Abstract
Objective
Preoperative brain injury, particularly stroke and white matter injury (WMI), is common in newborns with congenital heart disease (CHD). The objective of this study was to determine the risk of hemorrhage or extension of preoperative brain injury with cardiac surgery.
Methods
This dual-center prospective cohort study recruited 92 term newborns: 62 with transposition of the great arteries (TGA), and 30 with single ventricle physiology from two tertiary referral centers. Newborns underwent brain MRI scans before and after cardiac surgery.
Results
Brain injury was identified in 40 (43%) newborns on the preoperative MRI scan (median five days of life): stroke in 23, WMI in 21, and intraventricular hemorrhage in 7. None of the brain lesions presented clinically with overt signs or seizures. Preoperative brain injury was associated with balloon atrial septostomy (BAS) (P=0.003) and lowest SaO2 (P=0.007); in a multivariable model, only the effect of BAS remained significant when adjusting for lowest SaO2. On postoperative MRI in 78 newborns (median 21 days of life), none of the preoperative lesions showed evidence of extension or hemorrhagic transformation (0/40 [95% CI: 0-7%]). The presence of preoperative brain injury was not a significant risk factor for acquiring new injury on the postoperative MRI (P=0.8).
Conclusion
Clinically silent brain injuries identified preoperatively in newborns with CHD, including stroke, have a low risk of progression with surgery and cardiopulmonary bypass, and should therefore not delay clinically indicated cardiac surgery. In this multi-center cohort, BAS remains an important risk factor for preoperative brain injury, particularly stroke.
Keywords: Stroke, White matter injury, Term newborn, Congenital heart disease, Magnetic resonance imaging, Surgery, Preoperative injury
Introduction
Multiple studies using highly sensitive magnetic resonance imaging (MRI) have identified a high frequency of preoperative brain injury in newborns with congenital heart disease (CHD).(1-6) In particular, brain injuries such as stroke, white matter injury (WMI), and intraventricular hemorrhage (IVH) are seen in more than one third of term newborns with CHD scanned prior to surgery.(3, 4, 7) These lesions are typically clinically silent in the neonatal period, and usually not identified by routine cranial ultrasound scans.(3, 4) It is unknown whether heart surgery with cardiopulmonary bypass and peri-operative hemodynamic instability might worsen these areas of brain injury. Two studies published to date with pre- and postoperative MRI, noted a single patient with worsening of a preoperative hemorrhagic lesion(2) and worsening of periventricular leukomalacia in two patients.(1) In the latter cases, the authors did not distinguish between extension of existing WMI or addition of new WMI. Thus, the identification of these injuries poses a dilemma in the clinical care of these critically ill newborns: should heart surgery be delayed if brain injury is present preoperatively? The answer to this question must balance the need for and timing of life-saving surgery and the risk posed by exposing areas of injured brain to cardiopulmonary bypass and hemodynamic instability.
Most newborns with severe CHD, such as D-transposition of the great arteries (TGA), or single ventricle physiology (SVP), including the Hypoplastic Left Heart Syndrome (HLHS), require open heart-surgery with cardiopulmonary bypass to correct or palliate their heart defect.(8) Newborns undergoing cardiac surgery are exposed to the risks of intraoperative ischemia, postoperative low cardiac output syndrome (9) and anticoagulation required for cardiopulmonary bypass. Therefore, we hypothesized that preoperative brain injuries will be at significant risk of progression from the pre- to the postoperative scan, including hemorrhagic transformation of stroke or extension of WMI.
The objective of this study is to determine the frequency of progression of preoperative brain injuries with cardiac surgery in a prospective dual-centre cohort of newborns with TGA and SVP. This cohort also provides an opportunity to reexamine clinical risk factors previously identified for preoperative brain injury.
Material and Methods
Subjects
Between September 2001 and March 2008, this dual-centre prospective cohort study enrolled term newborns (>36 weeks gestation) with CHD delivered at or transferred to the University of California San Francisco Children's Hospital (UCSF) or British Columbia Children's Hospital University of British Columbia (UBC), both tertiary level cardiac referral centers,. The Research Ethics Board at each site approved the study protocol. The newborns were recruited and studied with the informed consent of their parents. Subgroups of this cohort were previously reported.(3-5, 7) Term newborns with TGA or SVP, including HLHS, were included in this study. They were excluded if they had a congenital infection or genetic malformation syndrome.
Clinical data thought to be potential predictors of brain injury or neurodevelopmental outcome were collected from the medical records at both sites as described previously (3-5, 7) Specifically, the lowest preoperative SaO2 value was obtained from the ICU nursing record. As this was an observational study, the newborns received routine clinical care for their cardiac lesions, including surgery, irrespective of the MRI findings. However at UCSF the surgery of the first two newborns was delayed empirically for one week after preoperative brain injury was found on the MRI scan.
MRI studies
MR studies were performed as soon as the newborns were sufficiently stable to be transported to the scanner by trained personnel and with the use of a specialized MRI-compatible isolette and a dedicated neonatal head coil.(10, 11) The transport team is composed of 2 intensive care nurses and critical care physician at UCSF, and of an intensive care nurse and a respiratory therapist at UBC. No adverse events occurred at either center with this scanning protocol. Newborns were examined by a pediatrci neurologist within 24 hours of the MRI scan, blinded to the MRI findings.
At UCSF, most subjects received pharmacological sedation with pentobarbital (1-2 mg/kg/dose up to a total of 6 mg/kg) and/or morphine sulfate (0.05 mg/kg/dose up to a total of 0.2 mg/kg), according to the institution's sedation guidelines. MRI studies were carried out on a 1.5 Tesla Signa Echo-Speed system (GE Medical System, Waukesha, W1) as described previously(3-5) and included (TR/TE/FieldOfView/SliceThickness/Gap): 1) T1-weighted sagittal spin echo images (600/8/20 cm/3mm/1mm), 2) dual-echo T2-weighted spin echo (3000/60/120/8×13.5 cm/4mm/2mm), 3) coronal volumetric 3D gradient echo images with RF spoiling (SPGR) images (36/3.5/22 cm/1mm/0), and (4) average diffusivity (Dav) maps Echo-planar acquisition (8000/150/36×27 cm/5mm/0).
At UBC, MRI studies were carried out without pharmacological sedation on a Siemens 1.5 Tesla Avanto using VB 13A software and included comparable imaging to that obtained at UCSF (TR/TE/FieldOfView/SliceThickness/Gap): 1) 3D coronal volumetric T1-weighted images (36/9.2/200 mm/1mm/0), 2) axial fast spin echo T2-weighted images (4610/107/160mm/4 mm/0.2mm). Average diffusivity (Dav) maps were generated from diffusion tensor imaging acquired with a multirepetition, single-shot echo planar sequence with 12 gradient directions (4900/104/160mm/3mm/0), b=0, 600 and 700s/mm2, and an in-plane resolution of 1.3 mm.
The MRI scans were reviewed by a neuroradiologist independently at each site (UCSF: AJB; UBC: KJP) blinded to the newborn's clinical condition except for gestational age. As previously described, the neuroradiologists scored each MRI scan for acquired injury (focal, multifocal, or global) or developmental brain abnormalities.(4) The severity of stroke, WMI, or IVH was recorded using previous scoring systems.(4) Single lesions in the white matter that measured (≤3mm in size) were classified as WMI, while larger lesions were considered stroke. The differentiation of these lesions is conceptual, with stroke resulting from occlusion of a vessel and being more likely to hemorrhage while WMI follows hypoxia-ischemia.(12) However, it is not clear that the two can be differentiated by MRI, and sensitivity analyses for the main findings were performed by reclassifying all lesions as either stroke or WMI. A single neuroradiologist (KJP) reviewed all pre and postoperative MRI scans in newborns with preoperative injury, blinded to the original scores. In the 4 cases of discrepancy (all solitary white matter lesions), the size definition was applied and consensus reached in all cases. “Progression” from the pre- to postoperative scans was defined as enlargement of the size of the injury, regardless of the presence of hemorrhage.
Anesthetic and Cardiopulmonary Bypass (CPB) Management
Newborns in this cohort underwent anesthesia and CPB according to a uniform clinical practice at each institution. At UCSF, anesthesia consisted of fentanyl (25–150 mg/kg total dose), midazolam (0.25–3 mg/kg total dose), and sevoflurane (up to 4% end-tidal concentration before and after CPB and isoflurane (up to 3% in the bypass sweep gas during CPB). Pancuronium was used for neuromuscular blockade. CPB was established with aortic and bicaval cannulation. Patients were cooled using alpha-stat pH management to a minimum nasopharyngeal temperature of 28° C for full flow bypass or 18 ° C for regional cerebral perfusion. The CPB prime included Normosol, methylprednisolone, cefazolin and albumin. Fresh whole blood or packed red blood cells and fresh frozen plasma were added to the prime to maintain hematocrit at 24-27%. Flow was maintained at 150 ml/kg/min except in cases requiring aortic arch reconstruction. For regional cerebral perfusion, the aortic cannula is removed and the innomonate artery cannulated with a 2 mm olive-tipped arteriotomy cannula through an incision in the aorta. Flows of 30-50 ml/kg/min are titrated to maintain pre-bypass regional cerebral saturations measured by near infrared spectroscopy (INVOS 5100A; Somanetics, Troy MI) and to keep pressure in the arterial line below 300 mmHg. After the repair and separation from CPB, all patients underwent modified ultrafiltration to remove the crystalloid priming volume. Aprotinin was used until 2007 (first 82 cases). At UBC, anesthesia was induced with fentanyl and rocuronium, and maintained with fentanyl (30 to 100 ug/kg total dose for the operation) and isoflurane (0.2-1.4% with titration to an appropriate blood pressure and level of sedation). The cardiopulmonary bypass prime included Plasmalyte A, albumin, sodium bicarbonate, and magnesium, and patients also received methylprednisolone and cefazolin intravenously prior to the initiation of bypass. Packed red blood cells and fresh frozen plasma were added to the prime to maintain a hematocrit of between 24 and 28%. Newborns in this cohort did not receive aprotinin. CPB was established with aortic and bicaval cannulation, and patients were cooled with a pH-stat management strategy to 32° C for full flow bypass or to 18° C for circulatory arrest or low-flow regional perfusion. Flow was established at 135 to 150ml/kg/minute for full bypass with arterial line pressures being maintained below 250mm Hg. For low-flow bypass a rate of 30 to 50 ml/kg/minute was accepted with arterial line pressures being maintained below 50 mm Hg. In this cohort, regional cerebral perfusion was not used as a predominant bypass strategy; in one of two patients with deep hypothermic circulatory arrest, anterograde cerebral perfusion was provided at a rate of 50 mg/kg intermittently for a total of 9 minutes. All patients were monitored with near infrared spectroscopy any any significant change from pre-bypass levels led to a reexamination of the bypass strategy or an increase in flow rate or hematocrit. After separation from bypass, all patients underwent a ten minute period of modified ultrafiltration.
Data Analysis
Data analyses were performed using Stata SE9.2 (Stata Corporation, College Station, TX). Clinical variables were compared in newborns with and without preoperative brain injury with the Mann Whitney U test for continuous or ordinal variables and the Fisher's exact test for categorical variables. Relative risks with 95% confidence intervals were calculated to describe the univariable association between preoperative injury and dichotomous clinical variables. Logistic regression was used to describe the univariable association between preoperative injury and continuous clinical variables. Clinical variables that were significantly associated with the risk of preoperative brain injury on univariable analysis were included in a multivariable logistic regression model.
Results
Study subjects
Ninety-two newborns were enrolled (Table 1): 69 at UCSF and 23 at UBC. Of these, 62 (67%) had TGA and 30 (33%) had SVP. Cardiac surgeries performed were arterial switch in 59 newborns, Norwood procedure in 28, Blalock-Taussig shunt in four, and one ductal stent with bilateral pulmonary artery banding. Three newborns required two operations prior to their second MRI: 2 needed coarction repair before the arterial switch, and one required reexploration for bleeding. The newborns had a preoperative MRI at a median age of 5 days of life (interquartile range: 3-7). Seventy-eight newborns had a postoperative MRI at a median age of 21 days (IQR: 16-27). Fourteen newborns were not scanned postoperatively: 9 expired, 4 needed permanent pacemaker wires, and 1 family withdrew from the study after a Blalock-Taussig shunt.
Table 1. Clinical description of the cohort by study centre: University of British Columbia (UBC) and University of California San Francisco (UCSF).
| Number (%), and Median (25th-75th percentile) | UBC | UCSF | |
|---|---|---|---|
| N=23 | N=69 | ||
| Preoperative factors | |||
| Gestational age at birth (wk) | 39 (38-40) | 39 (38-40) | |
| Birth weight (g) | 3250 (2955-4015) | 3364 (3000-3609) | |
| Male | 13 (57%) | 46 (67%) | |
| Apgar score at 5 minutes | 8 (7-9) | 8 (8-9) | |
| Caesarean delivery | 5 (22%) | 19 (28%) | |
| Heart lesion | TGA | 21 (91%) | 41 (59%) |
| SVP | 2 (9%) | 28 (41%) | |
| Days mechanically ventilated | 5 (3-7) | 6 (4-9) | |
| Intra-operative factors | |||
| Operation type | Arterial Switch | 19 (83%) | 40 (58%) |
| Norwood | 2 (9%) | 25 (36%) | |
| Other | 2 (9%) | 4 (6%) | |
| Bypass type | None | 2 (9%) | 3 (4%) |
| Low flow | 1 (4%) | 5 (7%) | |
| Full flow | 18 (78%) | 44 (64%) | |
| Regional cerebral perfusion | - | 12 (17%) | |
| Deep hypothermic cardiac arrest | 2 (9%) | 2 (3%) | |
| Other | - | 3 (4%) | |
| Postoperative factors | |||
| ECLS | 4 (17%) | 7 (11%) | |
| MRI 1 | |||
| MRI day (at day's of life) | 5 (4-12) | 5 (3-6) | |
| Acquired injury | 12 (52%) | 28 (41%) | |
| WMI | 7 (30%) | 14 (20%) | |
| IVH | 3 (13%) | 4 (6%) | |
| Stroke | 8 (35%) | 15 (22%) | |
| MRI 2 | |||
| MRI day (at day's of life) | 21 (17-29) | 20 (16-25) | |
| Acquired injury | 8 (47%) | 24 (40%) | |
| New WMI | 5 (29%) | 19 (32%) | |
| New IVH | - | 1 (2%) | |
| New stroke | 3 (18%) | 5 (8%) | |
Preoperative Brain Injury
Brain injury was identified in 40 (43%) newborns on preoperative MRI (Table 2a): stroke in 23 (Figure 1), WMI in 21 (Figure 1) and IVH in 7. The incidence of preoperative brain injury did not differ significantly by centre (P=0.3, Table 1). Eleven newborns had multiple lesions: 6 with stroke and WMI, 3 with stroke and IVH, and 2 with WMI and IVH. Strokes were solitary (23/23) and small (22/23) with most involving the middle cerebral artery (MCA) territory (19/23) and a minority in the posterior cerebral artery (PCA) territory (4/23). Strokes were clinically silent in all newborns. Ten newborns had solitary white matter lesions (Figure 2): 5 classified as strokes (> 3mm in size) and 5 as WMI (≤3mm in size). One newborn had evidence of watershed injury following perinatal depression (delivered “flat” following shoulder dystocia, Apgar of 5 at 5 minutes, cord ph=7.02); no other newborn had a pattern of injury typical of global hypoxia-ischemia in the term newborn.(13) Small subdural hemorrhages without mass effectwere seen in 13 newborns.
Table 2. Patterns of Brain Injury.
| A) Preoperative Brain Injury | ||||||||
|---|---|---|---|---|---|---|---|---|
| Preoperative Injury | Stroke | White Matter Injury | Intraventricular Hemorrhage | |||||
| < 1/3 | 1/3-2/3 | Mild | Moderate | Severe | Grade 1 | Grade 2 | ||
| TGA1 (62) | 28 (45%) | 17 (27%) | 1 (2%) | 9 (15%) | 3 (5%) | 1 (2%) | 2 (3%) | 2 (2%) |
| SVP2 (30) | 12 (40%) | 5 (17%) | - | 3 (10%) | 4 (13%) | 1 (3%) | - | 3 (10%) |
| Total (92) | 40 (43%) * | 23 (25%) | 21 (23%) | 7 (8%) | ||||
| B) New Postoperative Brain Injury | ||||||||
| Postoperative Injury | Stroke | White Matter Injury | Intraventricular Hemorrhage | |||||
| < 1/3 | 1/3-2/3 | Mild | Moderate | Severe | Grade 3 | |||
| TGA1 (56) | 20 (36%) | 3 (5%) | - | 12 (21%) | 3 (5%) | 2 (4%) | - | |
| SVP2 (22) | 12 (54%) | 4 (18%) | 1 (5%) | 1 (4%) | 6 (27%) | - | 1 (4%) | |
| Total (78) | 32 (41%) | 8 (10%) | 24 (31%) | 1 (1%) | ||||
The incidence of preoperative brain injury is not significantly different by heart lesion (P=0.7)
TGA: Transposition of the great arteries
SVP: Single ventricle physiology
Figure 1. Patterns of Brain Injury: stroke and white matter injury (WMI).
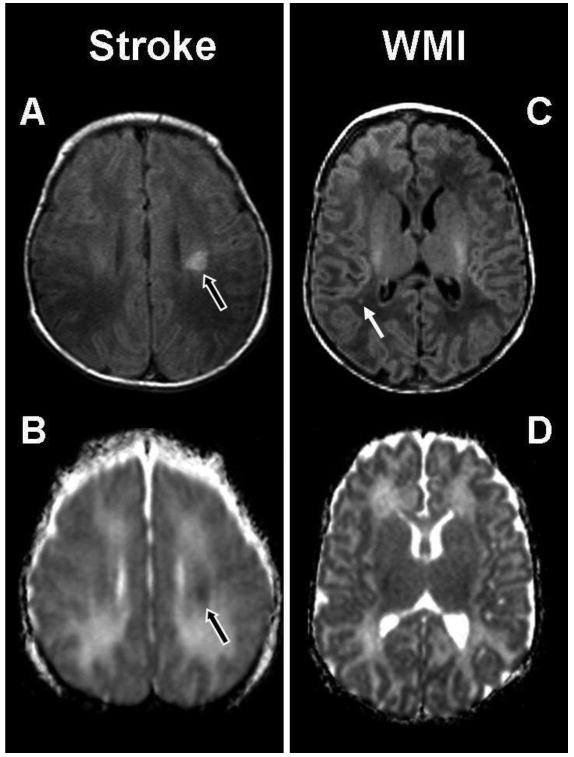
The abnormal hypersignal (black arrow) in the left basal ganglia is an example of a small preoperative stroke in a term newborn with TGA. It is localized in the middle cerebral territory and appears as a hyperintensity on the (A) T1-weighted image and an area of restricted diffusion on the (B) ADC map. The images on the right are an example of preoperative white matter injury (WMI) in a term newborn with transposition of the great arteries (TGA), and show a small focus (white arrow) of hyperintensity in the right parietal lobe on (C) the axial T1-weighted imaging. There is no corresponding area of restricted diffusion on (D) the ADC map.
Figure 2. Solitary white matter lesions.
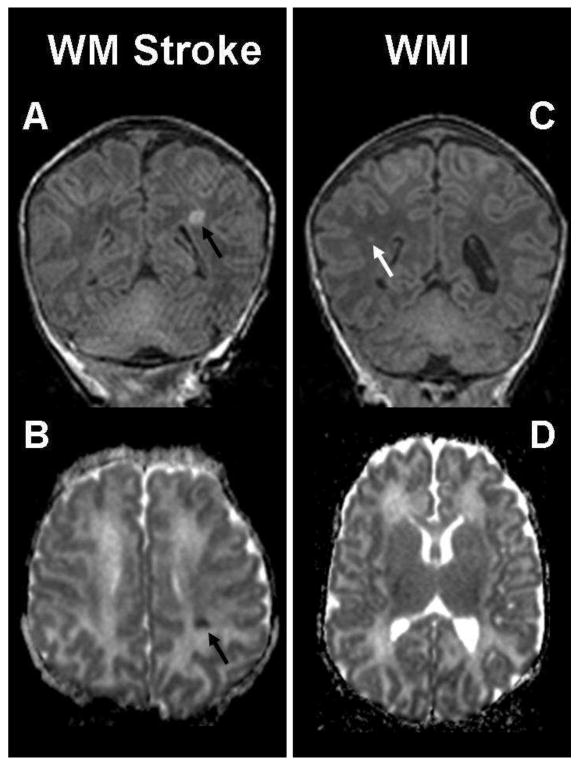
The coronal T1-weighted imaging (A) and axial ADC map (B) show a small preoperative white matter stroke (black arrow) in a term newborn with transposition of the great arteries (TGA). The lesion is localized in the left middle cerebral artery territory and characterized by an abnormal T1 hyperintensity (A) and an area of restricted diffusion (B) in left parietal lobe. The images on the right are an example of white matter injury (WMI) in another term newborn with TGA (same patient as in Figure 1), and show a small focus (white arrow) of hyperintensity in the right parietal lobe on (C) the coronal T1-weighted imaging. There is no corresponding area of restricted diffusion on (D) the ADC map.
Risk factors for Preoperative Brain Injury
Higher preoperative SaO2 was protective for preoperative brain injury (OR=0.96, 95%CI: 0.94-0.99, P=0.007). Balloon atrial septostomy (BAS) doubled the risk for preoperative brain injury (RR=2, 95%CI: 1.29-3.65, P=0.003) (Table 3). The risk of preoperative brain injury was not associated with the centre (P=0.3), the location (catheterization lab versus bedside) (P=0.2) nor the route of BAS (femoral versus umbilical vein) (p=0.9). When BAS and preoperative SaO2 were included in a multivariable model, BAS remained a significant risk factor for acquired brain injury (OR=3.6, 95%CI: 1.2-10.7, P=0.02) while SaO2 did not (OR=0.98, 95%CI: 0.95-1, P=0.3).
Table 3. Clinical description of newborns with preoperative brain injury (N=40) relative to those without preoperative brain injury (N=52).
| Number (%), and Median (25th-75th percentile) | Preoperative Brain Injury | No Preoperative Brain Injury | P Value | |
|---|---|---|---|---|
| Number | 40 | 52 | ||
| Gestational age at birth (wk) | 39 (38-40) | 39 (38-40) | 0.7 | |
| Birth weight (g) | 3300 (2880-3670) | 3418 (3000-3675) | 0.9 | |
| Male | 26 (65%) | 33 (63%) | 1.0 | |
| Apgar score at 5 minutes | 8 (8-9) | 8 (8-9) | 0.6 | |
| SNAP-PE | 15 (11-24) | 14 (10-19) | 0.1 | |
| Neonatal resuscitation score | 2 (1-4) | 2 (1-2) | 0.2 | |
| Caesarean delivery | 8 (20%) | 16 (31%) | 0.3 | |
| Heart lesion | TGA | 28 (70%) | 34 (65%) | 0.7 |
| SVP | 12 (30%) | 18 (35%) | ||
| Days mechanically ventilated | 6 (4-9) | 6 (4-8) | 0.9 | |
| Umbilical Artery Catheter | 26 (67%) | 32 (64%) | 0.8 | |
| Umbilical Venous Catheter | 25 (64%) | 26 (51%) | 0.3 | |
| Balloon septostomy | 27 (68%) | 18 (35%) | 0.003 | |
| Lowest SaO2 | 55 (45-70) | 70 (60-80) | 0.007 | |
| Preoperative arrest | 2 (5%) | - | 0.2 | |
| MRI day of life | 5 (4-6) | 5 (3-7) | 0.9 | |
| Surgery day of life | 9 (7-11) Range: 2-30 |
7 (5-11) Range: 2-38 |
0.1 | |
When examining specific brain injuries, the risk of stroke was even more strongly related to BAS (RR=4, 95%CI: 1.5-9.3, P=0.0015) and lower SaO2 (OR=0.95, 95%CI: 0.92-0.98, P=0.002). Neither the centre (P=0.3), location of the BAS procedure (P=0.3) nor the route (P=0.9) of BAS significantly impacted this risk. All 18 newborns with TGA and stroke required a BAS. Newborns with WMI were more likely to have caesarean section delivery (31% versus 10%, P=0.05) and preoperative cardiac arrest (10% versus 0%, P=0.05).
Given the difficulty coding a solitary white matter lesion as WMI or stroke, we repeated our main analyses recoding all solitary white matter lesions as strokes, and again considering them all as WMI. The relationship of BAS and SaO2 with stroke remained significant even if all the solitary white matter lesions were classified as strokes (BAS: P=0.002; SaO2: P=0.002) or WMI (BAS: P=0.015; SaO2: P= 0.005).
Progression of Brain Injury
Preoperatively identified brain injury did not progress or extended from the pre-to postoperative MRI (0/40 [95% CI: 0-7%](14)). Progression of injury was not observed in the two newborns with pre-operative injury who did not require cardiopulmonary bypass for surgery. The median time interval between the preoperative MRI scan and cardiac surgery was 2 days (IQR: 1-5), while the one between BAS and cardiac surgery was 7 days (IQR: 5-12), and that between the pre- and postoperative MRI scan was 14 days (IQR: 9-20). The age at surgery did not significantly differ in newborns with and without preoperative brain injury (Table 3). A number of the preoperative lesions became smaller and more difficult to detect on the postoperative MRI (3/23 strokes, 3/21 WMI, and 2/7 IVH).
New Postoperative Brain Injury
New postoperative brain injury was seen in 32 newborns (40%) (Table 2b): stroke in 8, WMI in 24 and IVH in 1. Strokes were solitary (8/8) and small (7/8) with 7 in the MCA territory (7/8) and one in the PCA territory. Strokes were clinically silent in all newborns. WMI was mild in 3 newborns, moderate in 9, and severe in 2. Patterns of injury suggestive of global hypoxia-ischemia were not seen. New subdural hemorrhage was seen in 12 newborns, but none showed mass effect. The incidence of new postoperative brain injury did not differ significantly by centre (P=0.8, Table 1). Of the five newborns requiring ECLS surviving to the second MRI scan, new postoperative brain injury was seen in 2 (40%); new postoperative brain injury was seen in 29 of 68 newborns not requiring ECLS (P>0.9). Of the three newborns not requiring cardiopulmonary bypass who underwent a second MRI scan, new postoperative brain injury was seen in 1 (33%; P>0.9 relative to the remainder of the cohort). New postoperative brain injury was seen in 13/33 newborns (39%) with preoperative brain injury and in 29/44 with normal preoperative scans. Thus, the presence of preoperative brain injury was not a significant risk factor for acquiring new injury on postoperative MRI studies (RR=0.9, 95% CI: 0.53-1.56, P=0.8).
Discussion
In this dual-centre prospective cohort study, preoperative brain injuries were commonly identified in newborns with CHD prior to heart surgery and were predominantly clinically silent, small focal lesions: stroke, WMI and IVH. The rate of these clinically silent injuries is substantially higher than that found in normative populations.(15) These lesions did not show signs of progression or extension on a repeat MRI scan following cardiac surgery with cardiopulmonary bypass. Furthermore preoperative brain injuries were not a significant risk factor for acquiring new postoperative lesions.
No progression of brain injury on MRI
The lack of progression of preoperative brain lesions with cardiac surgery and cardiopulmonary bypass is somewhat surprising. We anticipated that term newborns with CHD would be at high risk for injury progression due to the unavoidable exposure to some degree of ischemia with surgery and cardiopulmonary bypass. Previous studies have linked postoperative factors, such as hypotension, to the risk of WMI on postoperative MRI.(3, 16) These postoperative factors might lead to progression of existing brain injury. We also expected that anticoagulation would carry a risk for hemorrhage into existing strokes.
The small size of the preoperative lesions in this cohort might have attenuated the risk of hemorrhagic transformation with anticoagulation required for cardiopulmonary bypass. It is also possible that current surgical and bypass techniques, including the avoidance of prolonged deep hypothermic cardiac arrest (DHCA), helped to prevent a degree of ischemia sufficient to worsen preoperative brain lesions.(17-21) Despite these improvements in cardiopulmonary bypass, critically ill newborns, including those with CHD, are at risk of impaired cerebrovascular autoregulation,(22) making any exposure to ischemia more likely to be poorly tolerated. In fact, none of the preoperative brain injuries progressed from the pre- to the post-operative scans. The lack of progression at two centres with different bypass management strategies suggests a generalizability of our findings to other centres.
This present results cannot exclude the possibility that preoperative brain injuries worsen in a manner not detectable even by the optimized high resolution MRI techniques used in this study. Further evidence in support of this contention will require analysis of the relative associations of preoperative and postoperative brain injuries with subsequent late neurodevelopmental outcomes.
Absence of hemorrhagic transformation with cardiopulmonary bypass following preoperative stroke
Hemorrhagic transformation is one of the most feared complications of ischemic stroke in adults. In addition to treatment with recombinant tissue plasminogen activator, risk factors for hemorrhagic transformation include the size of the lesion, mechanism of the stroke (cardioembolic vs. atherothrombosis) (23), age, anticoagulation and systolic blood pressure. Molecular and cellular mechanisms thought to be important for hemorrhagic transformation include ischemia reperfusion with resulting oxidant injury to vascular endothelium. While hemorrhagic strokes account for half of all strokes in children,(24) risk factors for hemorrhagic transformation of pediatric stroke are less well described. (25) Although strokes are now commonly associated with cardiopulmonary bypass in both infants and adults, (26) these strokes are most commonly embolic and rarely hemorrhagic. Despite the potential for increased exposure with cardiopulmonary bypass to shared pathogenic mechanisms including ischemia-reperfusion, oxidative stress and inflammation, the present data suggests that the risk of hemorrhagic transformation of small focal preoperative strokes is low.
This study addresses a clinically relevant question posed in previous studies: when should newborns with CHD and preoperative brain injury undergo cardiac surgery so that the risk of preoperative brain injuries to progress is minimized.(4, 27, 28) Possible advantages of deferring surgery after identifying preoperative brain injury include the potential for recovery of autoregulation and lower risk of hemorrhagic transformation. Potential advantage of proceeding with surgery without delay is the observation that the risk of white matter injury increases with a longer interval from birth to surgery.(6) In the present study newborns underwent surgery with a median of 2 days between the preoperative MRI scan and surgery, and we did not observe injury progression. Our data suggest that it is not necessary to postpone clinically indicated cardiac surgery in newborns with these clinically silent focal brain injuries.
Risk factors for preoperative brain injury
In four previous reports, very few risk factors for preoperative brain injury have been identified to date: low oxygen saturation, brain immaturity and ballon atrial septostomy.(3, 4, 6, 29, 30) McQuillen at al. originally reported a link between BAS and preoperative stroke in a prospective cohort of newborns with TGA, an observation confirmed in a larger cohort with many types of congenital heart disease.(3) Subsequently two reports have failed to confirm this association. Petit et al. reported 26 newborns with TGA, of whom14 received BAS. In this study, stroke was not observed, yet WMI was detected in 10 and IVH in 6; only the time to surgery and low SaO2 were significant risk factors for preoperative brain injury.(6) Beca et al. reported preoperative MRI findings in 44 newborns with TGA (33 had BAS), 13 with HLHS and 7 with pulomary atresia.(29) Brain injury occurred in 19 (30%) infants: WMI in 17 and stroke in 3; clinical risk factors for preoperative brain injury were not detected. Despite differences in risk factors identified and variability in the patterns of brain injury across these cohorts, the rate of preoperative brain injury remains unacceptably high (30% or more).
In the present study, the rates of preoperative injury, including stroke, were similar at both centres. Consistent with earlier reports, all newborns with TGA who had acquired a stroke had received BAS.(3, 4) In addition to practice variability across neonatal cardiac centres, differences in stroke incidence and identified risk factors, may also relate to patient specific factors such as illness severity. Regardless the etiology, preoperatively identified brain injury has a low risk of progression. Another plausible reason for the difference in rates of stroke and WMI across cohorts is variability in the classification of solitary white matter lesions as either stroke or WMI. (3, 6, 28) In our study, we prospectively classified small punctuate white matter lesions as WMI and larger solitary lesions as stroke. As there is no objective imaging measure to distinguish these patterns of injury, we performed a sensitivity analysis by recoding the 10 solitary white matter lesions in this cohort uniformly as either stroke or WMI, and the main associations persisted. The clear benefits of BAS must be weighed against the risk of stroke and the procedure reserved for those newborns with significant hypoxemia, a consideration made even more difficult by the lack of information on the impact of small strokes for subsequent neurodevelopmental outcome.
Limitations
Despite being the largest reported group of newborns with CHD scanned with MRI pre- and postoperatively, given our sample size (0 of 40 injuries) we cannot completely exclude a low risk of progression of pre-operative focal brain lesions with surgery of up to7%. Unlike some previous studies in which newborns were scanned on the date of surgery, we were not able to assign a specific time frame from preoperative MRI to surgery, nor the time from surgery to post-operative MRI. Given this, very small increases in lesion size may have been missed by the time of the post-operative MRI; we would not expect these changes to be clinically relevant. This study also highlights the need for uniformity of both image acquisition and nomenclature describing and quantifying injuries for comparison across centers. As physiological data from intensive care monitoring, such as SaO2 values were collected from review of the nursing record and not stored electronically, more sophisticated analyses such as the effect of a specific duration of a value below a lower limit on the risk of brain injury could not be assessed. We also note that these data only apply to term newborns with CHD. Importantly, the association of both pre- and post-operative brain injuries with school age neurodevelopmental outcomes will be addressed as this cohort develops through childhood
Conclusion
In this study, clinically silent preoperative brain injuries in newborns with CHD, including stroke, have a very low risk of progression on imaging following cardiac surgery and cardiopulmonary bypass. Given this, the identification of similar lesions should not delay clinically indicated surgery.
Acknowledgments
This work is supported by a grant (5-FY05-1231) from the March of Dimes Foundation, a grant (0365018Y) from the American Heart Association, a grant (2002/3E) from the Larry L. Hillblom Foundation, grants (RO1 NS40117 and P50 NS35902) from the National Institutes of Health, a grant (5-M01-RR-01271) from the National Center for Research Resources, and a grant from the Canadian Institutes of Health Research (93780). Dr. Miller is supported by a Canadian Institutes of Health Research Clinician Scientist award, and a Michael Smith Foundation for Health Research Scholar award. We also thank the children and their parents who generously participated in this study. The authors have no potential financial conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002 Sep 24;106(12 Suppl 1):I109–14. [PubMed] [Google Scholar]
- 2.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006 Jan;131(1):190–7. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007 Feb;38(2 Suppl):736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 4.McQuillen PS, Hamrick SE, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006 Jan 17;113(2):280–5. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 5.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007 Nov 8;357(19):1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 6.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009 Feb 10;119(5):709–16. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller SP, McQuillen PS, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM, et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg. 2004 May;77(5):1698–706. doi: 10.1016/j.athoracsur.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman GM, Stuth EA, Jaquiss RD, Vanderwal PL, Staudt SR, Troshynski TJ, et al. Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg. 2004 Jan;127(1):223–33. doi: 10.1016/j.jtcvs.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Whitby EH, Griffiths PD, Lonneker-Lammers T, Srinivasan R, Connolly DJ, Capener D, et al. Ultrafast magnetic resonance imaging of the neonate in a magnetic resonance-compatible incubator with a built-in coil. Pediatrics. 2004 Feb;113(2):e150–2. doi: 10.1542/peds.113.2.e150. [DOI] [PubMed] [Google Scholar]
- 11.Dumoulin CL, Rohling KW, Piel JE, Rossi CJ, Giaquinto RO, Watkins RD, Vigneron DB, Barkovich AJ, Newton N. Magnetic resonance imaging compatible neonate incubator. Magn Reson Engineering. 2002;15:117–28. [Google Scholar]
- 12.Barkovich AJ. Pediatric neuroimaging. New York: Raven Press; 1990. [Google Scholar]
- 13.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005 Apr;146(4):453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Newman TB. If almost nothing goes wrong, is almost everything all right? Interpreting small numerators. JAMA. 1995 Oct 4;274(13):1013. [PubMed] [Google Scholar]
- 15.Bartha AI, Yap KR, Miller SP, Jeremy RJ, Nishimoto M, Vigneron DB, et al. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR Am J Neuroradiol. 2007 Jun-Jul;28(6):1015–21. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004 Mar;127(3):692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 17.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002 Sep 24;106(12 Suppl 1):I82–9. [PubMed] [Google Scholar]
- 18.Karl TR, Hall S, Ford G, Kelly EA, Brizard CP, Mee RB, et al. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2004 Jan;127(1):213–22. doi: 10.1016/j.jtcvs.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KC, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993 Oct 7;329(15):1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 20.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003 Nov;126(5):1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 21.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995 Mar 2;332(9):549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 22.Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000 Jul;48(1):12–7. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Kim BJ, Lee SH, Ryu WS, Kang BS, Kim CK, Yoon BW. Low level of low-density lipoprotein cholesterol increases hemorrhagic transformation in large artery atherothrombosis but not in cardioembolism. Stroke. 2009 May;40(5):1627–32. doi: 10.1161/STROKEAHA.108.539643. [DOI] [PubMed] [Google Scholar]
- 24.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: a population-based cohort study. Stroke. 2007 Oct;38(10):2658–62. doi: 10.1161/STROKEAHA.107.481895. [DOI] [PubMed] [Google Scholar]
- 25.Kelley RE. Hemorrhagic cerebral infarction in pediatric patients. Pediatr Neurol. 1986 Mar-Apr;2(2):111–4. doi: 10.1016/0887-8994(86)90068-8. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LJ, Davies RR, Rizzo JA, Davila JJ, Cooperberg MR, Shaw RK, et al. Stroke in surgery of the thoracic aorta: incidence, impact, etiology, and prevention. J Thorac Cardiovasc Surg. 2001 Nov;122(5):935–45. doi: 10.1067/mtc.2001.117276. [DOI] [PubMed] [Google Scholar]
- 27.Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005 Dec;110(6):563–78. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 28.McQuillen PS. Magnetic resonance imaging in congenital heart disease: what to do with what we see and don't see? Circulation. 2009 Feb 10;119(5):660–2. doi: 10.1161/CIRCULATIONAHA.108.835744. [DOI] [PubMed] [Google Scholar]
- 29.Beca J, Gunn J, Coleman L, Hope A, Whelan LC, Gentles T, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009 May 12;53(19):1807–11. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009 Mar;137(3):529–36. doi: 10.1016/j.jtcvs.2008.10.025. discussion 36-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


