Abstract
Background
Since osteosarcoma is extremely rare in children ≤ 5 years of age, we sought to investigate if tumor characteristics, treatment strategies, and outcomes differ compared to older patients.
Procedure
Patients < 20 years of age with high-grade osteosarcoma reported to national SEER database from 1973–2006 were separated into two groups based on age at diagnosis: ≤ 5 years (n=49) and 6–19 years (n = 1687). Patient, tumor, and treatment characteristics were compared using Fisher exact tests. Overall survival was estimated by Kaplan-Meier methods and compared using log-rank tests and Cox models.
Results
Patients ≤ 5 years had higher proportions of osteosarcoma arising from the upper limb compared to older patients (24.5% vs. 11.2%; p = 0.006). These very young patients had a significantly higher proportion of telangiectatic histology (10.2% vs. 2.9%; p = 0.017). Sex, metastatic status, race, or ethnicity did not differ by age. A higher proportion of very young patients was treated with amputation (55.2% vs. 27.3%; p = 0.002). Five-year overall survival was inferior for patients with localized osteosarcoma 5 years of age or younger compared to older children (51.9% vs. 67.3%; p = 0.03). After controlling for metastatic status, year of diagnosis, and tumor site, the hazard ratio for death in very young patients was 1.6 (95% confidence interval 1.02 – 2.36; p = 0.04) compared to older patients.
Conclusions
Tumor characteristics, treatment, and outcomes differ among children ≤ 5 years of age compared to older pediatric patients. These differences may reflect differences in tumor biology.
Keywords: osteosarcoma, young children, treatment, outcome, SEER
Introduction
Osteosarcoma is the most common childhood primary bone cancer [1]. Osteosarcoma incidence varies significantly with age [2]. The peak age for these tumors is in adolescence. Several groups have evaluated preadolescent patients (< 10–12 years of age at diagnosis) and have reported conflicting results. While some authors have found that survival in preadolescent patients is no worse than in adolescents [3–7], others have reported inferior outcomes in preadolescent patients [8,9].
Fewer studies have focused specifically on very young patients diagnosed with osteosarcoma at 5 years of age and younger. The clinical presentation and outcomes for very young osteosarcoma patients have not been well studied. The rarity of this tumor in very young patients has precluded comprehensive analyses. Published case series focused on this age group have only included small numbers of patients [10–13]. Given the marked difference in incidence of osteosarcoma in very young children, we hypothesized that these children might have different tumor characteristics, treatment approaches, and outcomes compared to older children and adolescents. Using data from the Surveillance, Epidemiology, and End Results Program (SEER), we evaluated this hypothesis in a cohort of children diagnosed with osteosarcoma at the age of 5 years or younger.
Methods
Patient Population
Patient data were extracted from the US National Cancer Institute’s SEER database, covering years 1973 – 2006. During this time period, the 17 population-based SEER registries have reported 1786 patients with osteosarcoma less than 20 years of age. The SEER system covers approximately 26% of the US population. Inclusion criteria for geographic areas to be selected for the SEER Program are based on their ability to operate and maintain a high quality population-based cancer reporting system and their epidemiologically significant population subgroups. Starting on January 1, 1973 the SEER Program began collecting and publishing cancer incidence and survival data as well as patient demographics, primary tumor site, tumor morphology, stage at diagnosis, first course of treatment and follow-up data for vital status.
Patients with histologically confirmed primary high-grade osteosarcoma between 0 and 19 years of age were eligible for the study. Patients with osteosarcoma arising in the setting of Paget disease (n=1) as well as parosteal (n=48) and well-differentiated osteosarcoma (n = 1) were excluded from the analyses. None of these excluded histologies were observed in patients 5 years of age or younger. Osteosarcoma was classified according to the International Classification of Childhood Cancer and/or the International Classification of Disease for Oncology, third revision (ICD-O-3). Anatomic site codes were classified according to the ICD-O coding. No additional detailed site descriptors or location distinctions, such as proximal or distal, were available.
Patient age at diagnosis was dichotomized into very young patients (age 0–5) and older patients (age 6–19). This cut-point was chosen a priori for our study based on our literature review indicating a paucity of published data in this age group. The SEER database included 49 patients ≤ 5 years of age, giving us a reasonable cohort to analyze.
Patient characteristics and clinical presentation were evaluated according to age group. Variables of interest included: sex; stage (metastatic vs. localized); year of diagnosis (in sequential 5-year blocks); race; ethnicity; primary tumor site (upper limb vs. lower limb vs. all other tumor sites); and histologic subtype. Other tumor sites included: cranial and facial bones (n=75); pelvic bones (n=56); chest wall bones (n = 20); vertebrae (n=18); and unspecified sites (n=22). Osteosarcoma histology was classified as described by the ICD-0-3 as chondroblastic, fibroblastic, telangiectatic, and not otherwise specified. Due to small numbers, periosteal, central, and small cell osteosarcoma were group into a category of “other histologies”.
Data on treatment received were also collected. Surgery was dichotomized as not used (except for diagnostic biopsy) or used as a component of local control. Patients who had surgical treatment were subdivided in two groups based on the use of limb-sparing surgery or amputation. Data regarding use of limb-sparing surgery vs. amputation were not available prior to 1986. Radiation therapy was dichotomized as not given or given if performed at any time point during treatment (including radioactive implants and radioisotopes). For analyses related to use of surgery or radiation, patients were excluded if they had missing data for surgery or radiation, respectively. Data regarding the use of chemotherapy were not available.
Statistical Methods
Patient, tumor, and treatment characteristics were evaluated for differences between age groups using Fisher exact tests. A logistic regression model was constructed to evaluate differences in frequency of amputation among very young patients while controlling for metastatic status, primary tumor site and year of diagnosis.
Overall survival was estimated by Kaplan-Meier methods and differences between age groups were evaluated using the log-rank test. Overall survival was expressed as Kaplan-Meier estimates with 95% confidence interval (CI). Overall survival time was calculated as the number of completed months between the date of diagnosis and whichever occurred first: the date of death; the date last known to be alive; or December 31, 2006. The median follow-up time for the analyzed cohort was 88 months.
Cox proportional hazard models were used to assess the effect of age on overall survival while controlling for known confounders. The proportional hazards assumption was tested using time-varying covariates. For models including metastatic status and/or year of diagnosis as variables, the proportional hazards assumption could not be confirmed. Subsequent models were stratified by metastatic status and year of diagnosis. These stratified models satisfied the proportional hazards assumption. The SEER database was accessed using SEER*Stat, version 6.4.4. All statistical analyses were performed using SAS, version 9 and STATA, version 10.
Results
Patient Characteristics
Between 1973 and 2006, the SEER database included 1786 patients with osteosarcoma diagnosed before age 20 years. After excluding 50 patients with osteosarcoma arising from Paget’s disease and parosteal or well-differentiated osteosarcoma, our study population included 1736 patients. Of these, only 49 (2.8%) patients were 5 years old or younger. Patient age among these very young patients was distributed as follows: < 1 year (n = 1); 1 year (n = 1); 2 years (n = 3); 3 years (n = 7); 4 years (n = 17); and 5 years (n = 20). The clinical characteristics of these very young patients are shown in Table I.
Table 1.
Prevalence of patient and tumor characteristics by age
| Characteristics | Age 0–5 years | Age 6–19 years | p-value |
|---|---|---|---|
| n = 49 | n = 1687 | ||
| Median age (range) | 4 years (0 – 5) | 14 years (6 – 19) | |
| Male | 46.9% (23) | 56.4% (952) | 0.19 |
| Stage | |||
| Metastatic | 12.2% (6) | 17.7% (299) | 0.48 |
| Localized | 77.6% (38) | 75.2% (1268) | |
| Unknown | 10.2% (5) | 7.1% (120) | |
| Histology | |||
| Osteosarcoma, NOS | 79.6% (39) | 78.7% (1327) | 0.098* |
| Chondroblastic | 8.2% (4) | 11.9% (200) | |
| Fibroblastic | 2% (1) | 4.1% (69) | |
| Telangiectatic | 10.2% (5) | 2.9% (50) | |
| Other histologies | 0% | 2.4% (41) | |
| Site | |||
| Upper limb | 24.5% (12) | 11.2% (189) | 0.006 |
| Lower limb | 59.2% (29) | 78% (1315) | |
| Other sites | 16.3% (8) | 10.9 (183) | |
| Race/Ethnicity | |||
| White non-Hispanic | 53.1% (26) | 54.8% (924) | 0.43 |
| Asian non-Hispanic | 4.1% (2) | 9.8% (165) | |
| Black non-Hispanic | 14.3% (7) | 14.2% (240) | |
| White Hispanic | 20.4% (10) | 16.9% (285) | |
| Unknown or Other | 8.2% (4) | 4.3% (73) | |
| Year of diagnosis | |||
| 1973 – 1977 | 6.1% (3) | 7.6% (128) | 0.76 |
| 1978 – 1982 | 12.2% (6) | 9.1% (153) | |
| 1983 – 1987 | 8.2% (4) | 8.7% (147) | |
| 1988 – 1992 | 16.3% (8) | 9.7% (164) | |
| 1993 – 1997 | 12.2% (6) | 14.2% (240) | |
| 1998 – 2002 | 20.4% (10) | 25.6% (431) | |
| 2003 – 2006 | 24.5% (12) | 25.1% (424) | |
P-value reflects overall difference in histologic subtypes between age groups. P-value for difference in telangiectatic subtype compared to all other histologies between age groups was 0.017.
Significant differences in clinical presentation among very young children were noted according to primary site and histology (Table I). A higher proportion of very young patients were diagnosed with osteosarcoma arising from the upper limb and other sites compared to older patients who were more likely to have lower extremity tumors (p = 0.006). A trend towards a difference in tumor histology was seen in the overall Fisher exact test (p = 0.098). This difference seemed to be most pronounced for the telangiectatic subtype. We therefore collapsed the data into telangiectatic versus all other histologies and repeated the Fisher exact test. This comparison confirmed a higher proportion of telangiectatic osteosarcoma among very young patients compared to older patients (p= 0.017). There were no differences in sex, metastatic status, race, or ethnicity according to age group.
Treatment Differences
The use of radiation therapy was uncommon (5.4% of all pediatric patients). The probability of receiving radiation therapy as a component of local control did not differ by age (6.1% in young group and 5.4% in older group). The proportion of patients undergoing surgical local control also did not differ according to age (87.5% in young group and 87.4% in older group).
Among those patients who underwent surgery, a higher proportion of very young patients had an amputation compared to older patients (55.2% in young group and 27.3% in older group; p = 0.002). The crude odds ratio for amputation for younger patients was 3.3 (95% confidence interval 1.6 – 6.9) compared to older patients. Patients with metastatic disease were more likely to undergo amputation compared to patients without metastatic disease (38% vs 26%; p = 0.003). In the very young patient group, 2 of 3 patients with metastatic disease and available data underwent amputation compared to 12 of 23 patients with localized disease. Given that the decision to perform an amputation may be confounded by other patient and tumor characteristics, we constructed a logistic regression model to control for these potential confounders. After controlling for metastatic status, year of diagnosis, and primary tumor site, very young patients continued to demonstrate higher amputation rates compared to older patients. The odds ratio for amputation for these very young patients was 3.5 (95% CI 1.54 – 7.77; p = 0.003) compared to older patients.
Patient Outcomes
Excluding patients with metastatic disease, overall survival was inferior for patients 5 years of age and younger compared to older patients (Figure 1). Patients with non-metastatic disease who were 5 years of age or younger had a Kaplan-Meier 5-year overall survival estimate of 51.9% (95% CI 36 – 66%) compared to 67.3% (95% CI 65 – 70%) for patients ages 6–19 years (p = 0.03 by log-rank test). Using a Cox proportional hazards model controlling for metastatic status, year of diagnosis, and tumor site, very young patients continued to demonstrate inferior overall survival compared to older patients. The hazard ratio for death in very young patients was 1.6 (95% CI 1.02 – 2.36; p = 0.04) compared to older patients.
Figure 1.
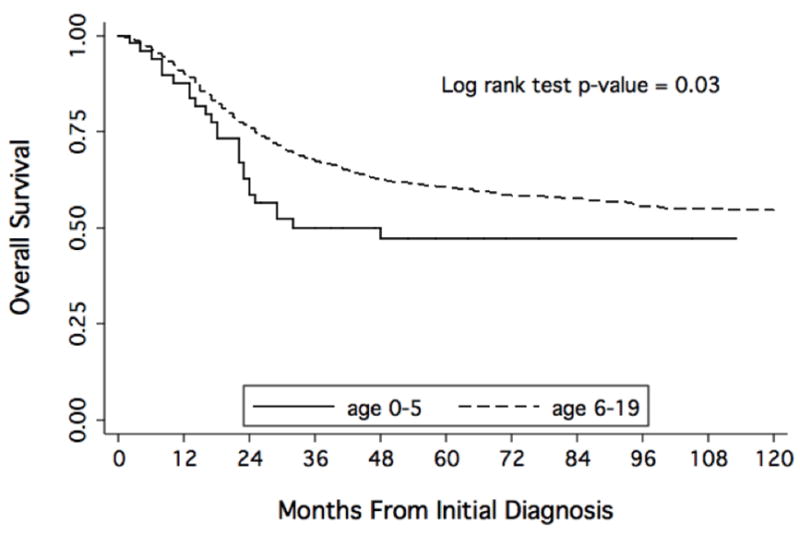
Kaplan-Meier estimates of overall survival from time of diagnosis according to age of diagnosis with localized osteosarcoma.
Discussion
This is the most comprehensive analysis of patient characteristics, treatment strategies, and outcomes in very young patients with osteosarcoma. Our results demonstrate differences in each of these domains for patients 5 years of age and younger compared to older patients.
Our findings confirm earlier observations from one case series in which patients 5 years and younger demonstrated a significantly higher incidence of telangiectatic osteosarcoma.[3] This series also reported a more common tumor location in the upper limb among very young patients compared to older patients [11]. Other case series have not specifically addressed these differences in this age group. The reason for these differences remains obscure, but suggest that osteosarcoma in these very young patients may have biological differences that drive these different clinical findings.
Surgical treatment of osteosarcoma has evolved over the past several decades [14]. Aggressive surgery of osteosarcoma, either by amputation or by limb-sparing surgery, is essential for curative treatment and to assure local control. Currently, approximately 80% to 90% of patients with musculoskeletal tumors undergo limb-sparing surgery rather than amputation [15–19]. For young skeletally immature patients, limb-sparing procedures can be especially challenging [20,21]. Given the challenges of limb-sparing procedures for these children, our finding that most very young patients with appendicular tumors are treated with amputation is not surprising.
Clinical outcomes for osteosarcoma patients age 5 years or younger have not been well described. Due to rarity of this tumor under the age of 5, single institution case series have been reported for these very young children, but comprehensive data analyses are lacking [11–13,22,23]. Our results indicate an unfavorable prognosis for children 5 years of age or younger at the time of initial diagnosis with osteosarcoma. Even after controlling for known prognostic factors, very young children demonstrated inferior overall survival compared to their older counterparts. This inferior outcome may reflect intrinsic biologic differences, differences in local control strategies due to extreme skeletal immaturity, or pharmacokinetic differences in drug metabolism [24–26]. Evaluation of these potential explanations cannot be made using the SEER database. The inferior outcome among very young patients reinforces the need to understand the factors driving this difference. Standard treatment in osteosarcoma has been defined from clinical trials enrolling mainly adolescences and young adults. These treatment approaches may not be well-suited to very young patients [10].
The impact of age on outcome in osteosarcoma has been a source of controversy. For older patients, some studies have reported equal rates of survival while others have described a worse prognosis for patients who are in adolescence or young adulthood [3,27,28]. Other groups have reported that pre-adolescent patients may have an inferior outcome [8,29,30]. Other studies found that the crude association of age and survival was significant, but age as prognostic factor did not maintain significance while controlling for known confounders [31]. Our finding that outcome is different among children 5 years of age or younger compared to older pediatric patients indicates that the relationship between age and outcome is complex. This complexity may account for some of the controversy in this field.
A main strength of this study is the relatively large number of osteosarcoma cases in very young children provided by using the SEER database. These patients were treated at many different areas of the United States and selection was not based on treatment or outcome. Furthermore, our control group of older pediatric patients includes a subgroup of patients (6 – 10 years of age) that others have suggested may have a worse prognosis [8,29,30]. The inclusion of these patients in our control group could have biased our findings towards the null hypothesis of no difference. Demonstrating a difference in survival using this control group supports our findings.
Limitations of analyzing data from SEER include those of any study relying on a tumor registry. No more data than those reported are available and the SEER database does not provide data on recurrence site. Tumor histology could not be independently confirmed. Moreover, data regarding osteosarcoma predisposition syndromes, such as Li-Fraumeni, Diamond-Blackfan anemia, and Rothmund-Thompson syndrome, were not available. A higher incidence of these syndromes among younger patients with osteosarcoma may help to explain some of the differences observed in our study [32–35]. Furthermore, in order to develop a large enough cohort of very young patients, we included patients treated in the 1970’s. Both the role of chemotherapy and surgical treatments evolved significantly over the past decades and some of our findings may not apply in the setting of current practice. In order to account for these treatment changes, we controlled for year of diagnosis in our regression models and were able to confirm our univariate findings.
Another specific limitation is the scarcity of tumor size data in SEER database, since tumor size is of prognostic value in patients with osteosarcoma [31]. In the SEER database, tumor size was unavailable in approximately half of the analyzed population and this variable was therefore not included in our analyses. Given our focus on very young children, this omission may not be as critical as in other analysis focused on larger patients. Studies in other sarcomas have shown that the risk associated with a given tumor size might not be the same in very young compared to older patients due to different body sizes [36].
This study demonstrates differences in clinical presentation, treatment strategy, and outcomes according to very young age even after controlling for prognostic factors. Osteosarcoma is extremely rare in patients 5 years of age or younger. This striking difference in incidence and our findings that distinct tumor characteristics differ among very young patients suggests that these tumors arising in very young patients may be biologically different. Further investigations into developmental changes and molecular genetics are required to evaluate the observed differences in osteosarcoma among very young children. Additional efforts should be directed at adjusting treatment strategies in these very young patients to improve their outcomes.
Acknowledgments
Support: Supported by the Campini Foundation and NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None
References
- 1.Gurney JG, Severson RK, Davis S, et al. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75(8):2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125(1):229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasser DB, Lane JM, Huvos AG, et al. Survival, prognosis, and therapeutic response in osteogenic sarcoma. The Memorial Hospital experience. Cancer. 1992;69(3):698–708. doi: 10.1002/1097-0142(19920201)69:3<698::aid-cncr2820690317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Rytting M, Pearson P, Raymond AK, et al. Osteosarcoma in preadolescent patients. Clin Orthop Relat Res. 2000;(373):39–50. doi: 10.1097/00003086-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Cho WH, Lee SY, Song WS, et al. Osteosarcoma in pre-adolescent patients. J Int Med Res. 2006;34(6):676–681. doi: 10.1177/147323000603400614. [DOI] [PubMed] [Google Scholar]
- 6.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15(1):76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Lee JA, Kim DH, Lim JS, et al. The survival of osteosarcoma patients 10 years old or younger is not worse than the survival of older patients: a retrospective analysis. Cancer Res Treat. 2007;39(4):160–164. doi: 10.4143/crt.2007.39.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler K, Beron G, Kotz R, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: results of a Cooperative German/Austrian study. J Clin Oncol. 1984;2(6):617–624. doi: 10.1200/JCO.1984.2.6.617. [DOI] [PubMed] [Google Scholar]
- 9.Delepine N, Delepine G, Jasmin C, et al. Importance of age and methotrexate dosage: prognosis in children and young adults with high-grade osteosarcomas. Biomed Pharmacother. 1988;42(4):257–262. [PubMed] [Google Scholar]
- 10.Rivera-Luna R, De Leon-Bojorge B, Ruano-Aguilar J, et al. Osteosarcoma in children under three years of age. Med Pediatr Oncol. 2003;41(1):99–100. doi: 10.1002/mpo.10281. [DOI] [PubMed] [Google Scholar]
- 11.Hartford CM, Wodowski KS, Rao BN, et al. Osteosarcoma among children aged 5 years or younger: the St. Jude Children’s Research Hospital experience. J Pediatr Hematol Oncol. 2006;28(1):43–47. [PubMed] [Google Scholar]
- 12.Luiz CP, al Kharusi W, Sethu AU, et al. Osteosarcoma in a 26-month-old girl. Cancer. 1992;70(4):894–896. doi: 10.1002/1097-0142(19920815)70:4<894::aid-cncr2820700428>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Sanchis-Alfonso V, Fernandez-Fernandez CI, Donat J, et al. Osteoblastic osteogenic sarcoma in a 13-month-old girl. Pathol Res Pract. 1994;190(2):207–210. doi: 10.1016/S0344-0338(11)80713-2. discussion 211. [DOI] [PubMed] [Google Scholar]
- 14.Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer. 1997;33(11):1725–1731. doi: 10.1016/s0959-8049(97)00252-9. [DOI] [PubMed] [Google Scholar]
- 15.Ruggieri P, De Cristofaro R, Picci P, et al. Complications and surgical indications in 144 cases of nonmetastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Clin Orthop Relat Res. 1993;(295):226–238. [PubMed] [Google Scholar]
- 16.Gebhardt MC, Flugstad DI, Springfield DS, et al. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;(270):181–196. [PubMed] [Google Scholar]
- 17.Rougraff BT, Simon MA, Kneisl JS, et al. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76(5):649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Anract P. Surgical management of primary bone cancer. Bull Acad Natl Med. 2009;193(1):107–126. [PubMed] [Google Scholar]
- 19.Rao BN, Rodriguez-Galindo C. Local control in childhood extremity sarcomas: salvaging limbs and sparing function. Med Pediatr Oncol. 2003;41(6):584–587. doi: 10.1002/mpo.10405. [DOI] [PubMed] [Google Scholar]
- 20.Neel MD, Wilkins RM, Rao BN, et al. Early multicenter experience with a noninvasive expandable prosthesis. Clin Orthop Relat Res. 2003;(415):72–81. doi: 10.1097/01.blo.0000093899.12372.25. [DOI] [PubMed] [Google Scholar]
- 21.Eckardt JJ, Kabo JM, Kelley CM, et al. Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res. 2000;(373):51–61. doi: 10.1097/00003086-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Graham NJ, Cairns RA, Anderson RA. Osteosarcoma in a 19-month-old girl. Can Assoc Radiol J. 1996;47(1):33–35. [PubMed] [Google Scholar]
- 23.Kozakewich H, Perez-Atayde AR, Goorin AM, et al. Osteosarcoma in young children. Cancer. 1991;67(3):638–642. doi: 10.1002/1097-0142(19910201)67:3<638::aid-cncr2820670319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Wang YM, Sutow WW, Romsdahl MM, et al. Age-related pharmacokinetics of high-dose methotrexate in patients with osteosarcoma. Cancer Treat Rep. 1979;63(3):405–410. [PubMed] [Google Scholar]
- 25.Wang YM, Fujimoto T. Clinical pharmacokinetics of methotrexate in children. Clin Pharmacokinet. 1984;9(4):335–348. doi: 10.2165/00003088-198409040-00003. [DOI] [PubMed] [Google Scholar]
- 26.Eyre R, Feltbower RG, Mubwandarikwa E, et al. Epidemiology of bone tumours in children and young adults. Pediatr Blood Cancer. 2009;53(6):941–952. doi: 10.1002/pbc.22194. [DOI] [PubMed] [Google Scholar]
- 27.Hudson M, Jaffe MR, Jaffe N, et al. Pediatric osteosarcoma: therapeutic strategies, results, and prognostic factors derived from a 10-year experience. J Clin Oncol. 1990;8(12):1988–1997. doi: 10.1200/JCO.1990.8.12.1988. [DOI] [PubMed] [Google Scholar]
- 28.Rosen G, Marcove RC, Caparros B, et al. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43(6):2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Age and dose of chemotherapy as major prognostic factors in a trial of adjuvant therapy of osteosarcoma combining two alternating drug combinations and early prophylactic lung irradiation. French Bone Tumor Study Group. Cancer. 1988;61(7):1304–1311. doi: 10.1002/1097-0142(19880401)61:7<1304::aid-cncr2820610705>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clin Orthop Relat Res. 2002;(397):40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Hicks MJ, Roth JR, Kozinetz CA, et al. Clinicopathologic features of osteosarcoma in patients with Rothmund-Thomson syndrome. J Clin Oncol. 2007;25(4):370–375. doi: 10.1200/JCO.2006.08.4558. [DOI] [PubMed] [Google Scholar]
- 34.Lipton JM, Federman N, Khabbaze Y, et al. Osteogenic sarcoma associated with Diamond-Blackfan anemia: a report from the Diamond-Blackfan Anemia Registry. J Pediatr Hematol Oncol. 2001;23(1):39–44. doi: 10.1097/00043426-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Aquino VM, Buchanan GR. Osteogenic sarcoma in a child with transfusion-dependent Diamond-Blackfan anemia. J Pediatr Hematol Oncol. 1996;18(2):230–232. doi: 10.1097/00043426-199605000-00030. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari A, Miceli R, Meazza C, et al. Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol. 2009;27(3):371–376. doi: 10.1200/JCO.2007.15.4542. [DOI] [PubMed] [Google Scholar]


