Abstract
Background
Formylpeptide receptors are implicated in a variety of immunological and inflammatory response cascades. Further understanding FPR-family ligand interactions could play an integral role in biological and therapeutic discovery. Fluorescent reporter ligands for the family are desirable experimental tools for increased understanding of ligand/receptor interactions.
Methods
The ligand binding affinity and fluorescent reporting activity of the peptide WK(FL)YMVm was explored though use of the high throughput HyperCyt® flow cytometric platform. Relative binding affinities of several known FPR and FPRL1 peptide ligands were compared in a duplex assay format.
Results
The fluorescent W-peptide ligand, WK(FL)YMVm, proved to be a high affinity, cross-reactive reporter ligand for the FPR/FPRL1 duplex assay. Ligand specificity was demonstrated for each receptor with known, selective peptide ligands. The binding site specificity of the reporter ligand was further verified by a fluorescent confocal microscopy internalization experiment.
Conclusions
The fluorescent peptide ligand WK(FL)YMVm bound with high affinity to both FPR and FPRL1. The differential affinities of known peptide ligands were observed with the use of this fluorescent probe in HTS flow cytometry.
Key terms: formylpeptide receptor, FPR, formylpeptide receptor-like 1, FPRL1, fluorescent ligand, cross-reactive, W-peptide, WKYMVm, flow cytometry, GPCR
Introduction
The G protein coupled formylpeptide receptor (FPR) is one of the first discovered members of the chemoattractant receptor superfamily (1,2). FPR is expressed in several cell types including neutrophils, monocytes, hepatocytes, immature dendritic cells, astrocytes, microglial cells, and the tunica media of coronary arteries (3-6). Two other FPR variants have been described; formylpeptide receptor-like 1 (FPRL1) and formylpeptide receptor-like 2 (FPRL2) (7). Also a 7-transmembrane G protein-coupled receptor (GPCR), FPRL1 shares 69% primary sequence identity with FPR (8). FPRL2 encodes a receptor that has 56% and 83% amino acid sequence identity to FPR and FPRL1, respectively. FPRL1 is expressed in an even greater variety of cell types than FPR including phagocytic leukocytes, hepatocytes, epithelial cells, T lymphocytes, neuroblastoma cells, astrocytoma cells, and microvascular endothelial cells (1,8-11). In addition, a recent study has documented expression of both FPR and FPRL1 on normal human lung and skin fibroblasts (12). The diverse tissue expression of these receptors suggests the possibility of as yet unappreciated complexity in the innate immune response and perhaps other unidentified functions for the receptor family. Exploring this diversity could be facilitated by molecular tools targeted at elucidation of ligand-receptor interactions. Based on known peptide ligands, our group sought a fluorescent reporter probe for use in flow cytometric analysis of FPR and FPRL1.
The most commonly studied class of FPR activators are protein/peptide based ligands. N-formylated peptides such as the E. coli derived N-formyl-Met-Leu-Phe (fMLF) are high affinity FPR ligands that elicit a variety of biologic activities in myeloid cells and it has been proposed that a primary FPR function is to promote trafficking of phagocytic myeloid cells to sites of infection and tissue damage where they exert anti-bacterial effector functions and clear cell debris. While FPR and its ligands have been studied in great depth (8,13), a growing awareness of the biological importance of FPRL1 makes it increasingly the subject of new, and joint FPR-family investigations. Many potent formylpeptide FPR agonists only weakly activate FPRL1 (14). N-formylated hexapeptides derived from the N-terminus of mitochondrial NADH dehydrogenase subunits 4 and 6 and cytochrome C oxidase subunit 1 prove to be an exception and are agonists of both FPR and FPRL1 (14). Non-N-formyl phagocyte chemotactic activation was shown with peptides of varied N-terminal substitution including free amino, acetyl, ureido, and carbamate functional groups (15-17). Despite the limited activation by N-formyl peptides, FPRL1 can be considered exceptionally promiscuous, responding to ligands of multiple origins spanning a wide range of structural diversity (1,18). A number of host-derived FPRL1 agonists have been identified that are associated with pathophysiological settings. These include amyloidogenic proteins, serum amyloid A (19), the 42 amino acid form of β amyloid, Aβ42 (20), and a prion protein fragment, PrP1206-26 (21), which are involved in chronic inflammation-associated systemic amyloidosis (22), Alzheimer's disease (23), and prion diseases (21), respectively. Since infiltration of activated mononuclear phagocytes is a common feature, cells responding to FPRL1 ligands may contribute to the inflammatory pathology observed in the diseased tissues (24). Other FPRL1 agonists include an enzymatic cleavage fragment of the neutrophil granule derived cathelicidin (25), and HIV-1 envelope protein domains which are also capable of binding FPR (26).
The present study was motivated by the need for a fluorescent probe with which to efficiently screen libraries of small molecules to identify selective FPR and FPRL1 ligands. The goal was to produce a high affinity probe that could simultaneously report binding interactions of test compounds with both receptors in a single assay volume. Baek et al. previously reported a class of peptides with an amino-terminal W residue, known as W-peptides, that were selective high affinity ligands for FPRL1 (27). In one series of W-peptides, it was shown that various substitutions could be made at the second amino acid residue without significantly affecting the FPRL1 binding interaction (28). We exploited the second residue binding ambiguity in this high affinity FPRL1 ligand class to generate a fluorescent, cross-reactive, high affinity FPR/FPRL1 probe. Incorporation of a amide conjugated fluoresceinyl-lysine residue at that position resulted in a fluorescent peptide probe, H2N-Trp-(Fluoresceinyl-Lys)-Tyr-Met-Val-(D-Met)-amide (WK(FL)YMVm), that not only retained high affinity for FPRL1 but also acquired a substantially increased affinity for FPR.
Materials and Methods
The W-peptide series (WK(FL)YMVm, WKYMVm, WKRMVm, and WKGMVm) were synthesized by New England Peptide, Inc. (Gardner, MA) and supplied at > 95% purity by HPLC with identity verified by MALDI-TOF MS. All peptide dilutions were done in DMSO prior to the final in well additions where the DMSO concentration was no more than 1%. Chemical reagents, including the formyl peptides N-formyl-Met-Leu (fML), fMLF, and N-formyl-Met-Leu-Phe-Phe (fMLFF), were obtained from Sigma (St. Louis, MO) unless otherwise specified. Flow cytometric analysis was done on a CyAn flow cytometer (Beckman-Coulter, Fullerton, CA). Fluorescence was excited at 488 nm and detected with 530/40 and 680/30 optical band pass filters for WK(FL)YMVm and FuraRed™, respectively. The resulting time-resolved data files were analyzed with IDLeQuery software to determine compound activity in each well. The HyperCyt® high throughput flow cytometry platform was used to sequentially sample cells from 384-well microplates (2 μL/sample) for flow cytometer presentation at a rate of 40 samples/min (29,30). The HyperCyt® platform and associated analysis software are commercially available from IntelliCyt™ (Albuquerque, NM). Ligand competition curves were fitted by Prism® software (GraphPad Software, Inc., San Diego, CA) using nonlinear least-squares regression in a sigmoidal dose response model with variable slope, also known as the four parameter logistic equation.
Rat Basophilic Leukemia (RBL-2H3) cells expressing human FPRL1 (RBL/FPRL1) were grown as adherent cell cultures in TCM supplemented with 2.5 μg/mL Amphotericin B (CellGro, Mediatech Inc., Manassas, VA). U937 cells expressing human FPR were grown as 100 mL suspensions in TCM. Unless otherwise indicated, U937 cells were used that expressed a mutant FPR with glycine and alanine substituted for serine and threonine residues in the C-terminal tail (ΔST) which do not internalize the receptor when stimulated with fMLF (31). Cultures were grown at 37°C in a 5% CO2 atmosphere, and passaged every 3 days. RBL/FPRL1 cells were detached with 0.25% Trypsin-EDTA (37° C, 2-5 min.), suspended in TCM, centrifuged 10 min. at 450×g and resuspended at 4 × 106 / mL in PDB. U937/FPR cells were centrifuged 10 min. at 450×g, resuspended in PDB at 5 × 105 cells/mL and color-coded by incubation 15 min. at 37°C with Fura Red™, AM (InVitrogen, Carlsbad, CA) at a final concentration of 6 μM. After two subsequent centrifugation washes in PDB to remove unincorporated dye, the cell pellet was resuspended by addition of the RBL/FPRL1 cell suspension to achieve a final U937/FPR cell concentration of 4 × 106 / mL, equal to that of the RBL/FPRL1 cells. The cell mixture was stored on ice until used in the assay.
Duplex Flow Cytometric Analysis
The experiment was performed in duplex format in which ΔST-U937 cells expressing FPR were tested together with RBL-2H3 cells expressing FPRL1. The FPR-expressing cells were stained red to allow them to be distinguished from the FPRL1-expressing cells during flow cytometric analysis. Assays were performed in polystyrene 384-well plates with small volume wells (#784101, Greiner, Monroe, NC). The assay optimized order of addition was done in the following sequence: 1) test compounds and control reagents, 5 μL/well; 2) a combination of FPR- and FPRL1-expressing cell lines; 3) fluorescent WK(FL)YMVm peptide (after 30 min., 4°C incubation, 5 μL/well). After an additional 45 min. at 4 °C incubation, plates were analyzed by flow cytometry. The assay response range was defined by replicate control wells containing unlabeled receptor-binding peptide (positive control) or buffer (negative control). The formyl peptide fMLFF was used as the FPR-blocking peptide and unlabeled WKYMVm as the FPRL1-blocking peptide. Assay wells were directly analyzed on the flow cytometer without wash steps. Supplemental material is available demonstrating the gating strategies used for these analyses. A recent publication describing the use of the fluorescent ligand described here in a screening campaign further illustrates these duplex-assay analysis methods (32). FPR/FPRL1 expression ranged from 100,000 to 200,000 receptors per cell as determined by comparison to standard curves generated with Fluorescein Reference Standard Microbeads (Bangs Laboratories, Fishers, IN). This corresponded to total FPR/FPRL1 concentrations of 0.6 to 1.2 nM. Potential quenching effects from both conjugation of fluorescein and receptor binding of the peptide were addressed by comparison to the previously characterized peptide, N-formyl -Met-Leu-Phe-Lys-fluorescein (fMLFK(FL)) (33). At saturation the fluorescence intensity of WK(FL)YMVm was 66% of that observed for fMLFK(FL) indicating that receptor number estimates were comparable within the indicated range.
Fluorescent Cross-Reactive Reporter Ligand Binding Affinity
The Kd of WK(FL)YMVm was determined in both FPR and FPRL1 expressing cell lines. To account for fluorescence from non-specific binding, the blocking peptides fMLFF and WKYMVm were used to saturate receptors prior to addition of WK(FL)YMVm. Each titration series of the fluorescent probe was done in duplicate and in total there were sixteen points per concentration over two separate days of data collection. The inhibitory peptide (5 μL) was added to the wells first, with final concentrations of 250 nM of fMLFF for FPR and 67 nM of WKYMVm for FPRL1, followed by 5 μL of cells. The plates were then incubated at 4°C for 30 min. followed by addition of 5 μL of the fluorescent WK(FL)YMVm ligand dilution series. The concentration of fluorescent ligand ranged in wells from 0.1 to 66.67 nM over a nine point span. The plates were incubated overnight (18 hrs.) at 4°C to allow receptor-ligand binding interactions to attain approximate equilibrium, then analyzed on the flow cytometer.
Comparative Formylpeptide Ligand Binding
The fluorescent WK(FL)YMVm peptide was then used as the reporter ligand for exploring relative binding affinities of known FPR and FPRL1 peptide ligands using the duplex protocol outlined above. Opposite to the Kd determination experimentation, the fluorescent ligand WK(FL)YMVm was held at a constant concentration (5 nM) and the peptide ligands were subjected to serial dilution. The nine point concentration range of the peptide ligands spanned from 1.0 nM to 6.7 μM. Incubation times and order of addition for compounds and cells were identical to the previous protocol.
Fluorescent Ligand Internalization
Stable FPRL1-transfected RBL cells were transiently transfected with RFP-tagged arrestin-3 using the Nucleofector transfection system with Solution L, Program T-020 (Amaxa Inc., Gaithersburg, MD). These dually transfected RBL cells were then plated on coverslips and allowed to recover overnight. The coverslips were incubated for 10 minutes at 37°C with 5 nM WK(FL)YMVm in growth medium (10% fetal bovine serum in RPMI 1640), washed, immediately fixed, and subsequently mounted. Images were acquired using a Zeiss laser scanning confocal fluorescence microscope (Thornwood, NY).
Results
WK(FL)YMVm Cross-Reactive Binding Affinity
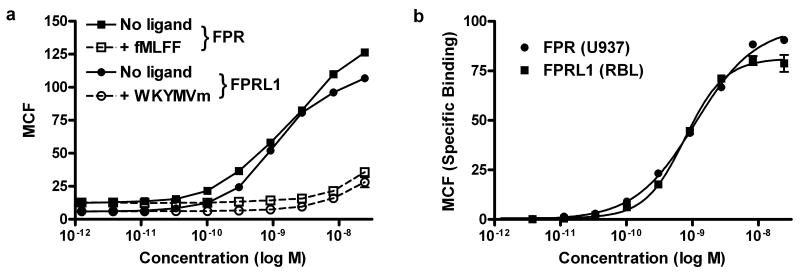
Prior to incorporation of fluoresceinated lysine, the W-peptide affinity for FPRL1 was approximately a hundred fold higher than for FPR (28). In equilibrium binding experiments with the W-peptide fluorescein conjugate, the Kd for FPR and FPRL1 were found to be 1.21 ± 0.36 nM and 1.82 ± 0.78 nM, respectively (Figure 1). To quantify non-specific binding of WK(FL)YMVm to cells, a solution of unlabeled high-affinity peptide ligands was added to the staining reaction. The concentration of inhibitory peptides used in the reported Kd determination experiment were optimized for use in the duplex system. The Kd values for each receptor were also determined independently in each cell line at 1 μM inhibitory peptide concentrations, more that 100 times the Kd of each peptide for its respective receptor. The values found were within the error range of those reported for the optimized duplex conditions (data not shown).
Figure 1.
Comparison of the binding affinity of WK(FL)YMVm on FPR and FPRL1. A: Raw median channel fluorescence values of bound receptor with and without the presence of nonfluorescent ligand (dashed and solid line, respectively). Receptor saturation was achieved in FPR (squares) with fMLFF and for FPRL1 (circles) with WKYMVm. B: Compensation for the nonspecific binding afforded Kd values of 1.21 ± 0.36 nM and 1.82 ± 0.78 nM, respectively, for FPR (solid circles) and FPRL1 (solid squares).
Receptor Binding Specificity
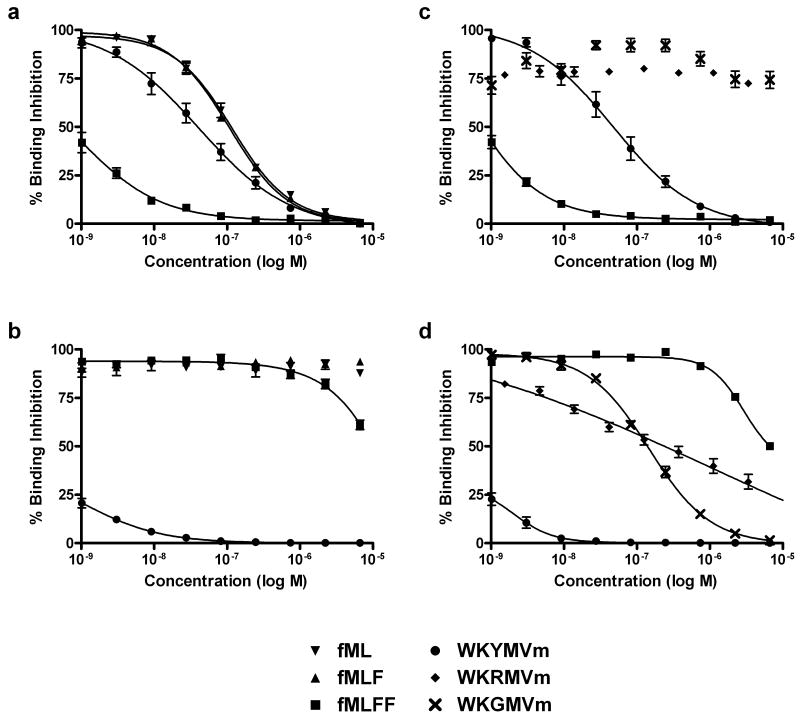
To evaluate the ligand affinity reporting capability of the probe at both receptors binding affinities of several unlabeled FPR and FPRL1 selective peptides were measured in competitive binding assays using the new fluorescent probe. The ligand dependent specificity was demonstrated by comparison of high affinity FPR binding formylpeptide ligands and FPRL1 selective WKYMVm (Figure 2 A, U937/FPR cells & B, RBL/FPRL1 cells). The formyl peptide ligands, fML, fMLF, and fMLFF, bound with moderate to high affinity to FPR in U937/FPR cells (EC50 values ranged from 110 nM for fML to 1 nM for fMLFF) but exhibited no detectable ligand activity for the FPRL1 expressing RBL cells (Table 1). Binding affinity measurements of WKYMVm for the two receptors yielded EC50 values of 43.8 nM and 1.8 nM for FPR and FPRL1, respectively. The single residue substitution peptides WKGMVm and WKRMVm showed exclusive binding for FPRL1 with EC50 values of 176 nM and 705 nM respectively (Figure 2 C, U937 cells & D, RBL cells). Bae et al. previously observed Ca2+ flux responses in FPR and FPRL1 expressing cell lines for a series of W-peptides including WKYMVm (EC50 = 47 nM and 0.6 nM respectively for FPR and FPRL1), WKGMVm (EC50 = 21 nM), and WKRMVm (EC50 = 2 nM) (28). Despite the apparent quantitative correlation of data across the two experiments for WKYMVm, the comparison of ligand binding affinity and the efficiency with which the ligand induces calcium flux does not necessarily have a direct association. Rather, it is important to note the qualitative relationship of the two data sets, particularly the cross-activity of WKYMVm for both receptors and the FPRL1 specificity seen for the residue analogs WKGMVm and WKRMVm.
Figure 2.
Direct comparison of unlabeled FPR and FPRL1 peptide ligands through a competitive binding assay with the fluorescent WK(FL)YMVm probe. A: This plot shows the direct comparison of the FPR selective formylpeptide ligands (fML, fMLF, fMLFF) with the FPRL1 selective WKYMVm in FPR expressing U937 cells. The high-affinity fMLFF stands out with a 1.0 ± 0.6 nM EC50 while WKYMVm is only 43.8 ± 4.3 nM. The other ligands, fML and fMLF, had EC50 values in the 100 nM range. B: In FPRL1 expressing RBL cells the WKYMVm ligand shows high affinity (EC50 = 1.8 ± 0.3 nM), whereas the formyl peptides are all effectively nonbinding. C: The W-peptides WKRMVm and WKGMVm show no binding in FPR expressing U937 cells. D: Moderate binding of the W-peptides is demonstrated in FPRL1 expressing RBL cells at EC50 values of 704.8 ± 384.1 nM and 175.6 ± 40.6 nM for WKRMVm and WKGMVm, respectively.
Table 1. Comparison of peptide ligand binding reported through the fluorescent probe WK(FL)YMVm. EC50 Values for Peptide Ligands.
| Peptide | U937 FPR EC50 (nM) |
RBL FPRL1 EC50 (nM) |
|---|---|---|
| fML | 110.0 ± 17.6 | - |
| fMLF | 79.0 ± 26.9 | - |
| fMLFF | 1.0 ± 0.6 | - |
| WKYMVm | 43.8 ± 4.3 | 1.8 ± 0.3 |
| WKRMVm | - | 704.8 ± 384.1 |
| WKGMVm | - | 175.6 ± 40.6 |
FPRL1/WK(FL)YMVm Internalization
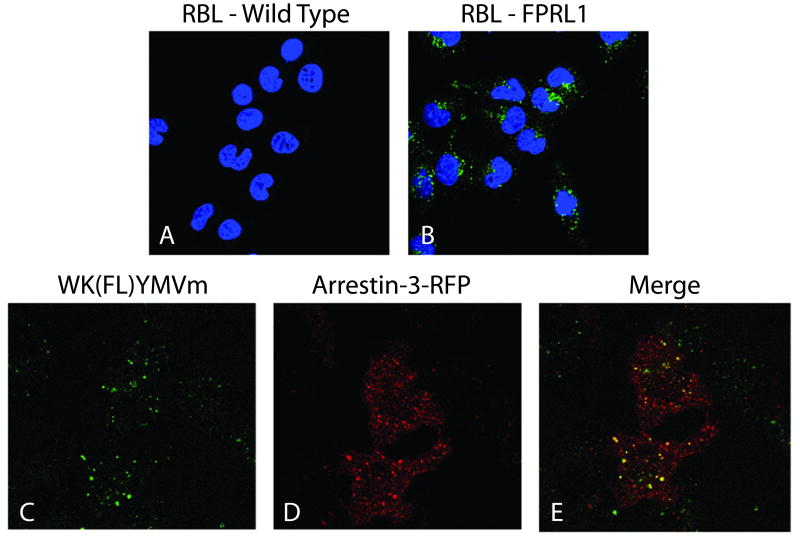
Arrestins are adaptor proteins that uncouple phosphorylated GPCRs from G proteins and regulate receptor internalization (34). FPR and FPRL1 agonists induce internalization of the receptors. Although FPR internalization is reportedly not dependent on the presence of arrestins (35), Huet et al. have demonstrated that upon agonist stimulation FPRL1 remains co-localized with arrestin during endocytosis (36). As a means of further validating FPRL1 binding specificity of the WK(FL)YMVm ligand, arrestin colocalization experiments were performed. Figure 3 shows the observed internalization data for WK(FL)YMVm in RBL cells. Slides A and B show both wild type and FPRL1 expressing RBL cell lines treated with 5 nM WK(FL)YMVm at 37°C to demonstrate receptor expression and internalization in the transfected cell line. Internalized ligand was not apparent in the wild type cells but is readily seen in cells expressing FPRL1. Internalized ligand was shown to colocalize extensively with arrestin-3, as demonstrated in Figure 3C-E.
Figure 3.
Internalization of fluorescent ligand bound FPRL1 and colocalization with arrestin-3 in RBL cells. The cells were all exposed to 5 nm WK(FL)YMVm for 10 min at 37°C before being washed and fixed. A,B: Internalization of the fluorescent WK(FL)YMVm ligand (green) is demonstrated in wild-type RBL cells (A) and FPRL1 expressing cells (B). Nuclei are stained with DAPI (blue). C_E: Colocalization of fluorescent ligand with arrestin-3. RBL cells transiently transfected with RFP-arrestin-3 (D) were allowed to internalize ligand as before (C); merged image in E.
Discussion
Baek et al. originally identified hexapeptide ligands with the consensus sequence XKYX(P/V)M, where X is one of 19 amino acids (cysteine excluded), that stimulate the formation of inositol phosphates (InoPs) in B lymphocyte cell types through the action of phospholipase C via a pertussis toxin sensitive G protein coupled cell-surface receptor (27). Since their discovery, this W-peptide family of ligands has been widely studied and modified to explore formylpeptide receptor family pharmacology. Specifically, the peptide WKYMVM was shown to initiate InoP generation in U266 (human B myeloma), U937 (human histiocytic lymphoma), and HL60 (human promyelocytic lymphoma) cell lines at 1 μM. Increased affinity was later seen after substitution of the L-Met6 with D-Met (WKYMVm) (37), with a hundred fold better affinity seen for FPRL1 over FPR (28). Expanding on the structure affinity relationship of the W-peptide series, Bae et al. demonstrated that the lysine residue of WKYMVm was less crucial to binding than any other position (28). Wan et al. also noted that the last two residues could be removed with little change in affinity and that the 4-peptide sequence with the highest affinity included a norleucine replacement of the lysine (WNleYM) (38). This primary amine free alkyl chain showed similar binding to its lysine counterpart, as well as the original hexapeptide (EC50: WKYMVm = 3.31 nM, WKYM = 43.6 nM, WNleYM =5.34 nM). The authors proposed that the binding pocket consisted of at least two aromatic interactions (tryptophan and tyrosine) as well as a hydrogen bonding region (methionine) and a hydrophobic interaction at the norleucine.
The high affinity binding and the potential to modify the second residue in the W-peptide family was taken advantage of to generate a fluorescent reporter ligand for two FPR family receptors. Modification of the FPRL1 ligand, WKYMVm, with a fluoresceinated lysine (WK(FL)YMVm) afforded an FPR/FPRL1 cross-reactive, high affinity reporter ligand. Addition of the large fluorescein carboxamide yielded a ligand with increased, affinity for both receptors. The ligand was also shown to internalize and co-localize with arrestin-3 by confocal microscopy in RBL cells, further demonstrating its formylpeptide receptor family specific binding.
To our knowledge there is no other cross-reactive fluorescent reporter ligand for both FPR and FPRL1. We have previously reported the use of fMLFK-FITC as a fluorescent reporter for FPR with a Kd of 3 nM, but this ligand does not bind FPRL1 (39). A radioactive iodinated WKYMVm ligand has been described as a binding affinity reporter for both FPR and FPRL1 (40). Although this probe can be used at subnanomoler concentrations, the need for physical sample processing (centrifugation through a 10% sucrose-PBS cushion) and the signal output (γ-ray emissions) are not conducive to HTS-methods. Replacing the natural amino acid residue with the bulky, relatively hydrophobic fluorescein group could have forced a more sterically favorable conformation change which in turn affected binding affinity. The fact that a fluorescein is involved in providing increased binding affinity suggests that there is potentially a large hydrophobic region involved in the interaction as suggested by Wan et al. (38).
The new fluorescent probe was then used in HTS flow cytometric analysis to demonstrate specific ligand interactions with both FPR and FPRL1 expressing cell lines in a single well. Known high affinity FPR formylpeptide ligands were shown to bind with specificity to FPR and the parent WKYMVm ligand was shown to be selective for FPRL1. Thus, the fluorescent WK(FL)YMVm reporter ligand can be used in a duplex format assay to explore chemical libraries for selective ligands for FPR and FPRL1 in a high content, high throughput manner. This fluorescent probe has been successfully used in the duplex competitive binding assay to screen more than 27,000 compounds from the NIH Small Molecule Repository and other sources to identify a number of novel and selective small molecule antagonists for FPR and FPRL1 (32).
Supplementary Material
Footnotes
This work was supported by NIH R03 MH076381 (BSE), NIH R01 AI36357 (ERP) and NIH U54 MH074425 (LAS), the New Mexico Molecular Libraries Screening Center, the University of New Mexico Shared Flow Cytometry Resource and Cancer Research and Treatment Center.
Literature Cited
- 1.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 2.Oppenheim JJ, Zachariae COC, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene \“intercrine\” cytokine family. Annu Rev Immunol. 1991;9:617–48. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 3.Keitoku M, Kohzuki M, Katoh H, Funakoshi M, Suzuki S, Takeuchi M, Karibe A, Horiguchi S, Watanabe J, et al. FMLP actions and its binding sites in isolated human coronary arteries. J Mol Cell Cardiol. 1997;29(3):881–894. doi: 10.1006/jmcc.1996.0291. [DOI] [PubMed] [Google Scholar]
- 4.Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 1995;61(1):71–8. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 5.McCoy R, Haviland DL, Molmenti EP, Ziambaras T, Wetsel RA, Perlmutter DH. N-formylpeptide and complement C5a receptors are expressed in liver cells and mediate hepatic acute phase gene regulation. J Exp Med. 1995;182(1):207–17. doi: 10.1084/jem.182.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155(7):3292–5. [PubMed] [Google Scholar]
- 7.Bao L, Gerard NP, Eddy RL, Jr, Shows TB, Gerard C. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homolog orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13(2):437–40. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- 8.Prossnitz ER, Ye RD. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74(1):73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 9.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon g and inhibits tumor necrosis factor a-induced IL-8 release. J Exp Med. 1998;187(8):1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Y, Hu J, Gong W, Shen W, Li B, Dunlop NM, Halverson DO, Blair DG, Wang JM. Expression of functional formyl peptide receptors by human astrocytoma cell lines. J Neuroimmunol. 2000;111(1-2):102–108. doi: 10.1016/s0165-5728(00)00373-8. [DOI] [PubMed] [Google Scholar]
- 11.Ye RD, Cavanagh SL, Quehenberger O, Prossnitz ER, Cochrane CG. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem Biophys Res Commun. 1992;184(2):582–9. doi: 10.1016/0006-291x(92)90629-y. [DOI] [PubMed] [Google Scholar]
- 12.VanCompernolle SE, Clark KL, Rummel KA, Todd SC. Expression and Function of Formyl Peptide Receptors on Human Fibroblast Cells. J Immunol. 2003;171(4):2050–2056. doi: 10.4049/jimmunol.171.4.2050. [DOI] [PubMed] [Google Scholar]
- 13.Le Y, Wang JM, Liu X, Kong Y, Hou X, Ruan L, Mou H. Biologically active peptides interacting with the G protein-coupled formylpeptide receptors. Protein & Peptide Letters. 2007;14(9):846–853. doi: 10.2174/092986607782110211. [DOI] [PubMed] [Google Scholar]
- 14.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35(8):2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 15.Derian CK, Solomon HF, Higgins JD, III, Beblavy MJ, Santulli RJ, Bridger GJ, Pike MC, Kroon DJ, Fischman AJ. Selective Inhibition of N-Formylpeptide-Induced Neutrophil Activation by Carbamate-Modified Peptide Analogs. Biochemistry. 1996;35(4):1265–9. doi: 10.1021/bi952087k. [DOI] [PubMed] [Google Scholar]
- 16.Gao JL, Becker EL, Freer RJ, Muthukumaraswamy N, Murphy PM. A high potency nonformylated peptide agonist for the phagocyte N-formylpeptide chemotactic receptor. J Exp Med. 1994;180(6):2191–7. doi: 10.1084/jem.180.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JD, III, Bridger GJ, Derian CK, Beblavy MJ, Hernandez PE, Gaul FE, Abrams MJ, Pike MC, Solomon HF. N-terminus urea-substituted chemotactic peptides: new potent agonists and antagonists toward the neutrophil fMLF receptor. J Med Chem. 1996;39(5):1013–15. doi: 10.1021/jm950908d. [DOI] [PubMed] [Google Scholar]
- 18.Le Y, Oppenheim JJ, Wang JM. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001;12(1):91–105. doi: 10.1016/s1359-6101(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 19.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101(4):1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 20.Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, Dunlop NM, Gao JL, Murphy PM, Oppenheim JJ, et al. Amyloid b42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001;21(2):RC123/1–RC123/5. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Y, Yazawa H, Gong W, Yu Z, Ferrans VJ, Murphy PM, Wang JM. Cutting edge: the neurotoxic prion peptide fragment PrP106-126 is a chemotactic agonist for the G protein-coupled receptor formyl peptide receptor-like 1. J Immunol. 2001;166(3):1448–1451. doi: 10.4049/jimmunol.166.3.1448. [DOI] [PubMed] [Google Scholar]
- 22.Snipe JD. In: The acute phase response Immunophysiology: The Role of Cells and Cytokines in Immunity and Inflammation. Shevac EM, editor. New York, NY: Oxford University Press; 1990. pp. 259–273. [Google Scholar]
- 23.Kalaria RN. Microglia and Alzheimer's disease. Curr Opin Hematol. 1999;6(1):15–24. doi: 10.1097/00062752-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Le Y, Yang Y, Cui Y, Yazawa H, Gong W, Qiu C, Wang JM. Receptors for chemotactic formyl peptides as pharmacological targets. Int Immunopharmacol. 2002;2(1):1–13. doi: 10.1016/s1567-5769(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 25.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Y, Jiang S, Hu J, Gong W, Su S, Dunlop NM, Shen W, Li B, Wang JM. N36, a synthetic N-terminal heptad repeat domain of the HIV-1 envelope protein gp41, is an activator of human phagocytes. Clin Immunol. 2000;96(3):236–242. doi: 10.1006/clim.2000.4896. [DOI] [PubMed] [Google Scholar]
- 27.Baek SH, Seo JK, Chae CB, Suh PG, Ryu SH. Identification of the peptides that stimulate the phosphoinositide hydrolysis in lymphocyte cell lines from peptide libraries. J Biol Chem. 1996;271(14):8170–5. doi: 10.1074/jbc.271.14.8170. [DOI] [PubMed] [Google Scholar]
- 28.Bae YS, Song JY, Kim Y, He R, Ye RD, Kwak JY, Suh PG, Ryu SH. Differential activation of formyl peptide receptor signaling by peptide ligands. Mol Pharmacol. 2003;64(4):841–847. doi: 10.1124/mol.64.4.841. [DOI] [PubMed] [Google Scholar]
- 29.Kuckuck FW, Edwards BS, Sklar LA. High throughput flow cytometry. Cytometry. 2001;44(1):83–90. [PubMed] [Google Scholar]
- 30.Ramirez S, Aiken Charity T, Andrzejewski B, Sklar Larry A, Edwards Bruce S. High-throughput flow cytometry: validation in microvolume bioassays. Cytometry. 2003;53(1):55–65. doi: 10.1002/cyto.a.10035. [DOI] [PubMed] [Google Scholar]
- 31.Prossnitz ER. Desensitization of N-formylpeptide receptor-mediated activation is dependent upon receptor phosphorylation. J Biol Chem. 1997;272(24):15213–15219. doi: 10.1074/jbc.272.24.15213. [DOI] [PubMed] [Google Scholar]
- 32.Young SM, Bologa CM, Fara D, Bryant BK, Strouse JJ, Arterburn JB, Ye RD, Oprea TI, Prossnitz ER, Sklar LA, et al. Duplex high-throughput flow cytometry screen identifies two novel formylpeptide receptor family probes. Cytometry. 2008 doi: 10.1002/cyto.a.20645. Early View. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fay SP, Posner RG, Swann WN, Sklar LA. Real-time analysis of the assembly of ligand, receptor, and G protein by quantitative fluorescence flow cytometry. Biochemistry. 1991;30(20):5066–75. doi: 10.1021/bi00234a033. [DOI] [PubMed] [Google Scholar]
- 34.Prossnitz ER. Novel roles for arrestins in the post-endocytic trafficking of G protein-coupled receptors. Life Sci. 2004;75(8):893–899. doi: 10.1016/j.lfs.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, Kohout TA, Lefkowitz RJ, Prossnitz ER. N-Formyl Peptide Receptors Internalize but Do Not Recycle in the Absence of Arrestins. J Biol Chem. 2003;278(43):41581–41584. doi: 10.1074/jbc.C300291200. [DOI] [PubMed] [Google Scholar]
- 36.Huet E, Boulay F, Barral S, Rabiet M-J. The role of b-arrestins in the formyl peptide receptor-like 1 internalization and signaling. Cell Signal. 2007;19(9):1939–1948. doi: 10.1016/j.cellsig.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Seo JK, Choi SY, Kim Y, Baek SH, Kim KT, Chae CB, Lambeth JD, Suh PG, Ryu SH. A peptide with unique receptor specificity. J Immunol. 1997;158(4):1895–1901. [PubMed] [Google Scholar]
- 38.Wan HX, Zhou C, Zhang Y, Sun M, Wang X, Yu H, Yang X, Ye RD, Shen JK, Wang MW. Discovery of Trp-Nle-Tyr-Met as a novel agonist for human formyl peptide receptor-like 1. Biochem Pharmacol. 2007;74(2):317–326. doi: 10.1016/j.bcp.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Edwards BS, Young SM, Oprea TI, Bologa CG, Prossnitz ER, Sklar LA. Biomolecular screening of formylpeptide receptor ligands with a sensitive, quantitative, high-throughput flow cytometry platform. Nature Protocols. 2006;1(1):59–66. doi: 10.1038/nprot.2006.9. [DOI] [PubMed] [Google Scholar]
- 40.Bae YS, Yi HJ, Lee HY, Jo EJ, Kim JI, Lee TG, Ye RD, Kwak JY, Ryu SH. Differential Activation of Formyl Peptide Receptor-Like 1 by Peptide Ligands. J Immunol. 2003;171(12):6807–6813. doi: 10.4049/jimmunol.171.12.6807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.