Abstract
Cancer immunotherapies can be guided by cellular imaging techniques, which can identify the presence or absence of immune-cell accumulation in the tumor tissue in-vivo and in real time. This review summarizes various new and evolving imaging techniques employed for tracking and monitoring of adoptive NK-cell immunotherapies.
Introduction
Adoptive transfer of in-vitro activated or genetically-modified NK-cells represents an evolving approach of cancer immunotherapy. The non-MHC-restricted killing of tumor cells by NK-cells provides advantages over T-cells and makes them appealing as potential effectors for immunotherapy. The capability of ex-vivo expanded NK-cells of homing to tumors and retaining their cytotoxicity once transferred from in-vitro to in-vivo tumor microenvironment is frequently questioned by oncologists (1). Strategies aimed at maximizing NK-cell trafficking to tumors or secondary lymphoid organs are being employed (e.g. NK-cell combination with bortezomib, or with lymphodepleting chemotherapy of high-dose cytoxan and fludarabine) to exploit the full therapeutic potential of NK-cell-based immunotherapy for cancer (1). Major obstacles in developing new immunotherapeutic approaches include the lack of a non-invasive tool for in-vivo monitoring of immune effector cells, quantifying effector-to-target (NK-cell: tumor) ratios and cytotoxicity outcomes (2). To date, the identification of responders and non-responders to NK-cell based immunotherapies relies on a decline in tumor markers or a reduction in tumor size, weeks or months after initiation of treatment (3–6). With imaging techniques, the presence, quantity and distribution of NK-cells in target tumors can be monitored non-invasively, instantly and in real-time (3–10). The acquired imaging data may serve as a surrogate marker for tumor response. Thus, NK-cell imaging could immediately and positively impact preclinical assessments of new combination/adjuvant immunotherapies, the design of related clinical trials, and ultimately, assessment of these therapies in clinical practice.
Labeling of NK-cells with tracers or contrast agents for in-vivo tracking
NK-cells can be tracked with Radioisotope imaging techniques-Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT); Magnetic Resonance (MR) imaging techniques and Optical imaging (OI)- Fluorescence and Bioluminescent imaging (BLI). For tracking with PET, NK-cells were labeled with positron emitting radionuclides-18F and 11C (11, 12). NK-cells were labeled with γ-photon emitting radioisotope-111Indium (111In) for SPECT imaging (3–5). Both PET and SPECT provide a high sensitivity (single-cell) for NK-cell depiction, high target-to-background contrast, in-vivo quantification of labeled cells, immediate clinical applicability due to FDA-approved labels and established whole body imaging techniques (3–5, 10, 11). Limitations comprise high expense, low resolution when used without CT (1–2mm), radiation exposure and tracer decay, limiting follow-up studies to 2–3 hours with 18FDG or 4–7 days for 111In (3–5, 10). The low resolution of PET and SPECT images can be improved by integration with high resolution images delivered by CT. This allows for better correlation of PET/SPECT signals to anatomical structures visualized at CT. The given resolution for CT part of hybrid PET/CT or SPECT techniques is 200–400μm. Of note, different SPECT radionuclides emit positrons at different energy levels, which can be distinguished by the y-cameras. This can be utilized to label two different cell populations (e.g. NK-cells and T-cells) with different SPECT isotopes and track them at once in the same subject. Conversely, all PET radioisotopes emit two γ-photons of the same energy (511 keV), thereby omitting parallel tracking of different cell types (3–5, 10, 13).
NK-cell tracking with OI requires either (A) labeling of target cells with an exogenous fluorescent dye for fluorescence reflectance imaging (9) (Figure 1) or (B) transfection of target cells with a gene encoding the synthesis of either (B1) a fluorescent protein (e.g. green fluorescent protein, gfp) detectable with fluorescence imaging or (B2) luciferase detectable with BLI (9). Methods (B1) and (B2) offer the distinct advantage of providing information regarding the viability of the labeled cells because the fluoroscence or bioluminescence signal disappears when the labeled cell undergoes apoptosis (8, 9, 14). Only method (A) is in principle clinically applicable, when using the FDA-approved label indocyanine green (ICG). OI offers inexpensive, fast and radiation-free in-vivo monitoring of NK-cells with high sensitivity and low background noise (especially with near-infrared (NIR) fluorophores) (9, 14). Limitations include limited tissue penetration (1cm with fluorescence imaging and 3cm with bioluminescence imaging), low resolution (2–3mm), 2D projection technique and under-developed human imaging devices (8, 9). Some of these obstacles are being addressed with the development of new handheld, endoscopic and tomographic imaging systems, such as Fluorescence molecular tomography (FMT), FMT-CT and FMT-MRI (8, 9). A prototype clinical OI scanner is currently investigated for breast cancer imaging in patients (15).
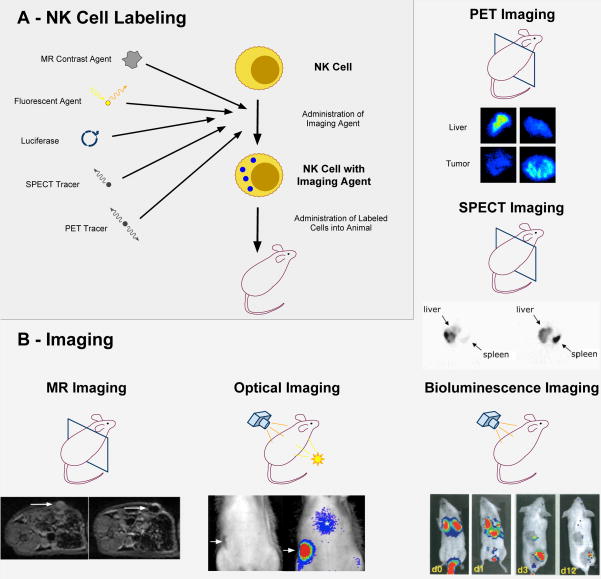
Figure 1.
a. NK-cells are labeled with a radiotracer (111In or 18FDG), contrast agent (Ferumoxides or DiD) or transfected with DNA encoding a luciferase reporter. b. Representative examples depicting NK-cell tumor accumulation with various imaging modalities. Sections through mice represent axial imaging modalities: Clockwise, ex-vivo PET images obtained 2 hours after Her-2/neu-targeted NK-cell injection show increased signal in tumor, while no signal is noted in the tumor after injection of parental cells. SPECT images show tumor accumulation of NK-cells in liver metastases (arrow) after intra-arterial (I.A.) injections in a clinical model. (Reproduced with permission from Matera et al, Journal of Translational Medicine (2006)). Bioluminescent imaging shows accumulation and persistance of NK-cells in tumor xenografts from days 0–12. (Reproduced with permission from Edinger et al, Blood (2003)). Axial MR images before and 24 hours after injection of ferumoxides labeled NK-cells: Post-injection MR image shows a decline in signal intensity due to NK-cell accumulation in the tumor (arrow).
MR imaging detects relaxivity of protons within a magnetic field as signal. MR contrast agents alter proton relaxivity leading to signal enhancement on MR images without being imaged directly. In order to track NK-cells with MR imaging, they are typically labeled with iron-oxide nanoparticles producing a strong negative (dark) enhancement on T2-weighted images (Figure 1). Several iron-oxide nanoparticles are suitable for clinical use, such as Ferumoxides (FDA-approved in the US, Europe and Japan for liver imaging), Ferumoxytol (FDA-approved for treating iron deficiency) and Ferucarbotran (approved for liver imaging in Europe and Japan) (7, 16, 17). MR imaging offers many advantages such as readily available clinical translation, high resolution (100μm in plane), high soft-tissue contrast, absent radiation exposure and longer persistence of the signal (2–4 weeks) (14, 18). Limitations of MR-cell-tracking techniques comprise high costs, long scan times and limited sensitivity (detects 40–50 cells) (14, 18). Additionally, the MR signal is not directly related to contrast agent or cell concentrations, making it difficult to quantify target cells in-vivo (14, 18).
Monitoring NK-cell tumor accumulation: Preclinical in-vivo studies
Radiotracers been extensively utilized for tracking leukocytes to inflammations and tumors. PET utilizing the [11C]methyl iodide label (half-life=20 minutes) was used to track activated NK-cells and non-activated lymphocytes to fibrosarcoma xenografts (Supplemental table 1, Figure 1) (11). At 1 hour post-injection, 4–30% of the activated NK-cells had accumulated in the tumor compared to 3–4% of non-activated cells. This study pioneered initial applications of NK-cell tracking to target tumors with PET (11). As a translational approach, FDA-approved 18FDG was used to label genetically-engineered Her2neu-directed NK-cells and track them to Her2neu-expressing breast sarcoma xenografts (10). Ex-vivo autoradiography scans showed 2.9 times higher radioactivity in tumors treated with genetically-engineered NK-cells than parental NK-cells (Supplemental table 1). This study furnished proof-of-concept for NK-cell tracking with a widely available, clinically applicable PET tracer (10). However, the short half-life of 18FDG (109 minutes) limits in-vivo monitoring of 18FDG labeled NK-cells to 2–3 hours (10). Other PET tracers with longer half-lives (e.g. 124I (t1/2=4.18 days), 89Zr (t1/2=3.27 days) and 86Y (t1/2=2.67 days) have been used to track labeled antibodies (bevacizumab, trastuzumab) and are yet to be exploited for cell-tracking (19).
BLI has been utilized to track gfp-luc transfected NK-cells to A-20 lymphoma xenografts (Figure 1). Since a genetic modification led to the luminescent signal, it did not fade with cell proliferation and allowed for long term cell-tracking after NK-cell administration. After injection of 5×106 NK-cells (25%gfp+) in these mice, the tumors completely regressed at day 12 and remained undetectable at 6 month follow-up (Supplemental table 1) (8). Positive luminescence concluded the viability of the labeled NK-cells (8). Limitations of BLI include relatively low transduction efficiency of retroviral vectors for primary lymphocyte populations and the risk of gene silencing (8). Additionally, some reporter genes could become immunogenic, as described for gfp (8, 14). The use of lentiviral vector systems and the generation of transgenic animals with constitutive or inducible expression of bioluminescent reporter genes are important steps in overcoming these limitations (8). The necessary genetic modification for obtaining bioluminescent cells limits this technique to experimental applications.
Fluoroscence reflectance imaging of NK-cells labeled with exogenous fluorescent markers has been exploited in experimental and clinical contexts. The NIR dyes, e.g., DiD (1,1′-dioctadecyl-3, 3, 3′, 3′ tetramethylindodicarbocyanine), DiO (3,3′-dilinoleyloxacarbocyanine), DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) or VT680 (VivoTag 680) provide quick, inexpensive and uncomplicated (simple incubation for few minutes) cell labeling for experimental applications. Other fluorescent labels for cell tracking have been reviewed by Sutton et al (14). DiD labels remain stable for several weeks, can be depicted with both OI and fluorescence microscopy and have been applied to track tumor-targeted NK-cells to prostate cancer xenografts (9). This allowed for close observations of the in-vivo distribution of systemically injected NK-cells (Supplemental table 1, Figure 1) (9). ICG, which is FDA-approved for measuring tissue blood volumes, cardiac output, hepatic function and tumor perfusion (12), is currently being investigated in our lab with the goal to develop clinically applicable optical NK-cell tracking techniques.
Lim et al provided proof-of-concept of cell tracking with quantum dots (QDs) by labeling NK92MI-cells with anti-CD56 antibody coated QD705 and tracking the labeled cells up to 12 days after intra-tumoral injections with optical imaging (Supplemental table 1). The authors found a decreased size of tumors treated with NK92MI-cells compared to controls (20). Labeling with QDs provides high quantum yield, high sensitivity, stable cell labeling for months and a very narrow excitation and emission spectrum. This allows for “multiplexing”, i.e. simultaneous tracking of two or more cell populations, labeled with quantum dots of different colors, in the same animal (20). Quantum dot labeling may be the most suited imaging technique for tracking different cell populations at once. Although currently available compounds are not clinically applicable because of toxic cadmium cores or other non-degradable components (21), cadmium-free or biodegradable quantum dots are currently being developed.
MR imaging has been exploited sparsely for NK-cell tracking, probably due to its high cost. However, MR imaging would be immediately clinically applicable and thus, deserves increased attention as clinical trials evolve. Our group used MR imaging to monitor NK-cell based immunotherapies for treatment of breast and prostate tumors (Supplemental table 1, Figure 1); we monitored the accumulation of ferumoxides-labeled HER2/neu-targeted NK-cells in HER2/neu-expressing breast sarcomas and the accumulation of ferumoxide-labeled EpCAM (epithelial cell adhesion molecule)-targeted NK-cells in EpCAM-expressing prostate cancers (Supplemental table 1) (7). In addition to confirming NK-cell tumor accumulation, MR imaging provided additional details about the distribution of the labeled NK-cells within the tumor tissue (7).
Clinical in-vivo applications
Clinically established SPECT techniques have been applied to evaluate the in-vivo distribution of systemically injected 111In labeled NK-cells in patients (3–5). Matera et al deduced the target tumor accumulation of NK-cells after intra-arterial and intravenous routes of NK-cell administration (Supplemental table 2, Figure 1). Signal within the tumor corresponding to delivery of cells was noted only after intra-arterial and not intravenous injections (5). Conversely, other authors found that intravenously injected 111In-labeled NK-cells initially accumulated in the lungs, then redistributed to the liver, spleen and bone marrow, and finally accumulated in metastases in lungs and liver at 24 hours after injection (Supplemental table 2) (3, 4). 111In labeled NK-cells could be tracked up to 6 days (half-life=2.8 days) (3, 4). This is in concordance with the patterns noted in our lab with various imaging modalities and prove that NK-cells do reach the tumors and are not immunologically destroyed in the circulation (3, 4). Thus, SPECT provides an immediately clinically applicable tool to monitor and tailor immunotherapy protocols with regard to the best route, quantity and timing of immune-cell administrations.
MR imaging and OI may offer less invasive, radiation-free alternatives and are ready for monitoring of NK-cell based clinical trials. Proof-of-concept for MR imaging has been provided for dendritic cells in patients with melanoma: Ferumoxides and 111In-labeled dendritic cells have been tracked from subcutaneous tissue injections to local lymph nodes using MR imaging, providing congruent information to simultaneously obtained SPECT scans (6). MR imaging allowed better assessment of the accuracy of dendritic cell delivery and cell migration patterns (6).
Future Directions
NK-cell immunotherapy has advantages over other immunotherapy approaches in being non-MHC-restricted, non-immunogenic and highly cytotoxic. NK-cell based strategies promise greatest benefit when used in the setting of minimal residual disease or as an adjunct to other non-immune-based therapies. Non-invasive imaging techniques allow for an in-vivo evaluation of the accumulation, distribution and quantity of NK-cells within the tumor parenchyma as early as 2–4 hours after systemic administration (9, 10). This may help to understand the in-vivo kinetics of parental and retargeted NK-cells and facilitate the development of successful and efficacious NK-cell therapies against chemotherapy-insensitive cancers. None of the described labels have been noted to interfere with NK-cell function (7,9,10). The best suited imaging modality for tracking NK cell therapies depends on the desired sensitivity, follow-up interval, radiation tolerance as well as available imaging technologies. Of the modalities described, PET and MR imaging combine clinically important attributes of high sensitivity (PET), high resolution (MR) and established imaging protocols for tumor imaging in patients. Thus, emerging developments of hybrid PET/MR techniques may represent the most promising approach for future clinical applications. Optical imaging and PET imaging provide for earlier though short follow-ups. If rapid image acquisition is required as in biokinectic studies, these modalities are most suitable. Given the longer stability of ferumoxides label, MR imaging can achieve long-term follow-up. Applying imaging-based surrogate markers for NK-cell tumor accumulation and tumor response would have a major impact on health care economics by avoiding ineffective treatment of patients who are unknowingly therapy resistent. Since clinical trials of new immunotherapies are expensive and take years to complete, the immediate value and impact of the described imaging techniques is apparent. NK-cell tracking techniques could be in principle readily applied in a clinical setting, hold the potential to improve therapy management and, ultimately, may provide a new tool to improve long term cancer regression and patient survival.
Supplementary Material
Acknowledgments
This work was supported by Award Number R21CA129725 from the National Cancer Institute.
References
- 1.Srivastava S, Lundqvist A, Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy. 2008;10:775–83. doi: 10.1080/14653240802648181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JS. Should natural killer cells be expanded in vivo or ex vivo to maximize their therapeutic potential? Cytotherapy. 2009;11:259–60. doi: 10.1080/14653240902888000. [DOI] [PubMed] [Google Scholar]
- 3.Meller B, Frohn C, Brand JM, et al. Monitoring of a new approach of immunotherapy with allogenic (111) In-labelled NK cells in patients with renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:403–7. doi: 10.1007/s00259-003-1398-4. [DOI] [PubMed] [Google Scholar]
- 4.Brand JM, Meller B, Von Hof K, et al. Kinetics and organ distribution of allogeneic natural killer lymphocytes transfused into patients suffering from renal cell carcinoma. Stem Cells Dev. 2004;13:307–14. doi: 10.1089/154732804323099235. [DOI] [PubMed] [Google Scholar]
- 5.Matera L, Galetto A, Bello M, et al. In vivo migration of labeled autologous natural killer cells to liver metastases in patients with colon carcinoma. J Transl Med. 2006;4:49. doi: 10.1186/1479-5876-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–13. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 7.Daldrup-Link HE, Meier R, Rudelius M, et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15:4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 8.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–8. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 9.Tavri S, Jha P, Meier R, et al. Optical imaging of cellular immunotherapy against prostate cancer. Mol Imaging. 2009;8:15–26. [PubMed] [Google Scholar]
- 10.Meier R, Piert M, Piontek G, et al. Tracking of [18F]FDG-labeled natural killer cells to HER2/neu-positive tumors. Nucl Med Biol. 2008;35:579–88. doi: 10.1016/j.nucmedbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Melder RJ, Brownell AL, Shoup TM, Brownell GL, Jain RK. Imaging of activated natural killer cells in mice by positron emission tomography: preferential uptake in tumors. Cancer Res. 1993;53:5867–71. [PubMed] [Google Scholar]
- 12.Meier R, Boddington S, Krug C, et al. Detection of postoperative granulation tissue with an ICG-enhanced integrated OI-/X-ray System. J Transl Med. 2008;6:73. doi: 10.1186/1479-5876-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaise C, Itti E, Petegnief Y, et al. [F-18]-Fluoro-2-deoxy-D: -glucose positron emission tomography as a tool for early detection of immunotherapy response in a murine B cell lymphoma model. Cancer Immunol Immunother. 2007;56:1163–71. doi: 10.1007/s00262-006-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton EJ, Henning TD, Pichler BJ, Bremer C, Daldrup-Link HE. Cell tracking with optical imaging. Eur Radiol. 2008;18:2021–32. doi: 10.1007/s00330-008-0984-z. [DOI] [PubMed] [Google Scholar]
- 15.Poellinger A, Martin JC, Ponder SL, et al. Near-infrared laser computed tomography of the breast first clinical experience. Acad Radiol. 2008;15:1545–53. doi: 10.1016/j.acra.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Wu YJ, Muldoon LL, Varallyay C, Markwardt S, Jones RE, Neuwelt EA. In vivo leukocyte labeling with intravenous ferumoxides/protamine sulfate complex and in vitro characterization for cellular magnetic resonance imaging. Am J Physiol Cell Physiol. 2007;293:C1698–708. doi: 10.1152/ajpcell.00215.2007. [DOI] [PubMed] [Google Scholar]
- 17.Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–7. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 18.Henning TD, Wendland MF, Golovko D, et al. Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5T and 3T: discrimination of viable from lysed cells. Magn Reson Med. 2009;62:325–32. doi: 10.1002/mrm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munnink T, Nagengast W, Brouwers A, et al. Molecular imaging of breast cancer. Breast. 2009;18 (Suppl 3):S66–73. doi: 10.1016/S0960-9776(09)70276-0. [DOI] [PubMed] [Google Scholar]
- 20.Lim YT, Cho MY, Noh YW, Chung JW, Chung BH. Near-infrared emitting fluorescent nanocrystals-labeled natural killer cells as a platform technology for the optical imaging of immunotherapeutic cells-based cancer therapy. Nanotechnology. 2009;20:475102, 8. doi: 10.1088/0957-4484/20/47/475102. [DOI] [PubMed] [Google Scholar]
- 21.Rzigalinski BA, Strobl JS. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009 doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.