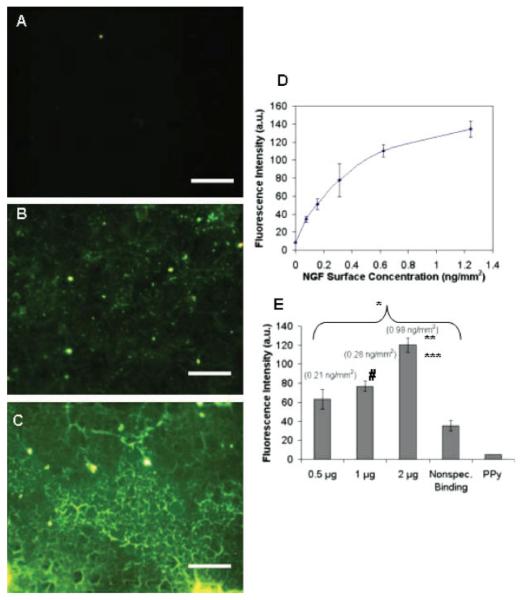
Figure 2.
Fluorescence images of PPy-NGF-FITC. A: Polypyrrole only; B: control sample without UV exposure and washes (1 μg of NGF-FITC added); C: experimental sample with UV exposure and washes (1 μg of NGF-FITC added); D: calibration curve for different concentrations of NGF-FITC on PPy (error bars correspond to standard deviation); E: quantitative analysis of fluorescence intensity for samples with different amounts of NGF-FITC added and controls (average ± SEM). Nonspecific binding control was performed without UV exposure for 1 μg of NGF-FITC added. The values in parenthesis correspond to the surface concentrations determined by comparing the average of the fluorescence intensity with the calibration curve in (D). *, **, ***Statistically significant difference with respect to PPy, 0.5 and 1 μg, respectively, using t-test with p < 0.001. #Statistically significant difference with respect to nonspecific binding control with p < 0.001. Scale bar = 200 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 2 illustrates fluorescence images of NGF-FITC immobilized via PAA-azido on PPy. By changing the concentration of protein cast, it was also possible to modulate the concentration of immobilized protein. In particular, 0.5–2 μg total protein added to the reaction yielded approximate surface concentrations between 0.2 and 1 ng/mm2 (determined from average of fluorescence intensity in Figure 2(E) and compared to calibration curve). These values are similar to previous reports where 6 fg/μm2 (6 ng/mm2) of NGF were immobilized on beads that successfully produced turning of DRG neurons.52 The nonlinearity of the results could be a result of (1) the fluorescence variability because of the nonuniform distribution of the protein, and (2) the analysis itself, as the calibration curve is not completely linear [Fig. 2(D)]. Although control samples with no UV light exposure revealed a relatively high nonspecific binding [Fig. 2(B)], the average concentration of nonspecifically adsorbed NGF (i.e., fluorescence intensity) was still statistically lower than the concentration on UV-exposed samples (p = 1 × 10−4) [Fig. 2(E)], which confirmed the effective activation of the azido groups. However, the reaction efficiency was low. At most, for an area of 1 cm2, the amount of immobilized protein corresponded to 100 ng, which was ~5% of the cast protein. Similar values have been reported for this type of chemistry in the literature.33
PPy-NGF characterization
Conductivity measurements were performed to analyze the changes in the electrical properties of the polymer as a result of the immobilization procedure. PPy-NGF had a conductivity of 9.3 ± 2 S cm−1 which represented a decrease of 30% compared to the value of 14.5 − 2 S cm−1 for PPy only (not statistically different, p 0.12 using t-test). PPy-NGF retained the electrical conductivity and the reduction was comparable with values from other surface chemistries, which vary between 16 and 60% depending on the amount of functionalization.16,23,25 Furthermore, this decrease in conductivity is minimal compared to differences in orders of magnitude found for PPy doped uniquely with biomolecules (~10−3 S cm−1).20 or for PPy derivatives synthesized with modified monomers having functional groups (~10−6 S cm−1).53
Stability studies were performed in PBS at 37°C to analyze the amount of NGF leaching out from the surface over time, as a result of nonspecific binding of the protein. This was an important factor to consider ensuring that the observed effects on cells were caused by the immobilized and not the soluble form of NGF. Using a standard sandwich ELISA assay, we analyzed sample volumes of PPy-NGF substrates, including slides with Plexiglas chambers for electrical stimulation, incubated in PBS at 37°C for 72 h. The average concentration of NGF leached into solution was 1.06 ± 0.28 and 1.02 6± 0.21 ng/mL (n = 3), for PPy-NGF on tissue culture dishes and inside Plexiglas chambers respectively; this concentration of NGF has been previously shown to have negligible cellular effects.54
To further confirm the presence of the immobilized protein, XPS analysis was performed on treated and untreated PPy substrates (Fig. 3). High resolution spectra of C1s [Fig. 3(A,C)] revealed a main peak for C—C polymer backbone at 285.2 eV and a peak at 286.2 eV attributed to C—N species. After the immobilization process, the C—N contribution increased and a new peak at 288.3 eV appeared, which corresponds to N—C=O species. Theincrease in C—N was expected from the presence of both the protein and PAA, whereas the peak at 288.3 eV was believed to correspond to amide bonds from NGF. The deconvoluted N 1s core level spectra showed two peaks for PPy corresponding to N—H species (400.2 eV), and one attributed to positively charged nitrogen C—N+ (401.7 eV).23,55-57 The modified polymer with NGF had three main peaks, one for N—H, one for C—N+ and one attributed to amide bonds (399.5 eV).58 The increase in the C—N+ contribution is not expected to be produced by the protein itself, as low levels of quaternary amines have been reported for proteins,59 and this peak was absent in spectra for positive control samples with cast protein (without washing). This suggests that the significant increase in C—N+ species could correspond to a modification of PPy with NGF tethering. We speculate that this increase might be a product of coupling of PAA via the nitrogen in the pyrrole ring and the corresponding formation of quaternary amines. An XPS semiquantitative analysis for finding N 1s/C 1s ratios also corroborated the presence of PAA and NGF, which revealed an increasing trend in the N 1s/C 1s ratio with 0.03 for PPy, 0.08 for PPy with PAA only, and 0.10–0.13 for PPy-NGF with 1–2 μg total protein cast.
Figure 3.
XPS spectra of PPy (A,C) and PPy-NGF (B,D). High resolution spectra of (A) C 1s of PPy only; (B) C 1s of PPy-NGF; (C) N 1s of PPy only; (D) N 1s of PPy-NGF. Both carbon and nitrogen spectra showed the increase in C—N and N—C=O peaks, as a result of the immobilized protein.
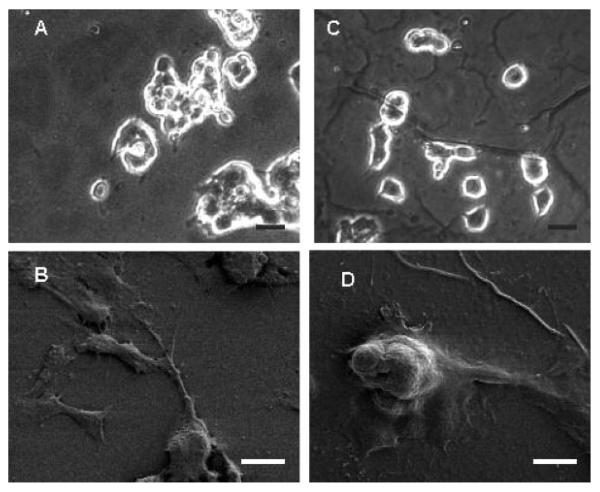
The immobilization process also produced a noticeable change in the surface topography of PPy as illustrated in Figure 4. Wrinkling of the surface was apparent at a macroscopic level, probably because of stresses induced by the drying steps and the conjugation chemistry.60 In addition, there was also a slight increase in roughness at the microscopic level because of the presence of PAA-Azido and NGF, as evidenced by the presence of bigger and more globular nodules on the surface in both SEM and AFM images. AFM measurements supported this increase, giving an RMS roughness of 60.0 and 14.6 nm for PPy-NGF and PPy, respectively. Roughness plays a role in cell interfacing, as it has been shown to affect cell adhesion.61-63 An increase in surface area has also been reported to be beneficial for neural electrodes.9 As a result, PC12 cells spread more on PPy-NGF than on PPy (Fig. 5), probably the result of a combination of the positively charged PAA layer and increased roughness. This represents a more intimate contact between cells and the polymer, which could be beneficial for either recording or stimulating neurons.3
Figure 4.
SEM and AFM of PPy and PPy-NGF. A,B: SEM images of PPy only (magnification 1 KX and 5 KX, respectively); D,E: SEM images of PPy-NGF (magnification = 1 KX and 5 KX, respectively); C,F: AFM images 5 × 5 μm2 scan for PPy and PPy-NGF, respectively. The increase in surface roughness is a consequence of the immobilization procedure. Scale bars = 10 μm (A,D) and 1.5 μm (B,E).
Figure 5.
Phase-contrast and SEM images of PC12 cells on PPy and PPy-NGF. A,B: PC12 cells on PPy; C,D: PC12 cells on PPy-NGF. Cells spread more on PPy-NGF as a result of increased surface roughness and the presence of PAA. Scale bars = 20 μm (A,C) and 10 μm (B,D).
PC12 cell and DRG neuron neurite extension
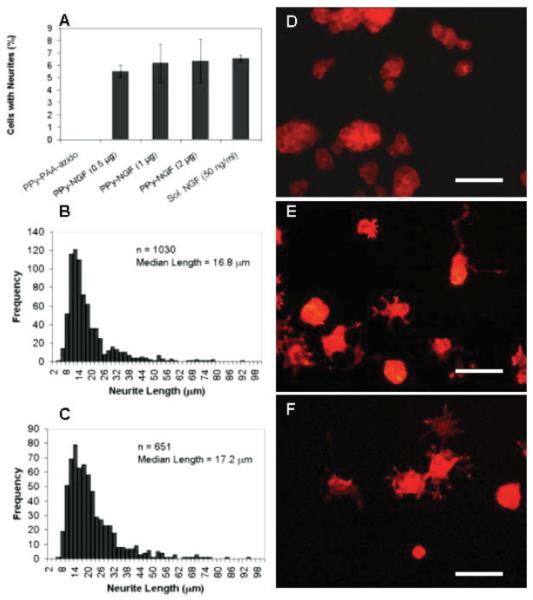
PC12 cells are neuron-like cells that differentiate and extend processes in the presence of NGF, and have been extensively studied for the effects and signaling of NGF.64,65 Figures 6 and 7 illustrate neurite extension results for these cells cultured on PPy-NGF with different NGF surface concentrations, after 2 or 10 days in culture, respectively (see all conditions in Table I). As controls, cells cultured on PPy with PAA-azido only (no immobilized NGF) were studied with and without NGF in the medium, to guarantee similar roughness and surface charge in all samples and rule out any possible advantages from these. Histograms of neurite lengths were obtained and the median for each of the samples calculated. Additionally, the percentage of cells with neurites longer than one cell body was calculated and summarized in Table I.
Figure 6.
Neurite extension from PC12 cells on PPy-NGF after 2 days in culture. A: Percentage of cells with neurites longer than one cell body (average ± SEM). PAA-azido corresponds to PPy modified with only PAA-azido but no NGF. NGF in solution corresponds to PPy-PAA-azido with soluble NGF. No neurites were observed for PPy-PAA-azido samples; B: neurite length histogram for PC12 cells cultured on PPy-NGF with 0.98 ng/mm2 (2 μg of NGF added); C: neurite length histogram for PC12 cells cultured on PPy-PAA-azido with soluble NGF (50 ng/mL). D–F: representative fluorescence images of PC12 cells cultured on PPy-PAA-azido, PPy-NGF (0.98 ng/mm2), and PPy-PAA-azido with soluble NGF (50 ng/mL), respectively. Cells were primed with NGF in solution for 1 week prior to experiments, seeded on substrates and fixed and stained with phalloidin-TRITC after 2 days in culture. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 7.
Neurite extension from PC12 cells on PPy-NGF after 10 days in culture. A: Percentage of cells with neurites longer than one cell body (average ± SEM). PAA-azido corresponds to PPy modified with only PAA-azido but no NGF. NGF in solution corresponds to PPy-PAA-azido with soluble NGF; B: neurite length histogram for PC12 cells cultured on PPy-NGF with 0.98 ng/mm2 (2 μg of NGF added); C: neurite length histogram for PC12 cells cultured on PPy-PAA-azido with soluble NGF (50 ng/mL).
TABLE I.
PC12 Cell Neurite Extension on PPy-NGF
| Experimental Conditions | Median Length (μm)b |
Percentage of Cells with Neuritesc |
|---|---|---|
| No electrical stimulationa | ||
| 2 Days in culture | ||
| PPy-PAA-azido | N/A | 0 (570) |
| PPy-NGF (0.5 μg) | 13.5 (636) | 5.52 ± 0.49 (945) |
| PPy-NGF (1 μg) | 13.4 (770) | 6.18 ± 1.50 (1333) |
| PPy-NGF (2 μg) | 16.8 (1030) | 6.35 ± 1.74 (1730) |
| PPy-PAA-azido + sol. NGF (1 ng/mL) | 9.2 (622) | 0.51 ± 0.18 (1229) |
| PPy-PAA-azido + sol. NGF (50 ng/mL) | 17.2 (651) | 6.55 ± 0.27 (932) |
| 10 Days in culture | ||
| PPy-PAA-azido | N/A | 0.52 ± 0.16 (715) |
| PPy-NGF (0.5 μg) | 8.4 (207) | 2.06 ± 1.00 (859) |
| PPy-NGF (1 μg) | 11.6 (451) | 4.38 ± 2.40 (1979) |
| PPy-NGF (2 μg) | 42.2 (556) | 8.95 ± 1.45 (3825) |
| PPy-PAA-azido + sol. NGF (50 ng/mL) | 42.1 (246) | 13.74 ± 0.58 (1056) |
| Electrical stimulationd | ||
| PPy-NGF (2 μg) with electrical stimulation | 12.0 (1383) | 1.44 ± 0.24 (3798) |
| PPy-NGF (2 μg) without electrical stimulation | 8.0 (765) | 0.30 ± 0.15 (2963) |
N/A, no neurite histogram was obtained because very few neurites were observed.
Cells were primed with soluble NGF for 1 week.
Neurite distribution was obtained (non-Gaussian) and the median length is reported. Values in parentheses indicate total number of neurites analyzed.
Cells with neurites longer than one cell body (average ± SEM of three samples). Values in parentheses indicate total number of cells analyzed.
Cells were primed with soluble NGF for 24 h and analyzed after 2 days as previously described in Ref. 10.
As suggested by the results, PC12 cells could successfully grow neurites on PPy with immobilized NGF. After 2 days in culture, PC12 cells cultured on PPy-NGF (2 μg NGF added to reaction, 0.98 ng/mm2) extended neurites with similar lengths (median length = 16.8 μm) as controls with PAA-azido and soluble NGF (median length = 17.2 μm, p = 0.51). Furthermore, when the percentage of cells with neurites was analyzed, a value of ~6% was obtained for all concentrations of immobilized NGF, as well as for soluble NGF (50 ng/mL) [Fig. 6(A)]. It is important to emphasize that these results were not a product of soluble NGF leaching out of the surface, as controls with PAA-azido and 1 ng/mL of soluble NGF (same concentration as detected with ELISA from stability studies) did not exhibit any significant neurite extension (Table I).
In addition, we also confirmed that PPy-NGF was not promoting additional adsorption of serum proteins from the medium that could indirectly enhance neurite extension. We repeated experiments for PPy-NGF with 0.98 ng/mm2 in serum-free conditions, and found that the median length was 18.02 μm (n = 776). This actually represented a statistically significant but small increase with respect to serum-containing media experiments (p = 0.002), which suggests that protein adsorption on the surface masked the immobilized NGF to a small extent.
PC12 cell neurite extension was also analyzed at a longer time point, 10 days, to further investigate the enduring effect of immobilized NGF. As summarized in Table I and Figure 7, immobilized NGF still supported neurite extension for a surface concentration of 0.98 ng/mm2 (median length = 42.2 μm, p = 0.8 with respect to soluble NGF), but was significantly decreased for 0.28 ng/mm2 (median length = 11.6 μm) and 0.21 ng/mm2 (median length = 8.4 μm). More importantly, the percentage of cells having neurites at this time-point was statistically lower for all NGF surface concentrations (p = 0.004, 0.03, 0.05 for 0.21, 0.28, 0.98 ng/mm2, respectively), when compared to controls on PAA-azido with soluble NGF [Fig. 7(A)]. These results suggest a possible detrimental effect resulting from the inhibition of NGF endocytosis and signaling (e.g., for differentiation),31 which at the same time might not markedly interfere with enhanced localized actin polymerization at the growth cone.
NGF has been immobilized with retained activity in many other studies, and effects on PC12 cells have been already reported by other groups.33,34,36-40 Here, we observed similar results for primed PC12 cells cultured on PPy-NGF, which exhibited comparable neurite extension to controls with soluble NGF for short-time points (2 days). However, we also observed some important differences for longer time points (10 days). Although neurite elongation was very similar between the highest concentration of immobilized NGF and the soluble form, there was a significant decrease in the total number of cells actually having neurites. We hypothesize that this discrepancy is a result of inhibition of endosome signaling by tethered NGF.
Previous studies showed that after NGF binds to TrkA receptors on the cellular membrane, the receptor-ligand complex is internalized and transported to the cell body, which triggers intracellular signaling cascades.65 However, not all signaling is dependent on internalization. Zhang et al. reported marked differences in intracellular pathways for internalized receptors versus “surface receptors”.31 This group demonstrated that noninternalized receptors exhibited phosphorylation of tyrosines after NGF binding that triggered a long-lasting activation of the PI 3-kinase pathway that is critical for survival. In contrast, they also demonstrated that endocytosis was essential for differentiation, and therefore neurite extension in PC12 cells, as these cells only extend neurites when differentiated. However, it has also been shown that PI 3-kinase regulates neurite extension. Gallo and Letourneau showed that axon sprouting on NGF-coated beads was dependent on PI 3-kinase activity,34 and more recently, Aoki et al. demonstrated that activation of Cdc42 and Rac1, GTPases that modulate actin polymerization, was locally controlled by PI 3-kinase.35
In light of these studies, we hypothesize that in early time points, primed PC12 cells are capable of easily extending neurites as a result of the priming (i.e., cells are already differentiated with soluble NGF for a week) and the local activation of PI-3 kinase pathway by immobilized NGF. However, for longer time points, two opposite effects could be present: (1) decreased differentiation and neurite extension as a result of endosome inhibition and (2) enhanced activation of PI-3 kinase pathways, which would locally stimulate actin reorganization for neurite growth. The combination of these two factors could explain the decrease in cells having neurites after 10 days in culture on PPy-NGF, but the similar neurite lengths of these processes.
As PC12 cells are not able to extend neurites without being differentiated, and this appears to depend on receptor endocytosis, we reconfirmed the proposed localized activation of neurite growth by PPy-NGF with another cell type that could extend neurites without having conflicting effects on the endocytosis pathway. Rat DRG neurons were cultured on PPy-NGF and controls and analyzed again for neurite extension after 2 days in culture. As depicted in Figure 8, PPy-NGF also promoted neurite outgrowth in DRG neurons when compared to PPy-PAA-azido only, and the response was statistically different but only slightly smaller than soluble NGF controls. This further supported the fact that immobilized NGF was active and capable of increasing neurite outgrowth from neurons.
Figure 8.
Neurite extension from DRG neurons on PPy-NGF after 2 days in culture. Neurite length histograms for neurons cultured on (A) PPy-PAA-azido; (B) PPy-NGF with 0.98 ng/mm2; and (C) PPy-PAA-azido with soluble NGF (50 ng/mL).
PC12 cell neurite extension with electrical stimulation
Previous studies on PPy have shown an increase in neurite length with electrical stimulation.10 Because of these results, we wanted to explore if this effect was also observed with PPy-NGF. For these experiments, electrical stimulation protocols were exactly followed as previously published.10 The experiments were performed with constant voltage to evaluate the overall characteristics of the modified material, including both the decrease in conductivity and the presence of immobilized NGF. As shown in Table I, there was an enhancement in neurite length for PPy-NGF with electrical stimulation compared to PPy-NGF without stimulation (12 and 8 μm, respectively; statistically different for p < 0.001). This result suggests an additive effect of electrical stimulation and chemical stimulation provided by the immobilized NGF. However, the median neurite length on PPy-NGF was lower than for unmodified PPy controls with NGF in solution. In that case, electrically-stimulated cells had a median length of 17 μm(n = 317), whereas cells with no stimulation had a median length of 8 μm(n = 244).
A possible explanation for this difference in median length when compared to controls could be again that PC12 cells exhibited restricted responses to immobilized NGF because of endosome signaling inhibition. Also, although the chemical grafting is a surface modification, there is a slight decrease in conductivity that correlates to a decrease in current passed through PPy-NGF, especially on the surface, compared to the current passed through unmodified PPy. However, it is important to emphasize that although the increase in neurite length with electrical stimulation on PPy-NGF was small, it was still statistically significant, which again suggests a positive impact from the combination of stimuli. Further optimization of electrical stimulation protocols will be necessary to maximize neurite extension.
The effects of electrical stimulation on neurite growth are still unclear. In these studies, current was continuously passed through PPy-NGF slides for 2 h at a constant voltage of 100 mV, as previously described.10 Although the mechanism for enhanced cell growth in these experiments is not known, this stimulation is different from conventional electrical stimulation with electrodes, where higher voltages or currents are used for very short pulses with the purpose of depolarizing cells or producing action potentials.52 In our case, electrical stimulation was constant for a longer time, which has been shown to have multiple effects, for example on protein adsorption.13 Patel and Poo also described in an early paper the effect of steady electric fields in neurite growth, and explained that electric fields of 0.10–10 V/cm produced intracellular potential differences of 0.01–1 μV and transmembrane potentials of 0.5–5 μV, which could produce continuous movement of charged molecules inside and outside the cell and modulate neuron behavior.66 In this same paper, the authors also suggested the effect of the electric field on surface glycoproteins. More recently, Howe showed that depolarization with high potassium chloride solutions increased the phosphorylation of TrkA receptors and neurite growth,67 which could have some influence in our experiments, although the degree of depolarization in our studies is probably very small.
Immobilization of NGF on PPy represents a significant modification of this biomaterial. Although electrical stimulation is a key factor to include in nerve repair therapies, it is desirable to include multiple cues to maximize cell responses. Growth factors such as NGF are important mediators that trigger a variety of cell behaviors. It is believed that the presence of NGF for nerve regeneration therapies might be crucial, and attempts to deliver it by different methods have been explored.68-70 Similarly, released NGF has also been used for neural probes to improve interfacing between the cells and the electrodes.3 Although NGF is a soluble factor, it has been immobilized with retained activity in several studies.33,34,36-40 The immobilized biomolecule could provide a more stable and long-lasting stimulus, as opposed to the release of soluble NGF, which might be drastically affected by rapid degradation. Even more, it has been proposed that cellular responses to immobilized growth factors are enhanced as a result of inhibition of down-regulation processes.71 Therefore, the immobilization of NGF could be an alternative method for the presentation of this protein for neural applications. With respect to PPy itself, the use of conducting polymers for nerve regeneration and electrodes has been proposed for several years.4-14 Here, we combined these two important stimuli to render a conducting polymer bioactive, which enhanced neurite outgrowth in vitro.
CONCLUSIONS
NGF was successfully immobilized on PPy using PAA-azido as a linking compound. Three different surface concentrations were studied, and it was determined that a surface concentration of 0.98 ng/mm2 produced similar neurite extension in PC12 cells compared to soluble NGF (50 ng/mL) after 2 days in culture. However, although immobilized NGF maintained the effect on neurite length after 10 days in culture, some detrimental effects were observed as a potential consequence of inhibition of endocytosis-dependent pathways. In addition, electrical stimulation using PPy-NGF further increased neurite length with respect to controls without stimulation. This suggested a possible additive effect of electrical and chemical stimuli, both exclusively provided by either the bulk or the surface properties of the material. Cell adhesion was also improved as a consequence of the positively charged PAA and the increased roughness from the immobilization procedure. This modified material could potentially be used for nerve regeneration strategies such as nerve conduits and neural electrodes to improve chemical interfacing with neurons.
Acknowledgments
The authors would like to thank Curt Deister for the assistance with the DRG dissection, and John Slater and Wolfgang Frey for the assistance with the AFM.
Contract grant sponsor: Gillson Longenbaugh
Contract grant sponsor: NIH; contract grant number: R01 EB004529
References
- 1.Chen J, Wise KD, Hetke JF, Bledsoe SC. A multichannel neural probe for selective chemical delivery at the cellular level. IEEE Trans Biomed Eng. 1997;44:760–769. doi: 10.1109/10.605435. [DOI] [PubMed] [Google Scholar]
- 2.Retterer ST, Smith KL, Bjornsson CS, Neeves KB, Spence AH, Turner JN, Shain W, Isaacson MS. Model neural prostheses with integrated microfluidics: A potential intervention strategy for controlling reactive cell and tissue responses. IEEE Trans Biomed Eng. 2004;51:2063–2073. doi: 10.1109/TBME.2004.834288. [DOI] [PubMed] [Google Scholar]
- 3.Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng. 2001;48:361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- 4.George PM, Lyckman AW, LaVan DA, Hedge A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials. 2005;26:3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24:777–787. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Abidian M, Martin DC. Conducting polymers grown in hydrogels scaffolds coated on neural prosthetic devices. J Biomed Mater Res A. 2004;71:577–585. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Martin DC. Microporous conducting polymers on neural microelectrode arrays I electrochemical deposition. Sens Actuators B. 2004;101:133–142. [Google Scholar]
- 8.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56:261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Cui X, Hetke JF, Wiler JA, Anderson DJ, Martin DC. Electro-chemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sens Actuators A. 2001;93:8–18. [Google Scholar]
- 10.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc Natl Acad Sci USA. 1997;94:8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RL, Doherty PJ. A preliminary assessment of poly (pyrrole) in nerve guide studies. J Mater Sci Mater Med. 1994;5:429–433. [Google Scholar]
- 12.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, Yao J, Chen F, Chen G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2004;68:411–422. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 13.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–1064. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen SJ, Wang DY, Yuan CW, Wang XD, Zhang P, Gu XS. Template synthesis of the polypyrrole tube and its bridging in vivo sciatic nerve regeneration. J Mater Sci Lett. 2000;19:2157–2159. [Google Scholar]
- 15.Garner B, Hodgson J, Wallace GG, Underwood PA. Human endothelial cell attachment to and growth on polypyrrole-heparin is vitronectin dependent. J Mater Sci Mater Med. 1999;10:19–27. doi: 10.1023/a:1008835925998. [DOI] [PubMed] [Google Scholar]
- 16.Cen L, Neoh KG, Li Y, Kang ET. Assessment of in vitro bioactivity of hyaluronic acid sulfated hyaluronic acid functionalized electroactive polymer. Biomacromolecules. 2004;5:2238–2246. doi: 10.1021/bm040048v. [DOI] [PubMed] [Google Scholar]
- 17.De Giglio E, Sabbatini L, Colucci S, Zambonin G. Synthesis, analytical characterization, and osteoblast adhesion properties on RGD-grafted polypyrrole coating on titanium substrates. J Biomater Sci Polym Ed. 2000;11:1073–1083. doi: 10.1163/156856200743580. [DOI] [PubMed] [Google Scholar]
- 18.Wong JY, Langer R, Ingber DE. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc Natl Acad Sci USA. 1994;91:3201–3204. doi: 10.1073/pnas.91.8.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner B, Georgevich A, Hodgson AJ, Liu L, Wallace GG. Polypyrrole-heparin composites as stimulus-responsive substrates for endothelial cell growth. J Biomed Mater Res. 1999;44:121–129. doi: 10.1002/(sici)1097-4636(199902)44:2<121::aid-jbm1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Collier JH, Camp JP, Hudson TW, Schmidt CE. Synthesis and characterization of polypyrrole-hyaluronic acid composite biomaterials for tissue engineering applications. J Biomed Mater Res. 2000;50:574–584. doi: 10.1002/(sici)1097-4636(20000615)50:4<574::aid-jbm13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt CE, Baier Leach J. Neural tissue engineering: Strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson AJ, John MJ, Campbell T, Georgevich A, Woodhouse S, Aoki T, Ogata N, Wallace GG. Integration of biocomponents with synthetic structures—Use of conducting polymer poly-electrolytes composites. SPIE. 1996;2716:164–176. [Google Scholar]
- 23.Cen L, Neoh KG, Kang ET. Surface functionalization of electrically conductive polypyrrole film with hyaluronic acid. Langmuir. 2002;18:8633–8640. [Google Scholar]
- 24.Li Y, Neoh G, Kang ET. Controlled release of heparin from polypyrrole-poly(vinyl alcohol) assembly by electrical stimulation. J Biomed Mater Res A. 2005;73:171–181. doi: 10.1002/jbm.a.30286. [DOI] [PubMed] [Google Scholar]
- 25.Cen L, Neoh KG, Kang ET. Surface functionalization of polypyrrole film with glucose oxidase and viologen. Biosens Bio-electron. 2003;18:363–374. doi: 10.1016/s0956-5663(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 26.De Giglio E, Sabbatini L, Zambonin PG. Development and analytical characterization of cysteine-grafted polypyrrole films electrosynthesized on Pt- and Ti-subtrated as precursors of bioactive interfaces. J Biomater Sci Polym Ed. 1999;10:845–858. doi: 10.1163/156856299x00919. [DOI] [PubMed] [Google Scholar]
- 27.Sanghvi A, Miller KP, Belcher AM, Schmidt CE. Biomaterials functionalization using a novel peptide that selectively binds to a conducting polymer. Nat Mater. 2005;4:496–502. doi: 10.1038/nmat1397. [DOI] [PubMed] [Google Scholar]
- 28.Song HK, Toste B, Ahmann K, Hoffman-Kim D, Palmore GTR. Micropatterns of positive guidance cues anchored to polypyr-role doped with polyglutamic acid: A new platform for characterizing neurite formation in complex environments. Biomaterials. 2006;27:473–484. doi: 10.1016/j.biomaterials.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Ebadi M, Bashir RM, Heidrick ML, Hamada FM, El Refaey H, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 30.Letourneau PC. Chemotactic response of nerve fiber elongation to nerve growth factor. Dev Biol. 1978;66:183–196. doi: 10.1016/0012-1606(78)90283-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campenot RB. NGF and the local control of nerve terminal growth. J Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- 33.Gallo G, Lefcort FB, Letourneau PC. The TrkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki K, Nakamura T, Matsuda M. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J Biol Chem. 2004;279:713–719. doi: 10.1074/jbc.M306382200. [DOI] [PubMed] [Google Scholar]
- 36.Ito Y. Regulation of cellular gene expression by artificial materials immobilized with biosignal molecules. Jpn J Artif Organs. 1998;27:541–544. [Google Scholar]
- 37.Kapur TA, Shoichet MS. Chemically-bound nerve growth factor for neural tissue engineering applications. J Biomater Sci Polym Ed. 2003;14:383–394. doi: 10.1163/156856203321478883. [DOI] [PubMed] [Google Scholar]
- 38.Kapur TA, Shoichet MS. Immobilized concentration gradients of nerve growth factor guide neurite outgrowth. J Biomed Mater Res A. 2004;68:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- 39.Maclnnis B, Campenot RB. Retrograde support of neuronal survival without retrograde transport of nerve growth factor. Science. 2002;295:1536–1539. doi: 10.1126/science.1064913. [DOI] [PubMed] [Google Scholar]
- 40.Chen P, Chen M, Sun J, Chen M, Tsai C, Lin F. Biocompatibility of NGF-grafted GTG membranes for peripheral nerve repair using cultured Schwann cells. Biomaterials. 2004;25:5667–5673. doi: 10.1016/j.biomaterials.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 41.Ito Y, Kondo S, Chen G, Imanishi Y. Patterned artificial juxtacrine stimulation on cells by covalently immobilized insulin. FEBS Lett. 1997;403:159–162. doi: 10.1016/s0014-5793(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Ito Y, Imanishi Y. Photoimmobilization of epidermal growth factor enhances its mitogenic effect by artificial juxtacrine signaling. Biochim Biophys Acta. 1997;1358:200–208. doi: 10.1016/s0167-4889(97)00065-7. [DOI] [PubMed] [Google Scholar]
- 43.Zisch AH, Chenk U, Schense JC, Sakiyama-Elbert SE, Hubbel JA. Covalently conjugated VEGF-fibrin matrices for endothelialization. J Control Release. 2001;71:101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 44.Patai S. The Chemistry of Azido Group. Interscience Publishers; London: 1971. [Google Scholar]
- 45.Scriven EF. Azides and Nitrenes, Reactivity and Utility. Academic Press; Orlando: 1984. [Google Scholar]
- 46.Matsuda T, Sugawara T. Photochemical protein fixation on polymer surfaces via derivatized phenyl azido group. Langmuir. 1995;11:2272–2276. [Google Scholar]
- 47.Ito Y, Chen G, Imanishi Y. Micropatterned immobilization of epidermal growth factor to regulate cell function. Bioconjug Chem. 1998;9:277–282. doi: 10.1021/bc970190b. [DOI] [PubMed] [Google Scholar]
- 48.Chen G, Ito Y. Gradient micropattern immobilization of EGF to investigate the effect of artificial juxtacrine stimulation. Biomaterials. 2001;22:2453–2457. doi: 10.1016/s0142-9612(00)00432-4. [DOI] [PubMed] [Google Scholar]
- 49.Chung TW, Lu YF, Wang SS, Lin YS, Chu SH. Growth of human endothelial cells on photochemically grafted Gly-Arg-Asp (GRGD) chitosans. Biomaterials. 2002;23:4803–4809. doi: 10.1016/s0142-9612(02)00231-4. [DOI] [PubMed] [Google Scholar]
- 50.Van der Pauw LJ. A method of measuring specific resistivity and hall effect of discs of arbitrary shape. Phillips Res Rep. 1958;13:1–9. [Google Scholar]
- 51.Randles RH, Wolfe DA. Introduction to the Theory of Non-parametric Statistics. New York: Wiley: 1979. [Google Scholar]
- 52.Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods. 1996;65:1–17. doi: 10.1016/0165-0270(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 53.Maeda S, Corradi R, Armes SP. Synthesis and characterization of carboxylic acid-functionalized polypyrrole-silica microparticles. Macromolecules. 1995;28:2905–2911. [Google Scholar]
- 54.Cao X, Shoichet MS. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001;103:831–840. doi: 10.1016/s0306-4522(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Roberge C, Wan Y, Dao LH, Guidoin R, Zhang Z. A biodegradable electrical bioconductor made of polypyrrole nanoparticle/poly(D,L-lactide) composite: A preliminary in vitro biostability study. J Biomed Mater Res A. 2003;66:738–746. doi: 10.1002/jbm.a.10037. [DOI] [PubMed] [Google Scholar]
- 56.Ebarvia B, Cabanilla S, Sevilla F., II Biomimetic properties and surface studies of piezoelectric caffeine sensor based on electro-synthesized polypyrrole. Talanta. 2005;66:145–152. doi: 10.1016/j.talanta.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Shi G, Rouabhia M, Wang Z, Dao LH, Zhang Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterials. 2004;25:2477–2488. doi: 10.1016/j.biomaterials.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 58.Xiao SJ, Textor M, Spencer ND, Wieland M, Keller B, Sigrist H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997;8:867–872. doi: 10.1023/a:1018501804943. [DOI] [PubMed] [Google Scholar]
- 59.Vermette P, Gengenbach T, Divisekera U, Kambouris PA, Griesser HJ, Meagher L. Immobilization and surface characterization of neutravidin biotin-binding protein on different hydrogel interlayers. J Colloid Interface Sci. 2003;259:13–26. doi: 10.1016/s0021-9797(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 60.Yuan W, O'Rear EA, Cho G, Funkhouser GP, Glatzhofer DT. Thin polypyrrole films formed on mica and alumina with and without surfactant present: Characterization by scanning probe and optical microscopy. Thin Solid Films. 2001;385:96–108. [Google Scholar]
- 61.He W, Gonsalves KE, Batina N, Poker DB, Alexander E, Hudson M. Micro/nanomachining of polymer surface for promoting osteoblast cell adhesion. Biomed Microdevices. 2003;5:101–108. [Google Scholar]
- 62.Huang HH, Ho CT, Lee TH, Lee TL, Liao KK, Chen FL. Effect of surface roughness of ground titanium on initial cell adhesion. Biomol Eng. 2004;21:93–97. doi: 10.1016/j.bioeng.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Kawakami H, Hiraka K, Nagaoka S, Suzuki Y, Iwaki M. Neuronal attachment and outgrowth on a micropatterned fluorinated polyimide surface. J Artif Organs. 2004;7:83–90. doi: 10.1007/s10047-004-0255-y. [DOI] [PubMed] [Google Scholar]
- 64.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valleta JS, Topp KS, Lavail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel N, Poo MM. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982;2:483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howe CL. Depolarization of PC12 cells induces neurite outgrowth and enhances nerve growth factor-induced neurite outgrowth in rats. Neurosci Lett. 2003;351:41–45. doi: 10.1016/s0304-3940(03)00915-7. [DOI] [PubMed] [Google Scholar]
- 68.Pu LL, Syed SA, Reid M, Patwa H, Goldstein JM, Forman DL, Thomson JG. Effects of nerve growth factor on nerve regeneration through a vein graft across a gap. Plast Reconstr Surg. 1999;104:1379–1385. doi: 10.1097/00006534-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 69.Lu GC, Yuan BJ, Liu JP, Zhao GR, Wu H. The promotional effect of nerve growth factor on regeneration of crushed sciatic nerve in rats. Zhongguo Xianyao Zazhi. 2003;12:1011–1013. [Google Scholar]
- 70.Xu X, Yee W, Hwang P, Yu H, Wan C, Gao S, Boon K, Mao H, Leong K, Wang S. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24:2405–2412. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 71.Ito Y. Tissue engineering by immobilized growth factors. Mater Sci Eng C. 1998;6:267–274. [Google Scholar]