Abstract
Professional antigen presenting cells, dendritic cells (DC) are responsible for initiation and maintenance of immune responses. Here, we report that a substantial proportion of DCs in tumor-bearing mice and cancer patients have increased levels of triglycerides. Lipid accumulation in DCs was caused by increased uptake of extracellular lipids due to up-regulation of scavenger receptor A. DCs with high lipid content were not able to effectively stimulate allogeneic T cells or present tumor-associated antigens. DCs with high and normal lipid levels did not differ in expression of MHC and co-stimulatory molecules. However, lipid-laden DCs had reduced capacity to process antigens. Pharmacological normalization of lipid levels in DCs with an inhibitor of acetyl-CoA carboxylase restored the functional activity of DCs and substantially enhanced the effects of a cancer vaccine. These findings support the regulation of immune responses in cancer by manipulation of lipid levels in DCs.
Introduction
DCs are antigen presenting cells specializing in acquisition, processing, and presentation of antigens to T cells. The defective function of DCs in cancer contributes greatly to tumor escape (rev. in 1,2). During investigation of effect of tumor-derived factors on DC differentiation, we unexpectedly observed accumulation of lipids in DCs generated in the presence of tumor cell conditioned medium (Supplementary Fig. 1). These findings prompted us to investigate the potential role of lipid accumulation in DC function in cancer.
Lipids represent a diverse group of low molecular weight molecules. Most lipid molecules contain fatty acid moieties3. Lipids are an important energy resource and a major structural component of the membrane matrix. Signaling functions of fatty acids are now considered a core component of the network, regulating different cells (rev in. 4).
The factors affecting storage, uptake and metabolism of different fatty acids in DC may impact the function of the immune system5. A number of studies have shown the negative effect of dietary lipids on DC function6–14 (see supplementary material). These data indicate that lipids could be an important factor regulating DC function. However, their exact role in DC activity remains unclear. There is very little information regarding the biological or clinical significance of lipid accumulation in DCs in cancer. Here, we report our novel finding that accumulation of lipids in DCs is not only a consistent phenomenon found in tumor-bearing hosts, but also has a profound effect on DC function.
Results
Lipid accumulation in DCs
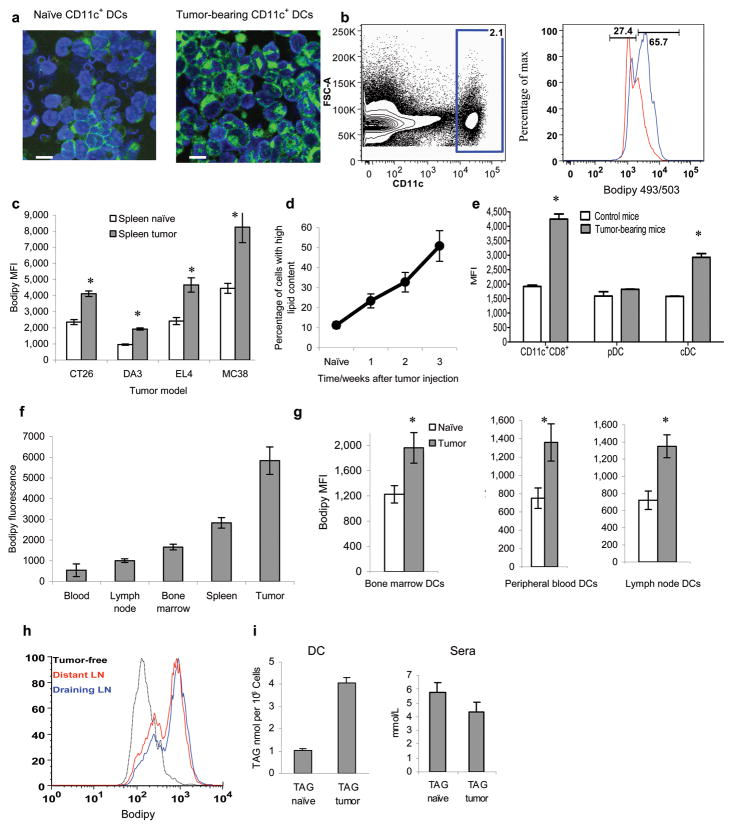
To evaluate the level of lipids in DCs we used the lipophilic fluorescent dye Bodipy 493/503. CD11c+ DCs isolated from spleens of mice bearing EL-4 tumor displayed higher fluorescence than their control counterparts (Fig. 1a). Using flow cytometry we detected two populations of CD11c+ DCs in tumor-bearing (TB) mice. One population termed DCs with normal lipid content (DC-NL) representing 30–50% of cells had a similar level of fluorescence to that in control DCs. The other population (50–70% of cells) referred to as DCs with high lipid content (DC-HL) had fluorescence higher than that in control DCs (Fig. 1b). The level of lipids in spleen DCs was elevated in all 4 tested tumor models in C56BL/6 and BALB/c mice as compared with DCs from control mice (Fig. 1c) and it increased with tumor progression (Fig. 1d). Increased lipid levels were observed in CD11c+CD8+ DCs and CD11c+CD11b+B220− conventional DCs (cDC) but not in CD11c+CD11b−B220+ plasmacytoid DCs (pDC) (Fig. 1e). DCs isolated from tumors had higher levels of lipids than DCs from blood and lymph nodes (LN) (Fig. 1f). DCs from the LN, bone marrow (BM), and blood from TB mice had higher levels of lipids than DCs from control mice (Fig. 1g). The lipid content was equally elevated in DCs from tumor-draining and distant LNs (Fig. 1h).
Figure 1. Lipid levels in DCs from TB mice.
a. Staining of CD11c+ from the spleens of naïve and EL-4 TB mice with Bodipy 493/503. 10μm scale bar shown. b. A typical example of the analysis of lipid level in CD11c+ DCs from spleens of control (red) or EL-4 TB mice (blue). c. Cumulative results of lipid staining of splenocytes from different tumor models. Geomean fluorescence intensity (MFI) of Bodipy 493/503 in CD11c+ DCs was measured. Each group includes 4-8 mice. Control and TB mice were evaluated in parallel in each experiment. d. Proportion of DCs with high lipid content in spleens during progression of CT-26 tumors. Each time point included 3 mice. Mean ± SD are shown. e. Lipid levels in different populations of DCs from the spleens of control or EL-4 TB mice. pDC –plasmacytoid DCs; cDC-conventional DCs. Each group had 3 mice. f. Lipid levels in CD11c+ DCs from different organs of CT-26 TB mice collected on week 3 after tumor inoculation (tumor size - 1.5 cm in diameter). Mean ±SD from 4 mice are shown. g. Lipid levels in CD11c+ DCs from naïve or CT-26 TB mice collected on week 3 after tumor inoculation. Mean ±SD from 3 mice per group are shown. h. Lipid level in CD11c+ DCs from draining (inguinal) and distant (contra-lateral axillary) LNs in EL-4 tumor-bearing mice. Three experiments with the same results were performed. i. The amount of triglycerides (TAG) per 106 CD11c+ DCs isolated from spleens of naïve BALB/c mice and CT-26 TB mice and in sera of TB mice. Each group included 3 mice. * - statistically significant differences (p<0.05) between values in DCs from control and TB mice.
To evaluate the nature of these lipids, CD11c+ DCs were isolated from spleens of naïve and TB mice. Total lipids were extracted and analyzed by high performance thin layer chromatography (HPTLC), and electrospray ionization mass spectroscopy (ESI-MS). DCs from TB mice showed dramatically higher levels of triglycerides (triacylglycerol, TAG) than cells from naïve mice. In contrast, no differences were found in serum TAG level between naive and TB mice (Fig. 1i). Most of the molecular species of TAG were elevated in DCs from TB mice as compared with their control counterparts (Supplementary Fig. 2a) and no differences were found in the profile of major molecular species of TAG between serum samples (data not shown). In contrast to TAG no changes were observed in the levels of cholesteryl-esters and phospholipids (Supplementary Fig. 2b,c).
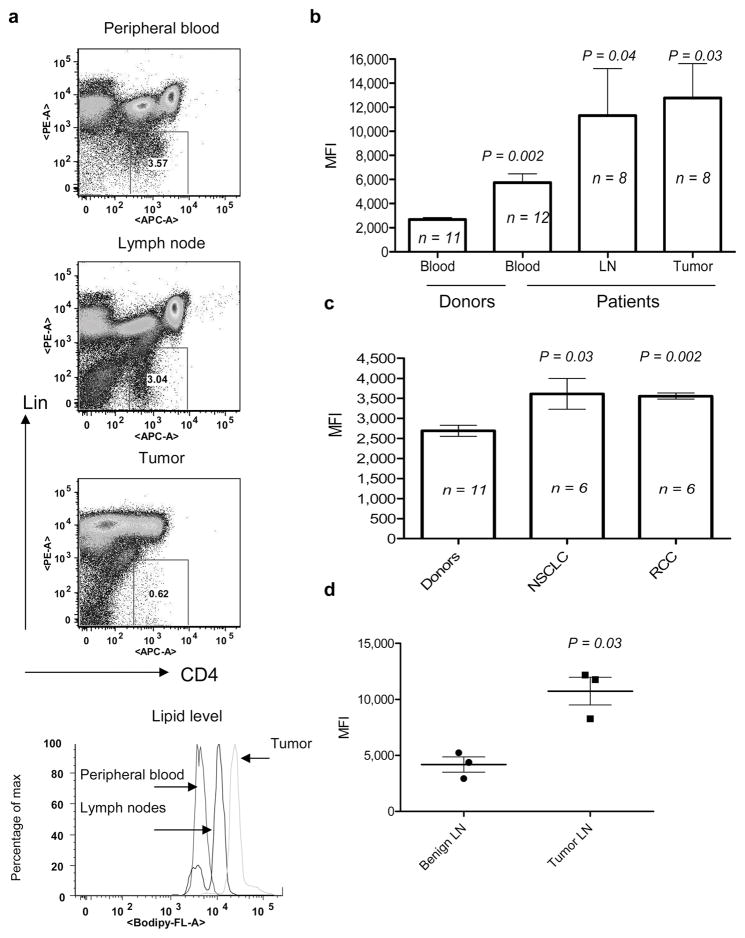
Lipid levels were evaluated in DCs from 12 individuals with head and neck cancer (HNC). Peripheral blood (PB), draining LN, and tumor tissues were collected during surgery. DCs were identified as Lineage−CD4+ cells15 (Fig. 2a). DCs from PB of persons with cancer showed significantly higher levels of lipids than PB DCs from the healthy individuals. The lipid level in DCs from LN and tumors was considerably higher than that in DCs from PB (Fig. 2b). Similar findings were obtained in the cohorts of patients with non-small lung cancer and renal cell cancer (Fig. 2c). In separate experiments we found that LN DCs from persons with HNC contained significantly higher level of lipids than LN DCs from individuals with benign tumors (Fig. 2d). Thus, these data strongly corroborate those obtained from mouse tumor models.
Figure 2. Lipid level in DCs from cancer patients.
a. Typical example of Bodipy staining of DCs from cancer patient. b. Cumulative results of lipid levels in DCs from patients with head and neck cancer. P values between the levels of lipids in blood DCs from control donors are shown; n = the number of samples analyzed. c. Lipid levels in peripheral blood DCs from patients with non-small lung cancer (NSCLC) and renal cell cancer (RCC). P values between the levels of lipids in blood DCs from control donors are shown n = the number of samples analyzed. d. Lipid levels in DCs from lymph nodes obtained from patients with head and neck cancer and benign tumors. P values between the levels of lipids in DCs from cancer patients and patients with benign tumors.
Mechanism of lipid accumulation in DCs
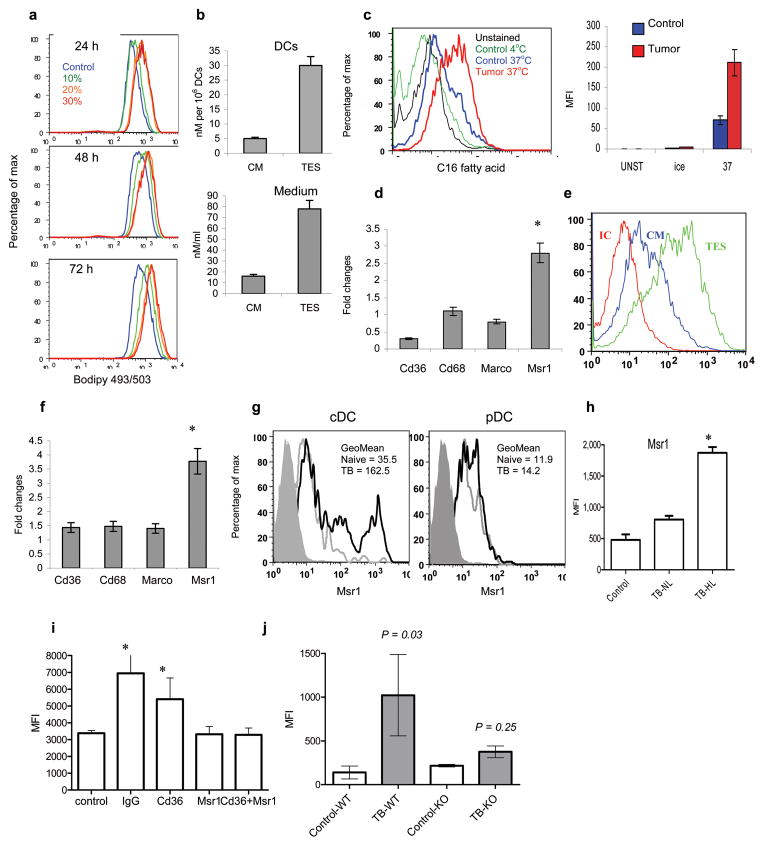
To study the mechanisms of lipid accumulation in DCs these cells were generated in vitro from BM progenitors (BMP) using GM-CSF and IL-4. Short 24 h incubation of DCs with tumor explant supernatants (TES) resulted in more than 3-fold increase in the lipid level (Fig. 3a). Analysis of lipid content by ESI-MS revealed that DCs generated in the presence of TES had almost 6-fold higher level of TAG than DCs generated in control culture medium (CM) (Fig. 3b). There was an increase in the level of all molecular species of TAG but no changes were observed in the level of phospholipids and cholesteryl-esters in these DCs (Supplementary Fig. 3). The total level of TAG in CT-26 TES was > 4 times higher than in CM (Fig. 3b). Despite variability in TAG level in TES obtained from different tumor cell lines they all caused comparable up-regulation of lipids in DCs (Supplementary Fig. 4). To directly evaluate fatty acid uptake, DCs cultured for 24 h with TES were incubated with fluorescently labeled palmitic fatty acid for 30 min at 37°C. DCs pre-incubated with TES had dramatically higher uptake of fatty acid than DCs incubated in control medium (Fig. 3c). Overnight incubation of DCs from spleens of TB mice in serum-free medium eliminated elevated level of lipids in these cells, while not affecting the level of lipids in control DCs (data not shown). These data suggested that increased uptake of lipids may be the major mechanism of lipid accumulation by DCs.
Figure 3. The mechanism of lipid accumulation in DCs.
a. Lipid level in DCs generated from enriched BM HPC and cultured in the presence of different concentrations of TES for indicated period of time. b. The total level of TAG using ESI-MS in CD11c+ DCs generated from BM HPC in the presence of 20% TES. Measurements were performed in triplicate. c. Uptake of fluorescently labeled C16 fatty acid by DCs generated from BM HPC and cultured for 24 hr with control medium or TES. The left panel is a typical example of staining after 30 min incubation with C16 fatty acid. The right panel shows cumulative results of 3 performed experiments (Mean±SD). Ice – cells incubated with fatty acid at 4°C; 37°C – cells incubate with fatty acid at 37°C. d. Gene expression of indicated SR in DCs cultured for 48 h with control medium or TES. Samples were evaluated in triplicate by qRT-PCR and the results were normalized to the level of expression of β-actin. The fold changes between DCs generated with TES and control were calculated. The results of from 3 performed experiments are shown. e. Expression of Msr1 on the surface of DCs cultured with control medium (CM) or TES. IC – isotype control. The typical examples of three performed experiments are shown. f. Gene expression of indicated scavenger receptors in DCs from naïve and EL-4 TB mice by qRT-PCR. The results were normalized to the level of expression of β-actin. Fold changes between EL-4 TB DCs and control DCs were calculated. The results of 3 performed experiments are shown. g. Expression of MSR1 within the population of conventional (cDC) or plasmacytoid (pDC) spleen DCs from naïve and EL-4 TB mice. Shaded histogram – isotype control; grey line – naïve mice, bold black line – TB mice. h. Expression of MSR1 in spleen DCs with normal lipid content (TB-NL) and high lipid content (TB-HL) from EL-4 TB mice. Control – DCs from control mice. Mean ± SD of 4 performed experiments are shown. * - statistically significant differences (p<0.05) from values in control DCs. Fluorescence of cells labeled with isotype control IgG was less than 20 in all samples. i. Lipid level in DCs generated in vitro and cultured for 3 days with TES in the presence of control IgG, or indicated antibodies against scavenger receptors. Mean ± SD from three experiments is shown. * - statistically significant differences (p<0.05) from values in control DCs. j. Lipid levels in DCs generated from enriched BM HPC of wild-type (WT) or MSR1 knockout (KO) C57BL/6 mice and transferred i.v. into sub-lethally irradiated congenic tumor-free (control) or EL-4 TB (TB) recipients. Lipids were measured in donor’s cells isolated from spleens of recipients two days after the transfer. Each group includes 4–8 mice. Geometric mean of fluorescence is shown. P values between control and TB mice were calculated using two-tailed Mann-Whitney test.
We then investigated why DCs from tumor-bearing hosts have higher lipid uptake than in controls. Scavenger receptors (SR) play an important role in intracellular transport of lipids. Currently little is known about changes in their expression on DCs from tumor-bearing hosts. Therefore, we evaluated the level of four major SR (Macrophage scavenger receptor, Msr1 (CD204), Cd36 (Srb), Cd68, and Marco). EL-4 TES caused significant up-regulation of the expression of Msr1 in DCs (Fig. 3d). This was associated with increased expression of this receptor on the surface of DCs (Fig. 3e). A similar effect was observed with TES from CT-26 and B16F10 tumors (data not shown). Msr1 was the only SR significantly elevated in DCs from TB mice (Fig. 3f). cDC from TB mice had 3-fold higher levels of Msr1 than their control counterparts. In contrast, no up-regulation of Sra was observed in pDC (Fig. 3g). No differences were found in the expression of Msr1 between DCs from naïve mice and DC-NL from TB mice. However, DC-HL expressed substantially higher levels of Msr1 (Fig. 3h). Similar results were obtained in experiments in vitro (Supplementary Fig. 5a). Elevated Msr1 expression was detected in DCs from all tested sites of TB mice and the level of Msr1 in DCs isolated from tumor was 20–50-fold higher than in other sites (Supplementary Fig. 5b). Thus, the pattern of Msr1 expression was similar to that of lipids and suggested that up-regulation of this receptor might be involved in lipid accumulation in DCs.
To directly test this hypothesis we used fucoidan, a soluble ligand which prevents binding of natural ligands to SRs. Fucoidan did not affect DC viability but completely abrogated the effect of CT-26 TES on lipid accumulation (data not shown). Pre-incubation of DCs with neutralizing antibodies to Msr1 completely abrogated the effect of TES on lipid accumulation, whereas Cd36-specific antibody had little effect (Fig. 3i). We generated DCs from BMP of wild-type and Msr1−/− C57BL/6 mice (CD45.2+). These DCs were transferred i.v. into sub-lethally irradiated congenic (CD45.1+) tumor-free or EL-4 TB recipients. Two days later the level of lipids was evaluated in CD45.2+ donor’s cells isolated from recipient spleens. Donor’s DCs in TB recipients demonstrated significantly higher levels of lipids than cells in tumor-free recipients. In contrast, DCs generated from Msr1−/− mice failed to accumulate lipid after transfer to TB recipients (Fig. 3j). Analysis of TAG content by ESI-MS showed that in contrast to wild-type DCs Msr1 deficient DCs did not accumulate TAG during the culture with CT-26 TES (Supplementary Fig. 6). Taken together these data indicate that up-regulation of Msr1 plays a major role in accumulation of lipids in DCs in cancer.
Functional activity of lipid laden DCs
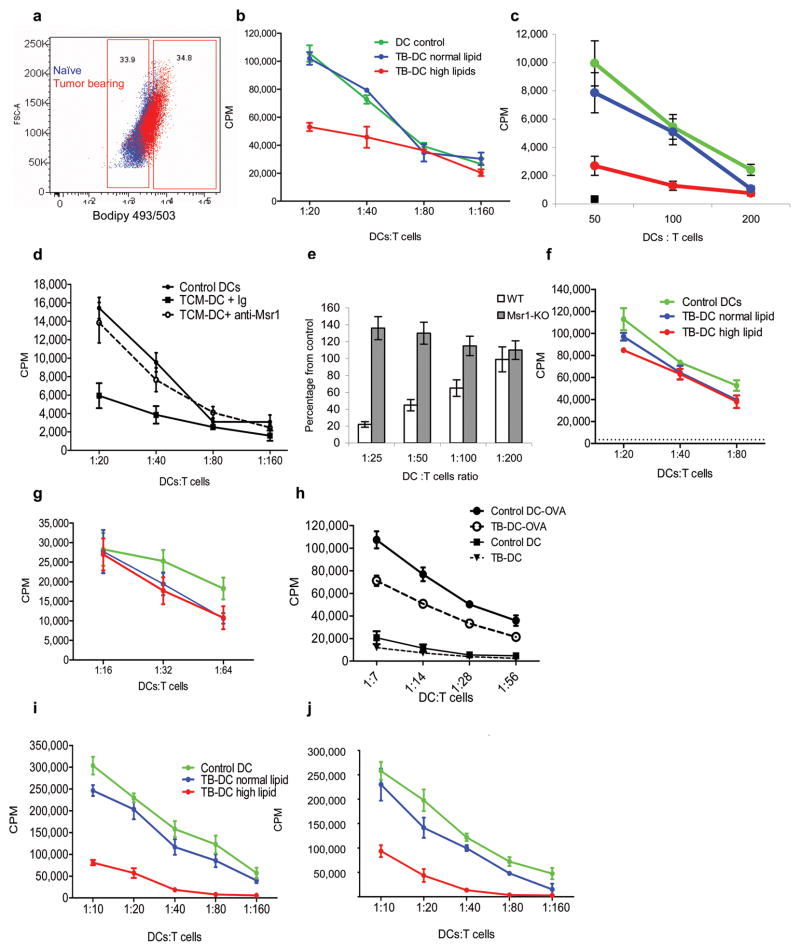
To investigate whether lipid accumulation in DCs has functional consequences, DC-HL and DC-NL from spleens of control and CT26 TB mice were sorted using the gates set around lipid levels in control DCs (Fig. 4a) and then used for stimulation of allogeneic T cells. DCs from control mice and DC-NL from TB mice had similar effects on allogeneic T-cell proliferation, whereas DC-HL from TB mice had substantially lower stimulatory activity (Fig. 4b). Similar results were obtained with in vitro generated DCs cultured for 48 h with TES (Fig. 4c).
Figure 4. Defective functional activity of DCs with high lipid content.
a,b. Stimulation of allogeneic T cell proliferation by DCs with different lipid content isolated from CT-26 TB mice. a. Examples of gates set for the sort of CD11c+ cells. Blue- naïve mouse, red–TB mouse. b. Stimulation of proliferation of allogeneic T cells by sorted DCs with high and low lipid content. Typical results of 4 performed experiments are shown. T cells alone had 3[H]-thymidine uptake of less than 500 CPM. c. Stimulation of allogeneic T cell proliferation by DCs generated from enriched BM HPC from naïve BALB/c mice, exposed for 2 days to CT-26 TES and sorted for DC-HL (red) or DC-NL (green) and compared to DCs cultured in control medium (blue). Three experiments with similar results were performed. T cells alone had 3[H]-thymidine uptake of less than 200 CPM. d. DCs were generated from HPC of C57BL/6 mice and cultured for 3 days with EL-4 TES in the presence of control IgG or anti-MSR1 antibody. After that time cells were cultured in triplicate with allogeneic T cells at indicated ratios and cell proliferation was evaluated by 3[H]-thymidine uptake. T cells alone had 3[H]-thymidine uptake of less than 500 CPM. e. DCs generated from BM HPC of wild-type and MSR1 KO mice in the presence of TES as described in Fig. 3j were used as stimulators in an allogeneic mixed leukocyte reaction. T-cell proliferation was measured by 3[H]-thymidine uptake. Two independent experiments were performed in triplicate. The values of T-cell proliferation induced by wild-type or MSR1-deifient DCs generated without the presence of TES were set as 100%. Values of T-cell proliferation induced by DCs generated in the presence of TES are presented as percentage from control level. f,g. DCs with high and normal lipid content were sorted from spleens of naïve and EL-4 TB mice and incubated at indicated ratios with T cells from OT-II (f) or OT-I (g) transgenic mice in the presence of 10 μg/ml of specific peptides. T cell proliferation was measured in triplicate by 3[H]-thymidine uptake. In the absence of peptides 3[H]-thymidine uptake in all samples was less than 5×103 CPM. Three experiments with the same results were performed. h. CD11c+ DCs isolated from spleens of naïve and EL-4 TB mice were cultured overnight with 1 mg/ml OVA or in medium alone and then used to stimulate T cells from OT-II transgenic mice. T cell proliferation was measured in triplicate by 3[H]-thymidine uptake. i.j. DCs with high and normal lipid content were sorted from the spleens of EL-4 TB mice and incubated overnight with 1 mg/ml OVA. Cells were used to stimulate T cells from OT-II (i) and OT-I (j) transgenic mice. T-cell proliferation was measured in triplicate using 3[H]-thymidine uptake. Two experiments with similar results were performed.
Msr1-specific antibody abrogated the negative effects of TES on DC function (Fig. 4d). TES inhibited ability of DCs generated from wild-type BMP to stimulate allogeneic T cells, whereas it did not affect this function of DCs generated from Msr1−/− BMP (Fig. 4e).
DC-NL and DC-HL isolated from EL-4 TB mice equally stimulated responses of transgenic CD4+ or CD8+ T cells to OVA-derived specific peptides (Fig. 4f,g). Different results were obtained when DCs were loaded overnight with soluble protein (OVA). CD11c+ DCs from TB mice had reduced ability to stimulate proliferation of OVA-specific CD4+ T cells (Fig. 4h). DC-NL from TB mice and control DCs showed similar activity in stimulation of OVA-specific CD4+ (Fig. 4i) and CD8+ (Fig. 4j) T cells, whereas DC-HL induced a substantially lower response. Thus DC-HL had substantially reduced capacity to stimulate T cells when it required processing of antigen.
Lipid-laden DCs have reduced capacity to process and present antigen
We compared the phenotype of CD11c+ DCs with normal and high lipid content from TB mice. We found no differences in the proportion of DCs expressing markers of macrophages or granulocytes (F4/80 and Gr-1) as well as in the level of expression of these markers between DC-NL and DC-HL (Supplementary Fig. 7a). Although DCs from TB mice had significantly lower levels of MHC class I, II, and co-stimulatory molecules (CD40, CD86, and CD80) than DCs from naive mice, no differences between DC-NL and DC-HL were found (Supplementary Fig. 7b).
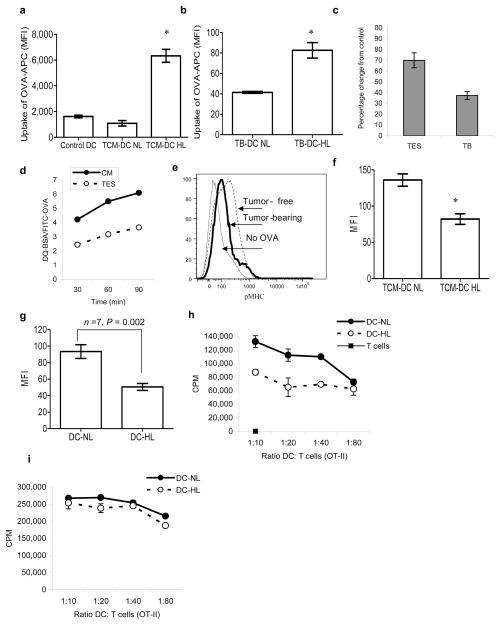
One possible explanation for these results could be a decreased uptake of soluble proteins by DC-HL. To test this possibility DCs generated from BMP in the presence of TES were incubated with fluorescently labeled OVA-APC. DC-NL had the same levels of OVA uptake as control DCs, whereas DC-HL showed significantly higher levels of OVA-APC (Fig. 5a). Similar results were obtained in vivo after injection of EL-4 TB mice i.p. with OVA-APC (Fig. 5b). Thus, DC-HL internalized more soluble protein than DC-NL.
Figure 5. Antigen processing in lipid-laden DCs.
a. One-hour uptake of OVA-APC by DCs generated from HPC in the presence of TES. The level of APC fluorescence was evaluated within the DC-NL and DC-HL populations. Mean±SD of three performed experiments are shown. * - statistically significant differences (p<0.05) from control DCs. b. Uptake of OVA-APC by DCs from EL-4 TB mice 2 h after i.p. injection of 100 μg of OVA-APC. Mean±SD of three mice per group is shown. c. DCs were either generated in vitro and then cultured for 48 h in control medium or TES, or isolated directly from spleens of naïve or EL-4 tumor-bearing (TB) mice. Cells were loaded with either 1 mg/ml DQ-BSA or 10 mg/ml FITC-OVA. DQ-BSA/FITC-OVA ratio was calculated within CD11c+ DCs after 1 h incubation. DQ-BSA/FITC-OVA ratio in control samples was expressed as 100%. Two experiments with the same results were performed. d. DCs generated in vitro as described above were loaded with DQ-BSA or FITC-OVA and incubated for the indicated time. Fluorescence in CD11c+ DCs was evaluated as described above. e. CD11c+ DCs were isolated from spleens of tumor-free and EL-4 tumor-bearing mice and loaded overnight with 1 mg/ml OVA. Cells were then stained with APC conjugated 25-d1.16 antibody. A typical result of one experiment out of 4 performed is shown. f. DCs were generated from progenitors in the presence of EL-4 TES and then loaded overnight with 1 mg/ml OVA. DCs were stained with Bodipy 493/503 and labeled with 25-d1.16 antibody. DCs with high and normal lipid content were analyzed. The mean fluorescence of cells stained with isotype control Ig was less than 20. * - statistically significant differences (p<0.05) between DC-NL and DC-HL. Cumulative results of 3 independent experiments are shown. g. Binding of 25-d1.16 antibody to DC-NL and DC-HL DCs from LN cells of EG-7 TB mice. The fluorescence of cells stained with isotype control Ig was less than 20. h.i. DCs with high and normal lipid content were sorted from LN of EG-7 TB mice and incubated with T cells isolated from OT-II transgenic mice in the absence of (h) or in the presence of 10 μg/ml of OT-II specific peptide (i). T cell proliferation was measured in triplicate by 3[H]-thymidine uptake. Two experiments with the same results were performed.
To assess the ability of DCs to process antigen we used BSA-DQ, a self-quenching conjugate of bovine serum albumin that exhibits fluorescence upon proteolytic degradation. To normalize for increased protein uptake by DC-HL we used the ratio of OVA-FITC and BSA-DQ fluorescence after loading DCs with these proteins. DCs isolated from TB mice or cultured with TES had substantially lower BSA-DQ/OVA-FITC ratio than DCs from control mice or cells cultured in control medium (Fig. 5c). This effect was observed at all tested time points (Fig. 5d). To verify these observations we used 25-d1.16 antibody that recognizes the OVA derived epitope bound to H2Kb (pMHC). CD11c+ DCs isolated from the spleens of EL-4 TB or control mice were loaded with OVA overnight and then stained with 25–D1.16 antibody. The binding of this antibody to DCs from TB mice was lower than to DCs from control mice (Fig. 5e). DCs generated from BMP in the presence of TES were loaded with OVA and then stained with Bodipy and pMHC antibody. DC-HL had considerably lower binding of the antibody than DC-NL (Fig. 5f).
We then addressed whether DC-NL and DC-HL differ in the presentation of tumor-associated antigen. Draining LN were isolated from mice with EG-7 tumors that express OVA and the level of pMHC in LN DCs was evaluated. DC-HL had lower expression of pMHC than DC-NL from the same mice (Fig. 5g). DC-HL and DC-NL cells were sorted from LN of EG-7 TB mice and cultured with OVA-specific OT-II T cells. DC-HL induced a lower response of OT-II T cells than DC-NL (Fig. 5h). In contrast, no difference in the T-cell response between DC-HL and DC-NL was seen when DCs were loaded with specific peptide prior to incubation with T-cells (Fig. 5i). Taken together these data indicate that DC-HL may have defects in processing of tumor-associated proteins.
Effects of regulation of lipid levels on DC function in cancer
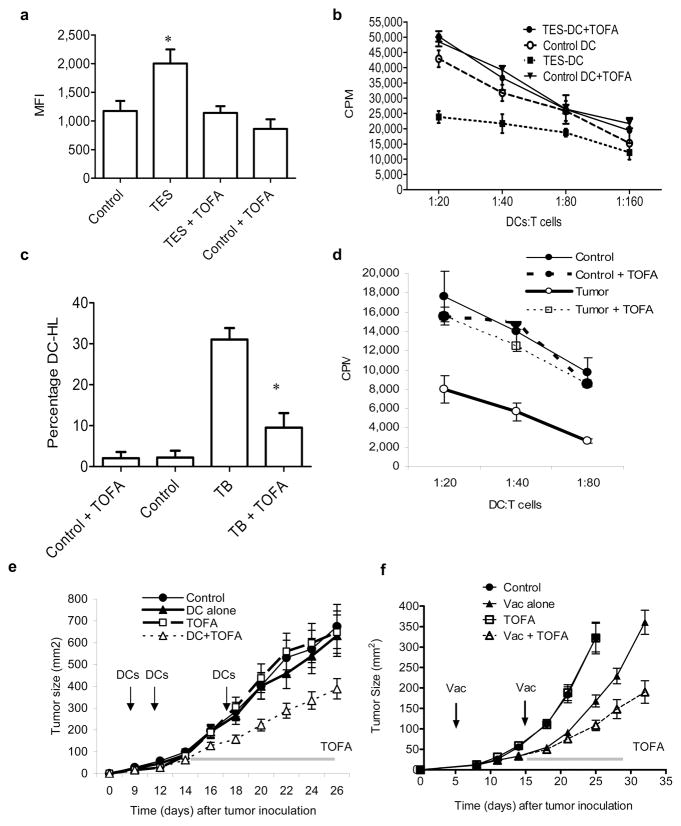
To identify the potential role of lipid accumulation in abnormal DC function in cancer we tried to regulate fatty acid levels using an inhibitor of acetyl-CoA carboxylase (ACC), 5-(tetradecycloxy)-2-furoic acid (TOFA)16. Since TAG undergo rapid degradation in the cells, the maintenance of their high level requires active fatty acid synthesis. We hypothesized that when synthesis is blocked, then cells will not be able to sustain high level of TAG even if lipid uptake is increased. When HPCs were cultured with TES in the presence of TOFA, it prevented the accumulation of lipids in DCs (Fig. 6a) without affecting the viability or causing up-regulation of co-stimulatory molecules or MHC class II (data not shown). Treatment with TOFA significantly improved the ability of DCs generated in the presence of TES to stimulate allogeneic T cells (Fig. 6b). To test the effect of TOFA in vivo, EL-4 TB mice were treated for 2 weeks with TOFA filled osmotic pumps. At the selected dose (1μg/hr) TOFA did not affect the number of DCs and did not cause changes in the expression of MHC class II or co-stimulatory molecules (data not shown) but it reduced the presence of lipid-laden DCs (Fig. 6c) and restored the ability of DCs to stimulate allogeneic T cells (Fig. 6d).
Figure 6. Effects of pharmacological regulation of lipid levels on DC function in cancer.
a. Lipid levels in DCs generated in vitro from HPC and cultured for 3 days with EL-4 TES in the presence of 5 μg/mL TOFA. b. Proliferation of allogeneic (BALB/c) T cells cultured with DCs generated from HPC in the presence of TES and TOFA. Proliferation was measured in triplicate by 3[H]-thymidine uptake. T cells alone had 3[H]-thymidine uptake of less than 103 CPM. c. Tumor-free (control) and EL-4 TB (TB) mice were treated for 2 weeks with TOFA using osmotic pumps (rate of release 1 μg/hr). Pumps filled with water were used as controls. The proportion of DCs with high lipid content was calculated. For each experiment 2 mice per group were used. Experiments were performed twice and the Mean±SD of the cumulative results is shown. * - statistically significant differences between TB and TB+TOFA groups. d. CD11c+ DCs isolated from the spleen of mice as described in Fig. 6c were incubated with allogeneic T cells and cell proliferation measured in triplicate by 3[H]-thymidine uptake. T cells alone had 3[H]-thymidine uptake of less than 103 CPM. e. EL-4 tumor was established in C57BL/6 mice.. Mice (5 mice per group) were immunized with 5×105 DCs transduced Ad-surv 4 days after tumor inoculation.. Vaccination was repeated twice at weekly intervals. Fourteen day osmotic pumps (Alzet, Cupertino, CA) (1μg/hr rate release) containing water or TOFA were implanted on day 14 after tumor cell injection. Tumor size was monitored and is presented as Mean ± SD per group. f. B16F10 tumor was established in C57BL/6 mice. Five days after tumor inoculation mice (8 mice per group) were immunized with a mixture of specific peptide and two Toll-like receptor ligands (TLR-L) [50 μg poly-IC, 100 μg CpG-1826]. Fourteen day osmotic pumps (1μg/hr rate release) filled with PBS were used in combination with lynch coil polyethylene tubing containing water or TOFA. Pumps were implanted on day 14 after tumor cell injection. Tumor size was monitored and results are presented as the mean tumor size (area in mm2) ± SD for every treatment group at various time points until the termination of the experiment.
To test the possibility that TOFA might improve antitumor response EL-4 TB mice were immunized with DCs transduced with Ad-survivin (Ad-surv)17. Vaccine or TOFA alone had little effect on growth of established tumor. Combination of TOFA with vaccine resulted in significant (p=0.041) decrease in tumor growth (Fig. 6e). In a different experimental system, C57BL/6 mice were injected s.c. with B16F10 melanoma cells and then vaccinated with the peptide derived from melanoma specific protein - tyrosinase 1. TOFA alone did not affect tumor growth. Vaccine alone caused a temporary decrease in tumor growth, but the combination of TOFA with vaccine provided for significantly (p<0.01) more potent antitumor effects (Fig. 6f).
Discussion
This study has demonstrated that a substantial proportion of DCs in TB hosts have increased amount of lipids, specifically triglycerides. These results were obtained using different methods of lipid detection in several tumor models and also in cancer patients across several diagnoses. These findings were reproduced by using conditioned medium from tumor explants indicating that tumor-derived factors were responsible for the observed phenomenon. In this study we have tried to address two main questions: what is the mechanism of lipid accumulation in DCs and whether it has any functional consequences for these cells.
Accumulation of lipids could be due to increased synthesis of fatty acids or because of increased lipid uptake from plasma. Our data suggested that the second explanation is more likely (Fig 3). Scavenger receptors represent a major route in acquiring fatty acids by DCs and macrophages 18–20 (see supplementary material). Our results indicate that DCs from TB mice had preferential up-regulation of Msr1. This was consistent with the data demonstrating increased expression of Sra on macrophages during co-culture with ovarian tumor cells21. DCs isolated from tumor tissues had substantially higher levels of Msr1 than cells isolated from spleens or LN, which paralleled the results with lipid level in these cells. Association between the expression of this receptor and lipid levels in DCs was further evident in the analysis of DC subsets. In our study accumulation of lipids and up-regulation of Msr1 was observed in cDC but not in pDCs. This was consistent with the results of a study that demonstrated that in contrast to cDCs, expression of Msr1 was not detectable in mouse and human pDCs22. Our experiments with blocking of Msr1 with the soluble SR ligand fucoidan23 and specific antibody, as well as experiments with Msr1−/− mice, have demonstrated that up-regulation of Sra was primarily responsible for increased uptake of exogenous lipids by DCs. It is likely that fatty acids are transferred to DCs in the form of modified lipoproteins. Different lipoprotein modifications have been described in cancer and those lipoproteins are likely abundant in tumor-bearing hosts24,25. It is likely that accumulation of lipids by DCs in vitro and in vivo is due to the presence of tumor-derived factors that up-regulate the expression of Sra on DCs, which enable these cells to pick up modified lipoproteins from serum. The nature of those factors remain unclear and require further investigations (see supplementary material).
In our studies lipid-laden DCs had a profound defect in ability to process and present soluble antigens. One possible explanation could be that lipid-laden DCs are immature cells, which are effective in picking up soluble proteins but have poor ability to present antigens. However, the fact that DC-HL and DC-NL expressed similar levels of MHC class II, and co-stimulatory molecules as well as the fact that peptide-loaded DC-HL and DC-NL stimulated specific T-cells equally well argues against this possibility. The molecular mechanisms by which fatty acids could affect antigen processing are currently not clear.
The results of the experiments with inhibition of fatty acid synthesis in TB mice demonstrated that this might improve DC function in TB host and antitumor immune responses and suggested that this approach could be useful in enhancing immune responses in cancer patients.
Methods
Human subjects
Several cohorts of human subjects have been analyzed. All individuals signed University of South Florida IRB approved consent forms. The description of cohorts and processing of samples is provided in supplementary materials.
Mice and tumor models
Female Balb/c or C57BL/6 mice were obtained from the National Cancer Institute. Tumor cell lines included CT-26 (on Balb/c mice) and MC38 (on C57BL/6 mice) colon carcinomas, B16F10 melanoma, EL4 lymphoma (both on C57BL/6 mice), and DA3 breast carcinoma (on Balb/c mice) described elsewhere26. All cells were maintained in Dulbecco’s modified eagle’s medium (Invitrogen Corp.) plus 10% fetal bovine serum (FBS, Sigma-Aldrich) at 37°C, 5% CO2. Tumors were injected subcutaneously (s.c.) at 5×105 cells per mouse and allowed to grow 3–4 weeks or until tumors reached 2 cm in diameter. OT-I TCR-transgenic mice (C57Bl/6-Tg(TCRaTCRb)1100mjb) have a T cell receptor that recognizes OVA residues 257–264 in the context of H2Kb. OT-II transgenic mice (C57BL/6-Tg(TcraTcrb)425Cbn/J) express the mouse T cell receptor that is specific for OVA323–339 in the context of I-Ab. These mice, as well as Msr1−/− mice (B6.Cg-Msr1tm1Csk/J), were obtained from Jackson Laboratories. Mice were housed and euthanized according to University of South Florida IACUC guidelines.
The following peptides were used in the studies: OT-I specific peptide – SIINFEKL, OT-II specific peptide – ISQAVHAAHAEINEAGR, control non-specific peptide – RAHYNIVTF. All peptides were obtained from the American Peptide Company (Vista, CA).
Generation and isolation of dendritic cells
DCs were generated from BM progenitor cells using culture with 10 ng/mL recombinant mouse GM-CSF (Invitrogen Corp), and 10 ng/mL interleukin 4 (IL-4) (R&D Systems) as described previously27.
Preparation of tumor conditioned medium
Tumor explants were prepared from freshly isolated subcutaneous tumors (see supplementary material).
Analysis of cell phenotype by flow cytometry
Cells were incubated with fluorescently labeled antibodies directed against cell surface markers. All antibodies were purchased from either BD Pharmingen or Serotec. 25-d1.16 antibody was obtained from eBioscience. Cell surface labeling was performed on ice for 30 min. Cells were then washed and re-suspended in 500 μL of Bodipy 493/503 at 0.5μg/mL in PBS. Cells were stained for 15 min at room temperature before the analysis. All experiments using Bodipy 493/503 staining were performed on LSRII or FACSAria (BD). For cell sorts, the gates defining normal lipid levels for discrimination of DC-NL vs. DC-HL in TB hosts were set using the fluorescence of control DCs as the background. A low pressure sort was performed using the BD FacsAria cell sorter with a 100 μm nozzle.
Lipid profiling by thin layer chromatography
Loading of DCs with fatty acid
DCs were incubated with fluorescently labeled (Bodipy FL) palmitic fatty acid (Molecular Probes) at 37°C for 15–30 min. Controls consisted of unloaded DCs or DCs loaded on ice. 5×106 DCs were re-suspended in 500μL of 10μg/mL fatty acid.
Functional assays
T cell proliferation was measured using a mixed leukocyte reaction or antigen specific proliferation. DCs were isolated as described above. T cells were isolated using R&D Systems Mouse T cell Enrichment columns, according to the manufacturer’s protocol (R&D Systems). T cells were plated at 105 T cells per well. DCs and T cells were mixed at different ratios. Antigen specific T cell proliferation was assessed with either OT-I or OT-II transgenic T cells. Isolated DCs (5×106 cells/mL) were incubated with 10 μg/mL of specific or control peptides for 90 minutes. Peptide loaded DCs were then washed and mixed with appropriate transgenic T cells at different ratios. Cells were incubated for 72 h. 3[H]-thymidine was then added at 1μCi per 200uL of cells per well for an additional 18 hours of incubation followed by cell harvesting and radioactivity count using a liquid scintillation counter.
Vaccination of mice
C57BL/6 mice were injected s.c. with 5×105 EL-4 or 3×105 B16F10 tumor cells. Four-five days later EL-4 TB mice were immunized with s.c. injection of 5×105 DCs transduced Ad-surv and vaccination was repeated twice with weekly interval. B16F10 TB mice were vaccinated twice weekly i.v. with a mixture of 150 μg optimized Trp1455–463/9M (TAPDNLGYM) peptide and two Toll-like receptor ligands (TLR-L) [50 μg poly-IC, 100 μg CpG-1826]. CpG-1826 was prepared by the Mayo Clinic Molecular Core Facility. Poly-IC (Hiltonol, a clinical grade stabilized formulation containing poly-L-lysine and carboxymethyl cellulose) was provided by Dr. Andres Salazar (Oncovir, Inc., Washington, DC).
Quantitative real-time polymerase chain reaction (qRT-PCR)
PCR was performed as described earlier 28 and details are provided in supplementary material.
Statistical Analysis
Statistical analysis was performed using 2-tailed Mann-Whitney or Wilcoxon non-parametric tests with significance determined at p<0.05. The geometric mean is reported for all fluorescence results. Tumor measurements were analyzed using two-way ANOVA test with Bonferroni post-test. All statistical analyses were performed using GraphPad PRISM 5 software (GraphPad Software, Inc. La Jolla, CA).
Supplementary Material
Acknowledgments
This work was supported by NIH grant 1R21AI070598 to DIG, NIH grants HL70755, HL094488 and OH008282 to VEK and in part by flow cytometry core of H. Lee Moffitt Cancer Center.
Abbreviation
- LN
lymph nodes
- BM
bone marrow
- OVA
ovalbumin
- BSA
bovine serum albumin
- TB
tumor-bearing
- TES
tumor explant supernatant
- HPC
hematopoietic progenitor cells
- TAG
triacylglycerol (triglycerides)
- SR
scavenger receptor
Footnotes
Authors’ contribution
DLH – performed initial experiments, participated in writing of the paper;
WC, YN, SVN, SN, AC, BL – performed experiments investigating the mechanism and immunological consequences of lipid accumulation in DCs, analyzed the data;
VK, VAT – designed and performed experiments with MS analysis of lipid content, analyzed the data, participated in writing the paper;
EC, HIC – designed and performed experiments with B16F10 model, analyzed the data;
SCK – participated in the design of the original experiments, participated in writing the paper,
TP, TVM, JCM, SA – participated in experiments evaluating human samples;
MF, RLF - participated in experiments evaluating human samples, participated in writing the paper;
DIG – designed the study, analyzed the data, and wrote the paper
Authors declare no competing financial interests.
Contributor Information
Donna L. Herber, Email: dherber@research.usf.edu.
Wei Cao, Email: wei.cao@moffitt.org.
References
- 1.Gabrilovich DI. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 2.Shurin M, Chatta G. Immunobiology of dendritic cells in cancer. In: Gabrilovich DI, Hurwitz A, editors. Tumor-induced immune suppression. Mechanisms and theraputic reversal. Springer; New York: 2008. pp. 101–130. [Google Scholar]
- 3.Calder PC, Burdge GC. Fattty acids. In: Nicolaou A, Kokotos G, editors. Bioactive lipids. Bridgewater: The Oily Press; 2004. pp. 1–36. [Google Scholar]
- 4.Shaikh SR, Edidin M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. The American journal of clinical nutrition. 2006;84:1277–1289. doi: 10.1093/ajcn/84.6.1277. [DOI] [PubMed] [Google Scholar]
- 5.Knight SC. Specialized perinodal fat fuels and fashions immunity. Immunity. 2008;28:135–138. doi: 10.1016/j.immuni.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson P, MacPherson GG, Jenkins CH, Calder PC. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J Leukoc Biol. 1997;62:771–777. doi: 10.1002/jlb.62.6.771. [DOI] [PubMed] [Google Scholar]
- 7.Touitou E, Godin B, Karl Y, Bujanover S, Becker Y. Oleic acid, a skin penetration enhancer, affects Langerhans cells and corneocytes. J Control Release. 2002;80:1–7. doi: 10.1016/s0168-3659(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 8.Saemann MD, et al. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J Leukoc Biol. 2002;71:238–246. [PubMed] [Google Scholar]
- 9.Zeyda M, et al. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280:14293–14301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- 10.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 11.Weatherill AR, et al. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 12.Shamshiev AT, et al. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8{alpha}-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angeli V, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Packard RR, et al. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrot I, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 16.Halvorson DL, McCune SA. Inhibition of fatty acid synthesis in isolated adipocytes by 5-(tetradecyloxy)-2-furoic acid. Lipids. 1984;19:851–856. doi: 10.1007/BF02534514. [DOI] [PubMed] [Google Scholar]
- 17.Nagaraj S, et al. Dendritic cell-based full-length survivin vaccine in treatment of experimental tumors. J Immunother. 2007;30:169–179. doi: 10.1097/01.cji.0000211329.83890.ba. [DOI] [PubMed] [Google Scholar]
- 18.de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- 19.Peiser L, Mukhopadhyay S, Gordon S. Scavenger receptors in innate immunity. Curr Opin Immunol. 2002;14:123–128. doi: 10.1016/s0952-7915(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 20.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 21.Hagemann T, et al. Ovarian cancer cells polarize macrophages toward a tumor- associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 22.Becker M, Cotena A, Gordon S, Platt N. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur J Immunol. 2006;36:950–960. doi: 10.1002/eji.200535660. [DOI] [PubMed] [Google Scholar]
- 23.Jin JO, et al. Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood. 2009;113:5839–5847. doi: 10.1182/blood-2008-10-184796. [DOI] [PubMed] [Google Scholar]
- 24.Delimaris I, et al. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clinical biochemistry. 2007;40:1129–1134. doi: 10.1016/j.clinbiochem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Motta M, et al. Antibodies against ox-LDL serum levels in patients with hepatocellular carcinoma. Panminerva medica. 2003;45:69–73. [PubMed] [Google Scholar]
- 26.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nefedova Y, et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–474. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 28.Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103:3503–3510. doi: 10.1182/blood-2003-07-2340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.