Abstract
Acid-sensing ion channels (ASICs) are densely expressed in broad areas of mammalian brains and actively modulate synaptic transmission and a variety of neuronal activities. To explore whether ASICs are linked to addictive properties of drugs of abuse, we investigated the effect of the psychostimulant amphetamine on subcellular ASIC expression in the rat forebrain in vivo. Repeated administration of amphetamine (once daily for 7 days, 1.25 mg/kg for days 1/7, 4 mg/kg for days 2–6) induced typical behavioral sensitization. At a 14-day withdrawal period, ASIC1 protein levels were increased in the defined surface and intracellular compartments in the striatum (both caudate putamen and nucleus accumbens) in amphetamine-treated rats relative to saline-treated rats as detected by a surface protein cross-linking assay. ASIC2 proteins, however, remained stable in the striatum. In the medial prefrontal cortex, repeated amphetamine administration had no effect on ASIC1 expression in either the surface or the intracellular pool. However, amphetamine selectively reduced the surface expression of ASIC2 in this region. These data identify ASICs as a sensitive target to repeated stimulant exposure. The region- and compartment-specific regulation of ASIC1 and ASIC2 expression may constitute a key synaptic adaptation in reward circuits critical for psychomotor plasticity.
Keywords: dopamine, striatum, caudate, nucleus accumbens, prefrontal cortex, behavioral sensitization, addiction
1. Introduction
Repeated drug exposure leads to profound drug addiction which is characterized by compulsive drug craving and ingestion despite severe adverse consequences. In rats, repeated, intermittent administration of the psychostimulant, amphetamine (AMPH), induces a progressive and enduring augmentation of behavioral responses to AMPH (Koob and Nestler 1997; Wolf, 1998; Vanderschuren and Kalivas, 2000; Hyman et al., 2006). This long-lasting behavioral adaptation known as behavioral sensitization corresponds to the intensification of drug craving in human addicts. It therefore serves as a valuable animal model to explore the underlying mechanisms for the pathological form of experience-dependent drug addiction.
Precise molecular mechanisms underlying persistent behavioral sensitization are poorly understood at present. Available data indicate that the complex molecular adaptations in specific neural circuits occur in response to repeated drug exposure and are critical for this behavioral plasticity (Nestler, 2005; Hyman et al., 2006). AMPH, as a drug that principally stimulates dopamine release from dopaminergic nerve terminals (Sharp et al., 1987; Butcher et al., 1988), has been demonstrated to affect mainly the dopaminergic pathways in the central nervous system. Specifically, AMPH enhances dopaminergic inputs to the striatum and medial prefrontal cortex (mPFC), the two major dopaminergic projection sites in the mesolimbic/mesocortical reward system of the forebrain. In doing so, the drug triggers major adaptive responses in these regions, leading to persistent mental illnesses. Indeed, the vast majority of the early work has concentrated on these dopaminergic reward systems and has revealed that the enhanced dopaminergic transmission contributes to drug-dependent psychomotor plasticity (reviewed in Vanderschuren and Kalivas, 2000; Anderson and Pierce, 2005).
In addition to the dopaminergic transmission, other synaptic proteins may be involved in drug action. One of potential proteins of this kind is the acid-sensing ion channel (ASIC), a channel that is specifically activated by a drop in the extracellular pH level (Kellenberger and Schild, 2002). So far, four genes (ASIC1-4) have been cloned to encode seven distinct subunits of ASICs (ASIC1a, 1b1, 1b2, 2a, 2b, 3, and 4) (Price et al., 1996; Waldmann et al., 1996; Garcia-Anoveros et al., 1997; Lingueglia et al., 1997; Chen et al., 1998; de Weille et al., 1998). Most of these subunits can form into homomeric and/or heteromeric channels. A functional ASIC is usually assembled as a trimer complex from mixtures of different subunits (Waldman and Lazdunski, 1998; Benson et al., 2002; Jasti et al., 2007). In brain neurons, three subunits (ASIC1a, ASIC2a, and ASIC2b) are usually found, and among them, ASIC1a is the dominant subunit (Krishtal 2003; Wemmie et al., 2006; Xiong et al., 2006; 2008). The homomeric ASIC1a is unique since they are permeable to Ca2+ in addition to Na+ (Waldmann et al., 1997; Chu et al., 2002; Yermolaieva et al., 2004). Functionally, ASIC1, as a channel enriched at synaptic sites (Wemmie et al., 2003), modulates synaptic plasticity related to learning and memory (Wemmie et al., 2002). Given the fact that ASIC1 is frequently associated with the pathogenesis of various psychiatric disorders (Wemmie et al., 2003; Ziemann et al., 2008; 2009), this channel likely plays a pivotal role in addictive properties of drugs of abuse.
The striatum represents a brain area where ASIC1 and ASIC2 are densely expressed (Biagini et al., 2001; Alvarez de la Rosa et al., 2003; Wemmie et al., 2003). Recent electrophysiological evidence also identifies the homomeric ASIC1a as a predominant subtype of ASICs in the medium spiny projection neurons of the mouse striatum (Jiang et al., 2009). Given the importance of the striatum in drug action, expression of the functional ASIC1a in this structure implies a potential involvement of the channel in drug effects. In this study, we examined gene expression of ASIC1 and ASIC2 in the rat forebrain in response to chronic drug exposure in vivo. In the rats showing behavioral sensitization to repeated AMPH administration, changes in ASIC1 and ASIC2 protein levels in specific subcellular compartments of striatal or cortical neurons were examined at a 14-day withdrawal period.
2. Materials and Methods
2.1. Animals
Adult male Wistar rats weighting 175–200 g (Charles River, New York, NY) were individually housed in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12/12 h light/dark cycle. Rats were allowed 6–7 days of habituation to the animal colony before experiments. All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
2.2. AMPH treatments and behavioral assessments
In a behavioral sensitization regimen modified from our recent study (Mao et al., 2009), rats were subjected to repeated AMPH administration. Briefly, rats were treated with a single daily intraperitoneal (i.p.) injection of AMPH (D-amphetamine sulfate; Sigma, St. Louis, MO) for 7 consecutive days in home cages. The first and last injections were given at a dose of 1.25 mg/kg. Starting at day 2, rats were injected at a dose of 4 mg/kg until day 6. Age-matched rats received 7 daily i.p. injections of saline (1 ml/kg), which served as controls. Before the start of daily drug treatment, all animals were routinely handled for 2 days to accommodate them to injection procedures. All drugs were freshly prepared at the day of injection. Amphetamine was dissolved in physiological saline and its dose was calculated as a salt. Behavioral responses to the first and last injections of AMPH (1.25 mg/kg, i.p.) were monitored with an infrared photocell-based automated Opto-Varimex-Micro apparatus (Columbus Instruments) as described previously (Liu et al., 2006; 2009). Horizontal locomotor activity was monitored. Stereotypy was detected using computer-generated stereotypy time recorded by VersaMax monitors, which refers to the total time that stereotypic behaviors (repetitive breaks of a given beam or beams with an interval less than 1 s) were observed.
2.3. Surface receptor cross-linking assays
Surface expression of ASICs was assayed using the membrane-impermeable cross-linking reagent bis(sulfosuccinimidyl)suberate (BS3) as described previously (Mao et al., 2009). Briefly, rats were anesthetized with 2.5–3% isoflurane and decapitated. Brains were rapidly removed and sliced into coronal sections (400 μm) with a vibratome (Leica, VT 1200 S). Selected brain structures, including the dorsal (caudate putamen, CPu) and ventral (nucleus accumbens, NAc) striatum and mPFC, were dissected and added to Eppendorf tubes containing ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 10 glucose, 124 NaCl, 3 KCl, 1.25 KH2PO4, 26 NaHCO3, 2 MgSO4, and 2 CaCl2, bubbled with 95% O2-5% CO2, pH 7.4. BS3 (Thermo Fisher Scientific Inc., Rockford, IL) which only cross-links proteins on the surface of live cells was added to the tubes to 2 mM for incubation with gentle agitation for 30 min at 4°C. The cross-linking reaction was terminated by quenching with 20 mM glycine (10 min, 4°C). The tissue was then washed four times in ice-cold ACSF (10 min each). Samples were removed into another tube containing ice-cold sample buffer (20 mM Tris-HCl, pH 7.4, 1 mM dithiothreitol, 10 mM NaF, 2 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 5 μM microcystin-LR, and 0.5 mM phenylmethylsulfonyl fluoride) and homogenized by sonication. The total protein homogenate was measured for its protein concentration and used in Western blot analysis. Of note, ASICs have a relatively simple membrane topology with two transmembrane domains, cytoplasmic N- and C-termini, and a large ectodomain between the transmembrane domains (Price et al., 1996; Waldmann et al., 1996; Garcia-Anoveros et al., 1997; Lingueglia et al., 1997; Chen et al., 1998; de Weille et al., 1998). Primary amines (−NH2) in the N-terminus and in the side chain of a large number of lysine residues in the ectodomain form stable amide bonds with BS3. Through those bonds, BS3 cross-links ASICs to form high-molecular weight aggregates.
2.4. Western blot analysis
Western blot analysis was conducted according to our standardized procedures (Yang et al., 2004; Mao et al., 2005). Briefly, the equal amount of protein was loaded on SDS-PAGE 4–12% Tris-glycine gels (Invitrogen, Carsbad, CA) for separation of proteins. Proteins on gels were then transferred to polyvinylidene fluoride membranes (Immobilon-P; 0.45 mm; Millipore, Bedford, MA) and blocked in blocking buffer (5% nonfat dry milk in PBS and 0.1% Tween 20) for 1 h. The blots were incubated in a primary rabbit polyclonal antibody against ASIC1 (Alomone Labs, Jerusalem, Israel) or ASIC2 (Alpha Diagnostic, San Antonio, TX), or a mouse antibody against α-actinin (Millipore, Bedford, MA) overnight at 4°C. The membrane was then incubated for 1 h in goat anti-rabbit horseradish peroxidase-linked secondary antibodies (Jackson Immunoresearch Laboratory, West Grove, PA) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ), and captured into Kodak Image Station 2000R. Kaleidoscope-prestained standards (Bio-Rad, Hercules, CA) and MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. The density of immunoblots was measured using the Kodak 1D Image Analysis software (Yang et al., 2004).
2.5. Statistics
The results are presented as means ± SEM. The data were evaluated using Student's t test or a one- or two-way analysis of variance, as appropriate. The analysis of variance was followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means. Probability levels of < 0.05 were considered statistically significant.
3. Results
3.1. Chronic AMPH administration induces behavioral sensitization
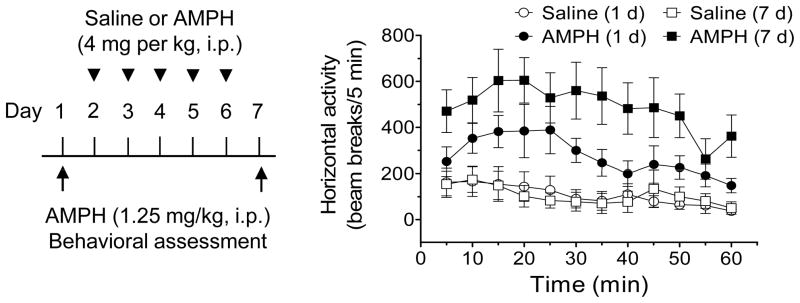
Chronic noncontingent administration of AMPH is known to induce behavioral sensitization in experimental animals (Wolf, 1998). To link behavior to ASIC expression following chronic AMPH administration, we subjected rats to a typical AMPH sensitization regimen (once daily i.p. injection of AMPH for 7 consecutive days; 1.25 mg/kg for the first and last injections and 4 mg/kg for the middle 5 daily injections). The locomotor behavioral responses to the first and last injections of AMPH were measured and compared to confirm the occurrence of behavioral sensitization. As shown in Fig. 1, the locomotor response to the last injection of AMPH at day 7 was significantly greater than that to the first injection of the drug at day 1. In addition to augmented locomotor responses, stereotypical behaviors characterized by repetitive sniffing, exploration, and rearing at day 7 were enhanced in AMPH-treated rats compared to day 1. These data validate the reliable development of behavioral sensitization to repeated injections of AMPH.
Fig. 1. Repeated AMPH administration induces behavioral sensitization in rats.
Rats were injected daily with saline or AMPH for 7 days (1.25 mg/kg for the first and last injections; 4 mg/kg for the middle 5 daily injections). Locomotor activity in response to the first and last injections of AMPH was tested at day 1 and 7, respectively (n = 8–10 per group). Note that the locomotor response to the last injection of AMPH observed at day 7 was significantly greater than that to the first injection of AMPH at day 1, confirming the development of behavioral sensitization following repeated drug administration.
3.2. ASIC1 and ASIC2 are normally expressed in both surface and intracellular pools
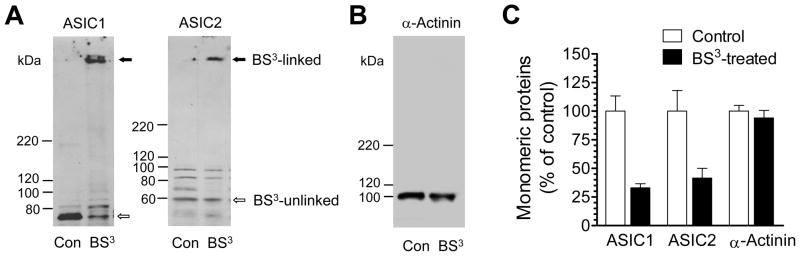
We first examined ASIC expression in the surface versus the intracellular pool in normal rats in vivo. This was done by performing surface protein cross-linking assays with membrane-impermeable reagent BS3. This reagent selectively cross-links surface membrane-bound channels to form high-molecular weight aggregates as opposed to unlinked intracellular channels with a normal molecular weight. These BS3-linked surface-expressed channels and BS3-unlinked intracellular channels can be readily separated by gel electrophoresis due to their different molecular weights. As shown in Fig. 2A, BS3-treated striatal tissue showed a high-molecular weight band of ASIC1 and ASIC2 (surface channels) and a monomeric molecular weight band of ASIC1 and ASIC2 (intracellular channels). Unlike BS3-treated tissue, non-cross-linked tissue displayed only a single monomeric molecular weight band (both surface and intracellular channels). The selectivity of BS3 was confirmed by 1) the lack of the BS3-cross-linking effect on α-actinin, an intracellular protein (Fig. 2B), and 2) a complete elimination of the monomeric ASIC1 and ASIC2 bands by treating striatal homogenates with BS3 (data not shown). Further quantification analysis reveals that a larger portion of ASIC1 and ASIC2 is expressed in the surface membrane (60–70% for both) relative to these channel levels in the intracellular pool (Fig. 2C). Similar subcellular distribution patterns for the two channels were also observed in the mPFC (data not shown).
Fig. 2. Normal subcellular expression of ASIC1 and ASIC2 in striatal neurons in vivo.
(A) Representative immunoblots of ASIC1 and ASIC2 from rat striatal tissue treated with BS3 or from control tissue without BS3 treatment. (B) Representative immunoblots of α-actinin from rat striatal tissue treated with BS3 or from control tissue without BS3 treatment. (C) The percentage of ASIC and α-actinin distributions in the intracellular pool. An equal amount of proteins (20 μg/each loading) was loaded for control and BS3-treated tissue. Total and intracellular proteins at the monomeric molecular weight level were measured from control tissue and BS3-treated tissue, respectively. The data are expressed in terms of means ± SEM (n = 3–4 per group).
3.3. Chronic AMPH administration increases ASIC1 expression in the CPu
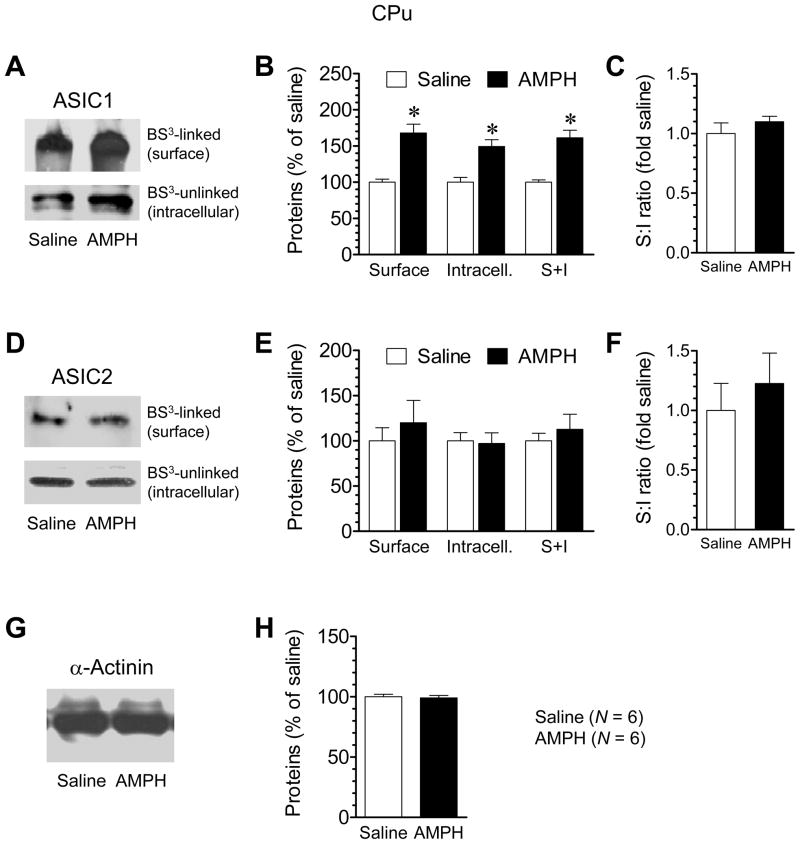
We then tested the effect of repeated AMPH administration on ASIC1 and ASIC2 expression in the distinctive surface and intracellular pools in the rat CPu. We performed these experiments at a 14-day withdrawal period. We selected this time point because behavioral sensitization to AMPH remained and reliable changes in expression of some key excitatory synaptic proteins were seen in AMPH-treated rats at this stage (Mao et al., 2009). At 14 days after discontinuation of drug treatment, we found a marked increase in the amount of ASIC1 in the surface pool of AMPH-treated rats compared to saline-treated rats in BS3-cross-linking experiments (Fig. 3A and 3B). Like ASIC1 in the surface pool, ASIC1 in the intracellular pool showed a significant increase in its abundance in AMPH-treated rats relative to saline-treated rats (Fig. 3A and 3B). Due to these parallel increases in both surface and intracellular pools, the total protein level of ASIC1 (surface plus intracellular) was elevated (Fig. 3B), while the surface:intracellular ratio of ASIC1 remained unchanged (Fig. 3C). In contrast to ASIC1, ASIC2 expression in the surface and intracellular pools was insensitive to AMPH since there was no difference in the amount of ASIC2 proteins in either pool between AMPH- and saline-treated animals (Fig. 3D and 3E). As a result, total ASIC2 levels and the surface:intracellular ratio of ASIC2 were not altered (Fig. 3E and 3F). No changes were seen in the levels of α-actinin (Fig. 3G and 3H). These results demonstrate a selective elevation of ASIC1 in the confined surface and intracellular pools of CPu neurons following chronic AMPH administration.
Fig. 3. Effects of repeated AMPH administration on ASIC1 and ASIC2 expression in the CPu.
(A and B) Repeated AMPH administration increased ASIC1 levels in surface (S) and intracellular (I) pools. (C) Repeated AMPH administration did not alter the S:I ratio of ASIC1. (D and E) Repeated AMPH administration had no effect on ASIC2 levels in surface and intracellular pools. (F) Repeated AMPH administration did not alter the S:I ratio of ASIC2. (G and H) Repeated AMPH administration had no effect on α-actinin expression. Rats were injected with saline or AMPH (once daily for 7 days; 1.25 mg/kg for the first and last injections and 4 mg/kg for the middle 5 daily injections). Animals were sacrificed at 14 days after the last injection. The CPu tissue was dissected for the surface cross-linking experiments with BS3. Data are expressed in terms of means ± SEM (n = 6 per group). *p < 0.05 versus saline.
3.4. Chronic AMPH administration increases ASIC1 expression in the NAc
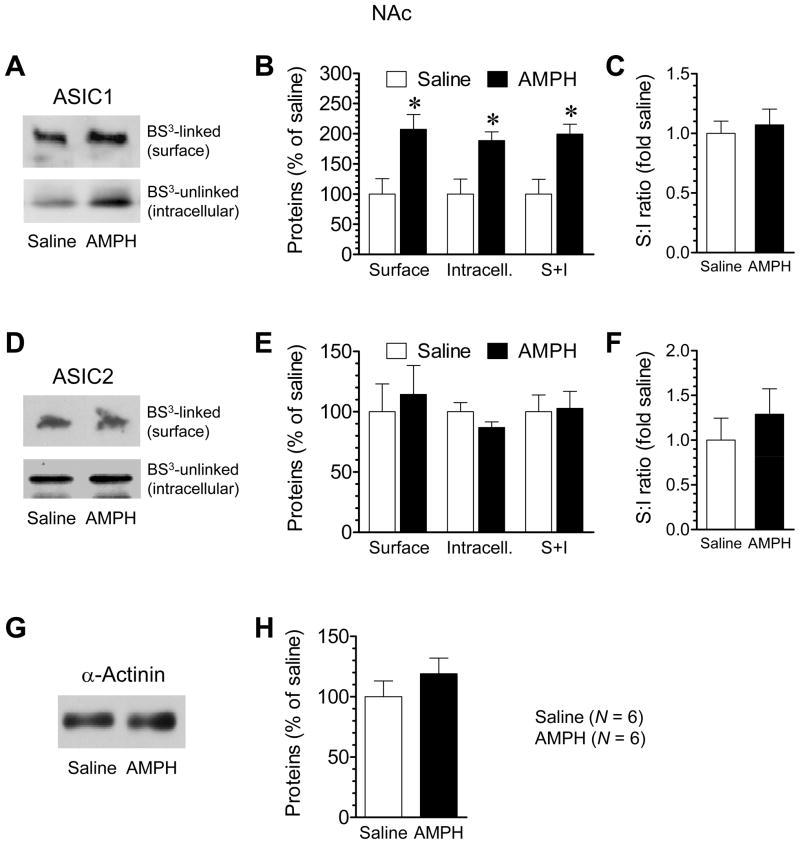
To determine if the addition of surface and intracellular ASIC1 is confined to the CPu, we examined changes in subcellular ASIC1 expression in the NAc following repeated AMPH administration. After a 14-day withdrawal period, a significant increase in the surface (BS3-linked) amount of ASIC1 proteins in the NAc was exhibited in AMPH-treated rats relative to saline-treated rats (Fig. 4A and 4B). The intracellular (non-BS3-linked) ASIC1 protein level in the NAc was also elevated to a comparable extent in AMPH-treated rats (Fig. 4A and 4B). These increases constitute an increased level of the total amount of ASIC1 (surface plus intracellular) in NAc neurons. No changes were observed in the surface:intracellular ratio of ASIC1 in this region (Fig. 4C). These results demonstrate that the NAc, like the CPu, is a sensitive site where repeated AMPH administration can upregulate ASIC1 expression in both surface and intracellular compartments. With regard to ASIC2, no significant changes were observed in its levels in either surface or intracellular fraction in AMPH-treated rats (Fig. 4D and 4E). The surface:intracellular ratio of ASIC2 and protein levels of α-actinin in AMPH-treated rats were not different from those in saline-treated rats (Fig. 4F–H). These data support a selective modulation of ASIC1 in the NAc in response to AMPH.
Fig. 4. Effects of repeated AMPH administration on ASIC1 and ASIC2 expression in the NAc.
(A and B) Repeated AMPH administration increased ASIC1 levels in surface (S) and intracellular (I) pools. (C) Repeated AMPH administration did not alter the S:I ratio of ASIC1. (D and E) Repeated AMPH administration had no effect on ASIC2 levels in surface and intracellular pools. (F) Repeated AMPH administration did not alter the S:I ratio of ASIC2. (G and H) Repeated AMPH administration had no effect on α-actinin expression. Rats were injected with saline or AMPH (once daily for 7 days; 1.25 mg/kg for the first and last injections and 4 mg/kg for the middle 5 daily injections). Animals were sacrificed at 14 days after the last injection. The NAc tissue was dissected for the surface cross-linking experiments with BS3. Data are expressed in terms of means ± SEM (n = 6 per group). *p < 0.05 versus saline.
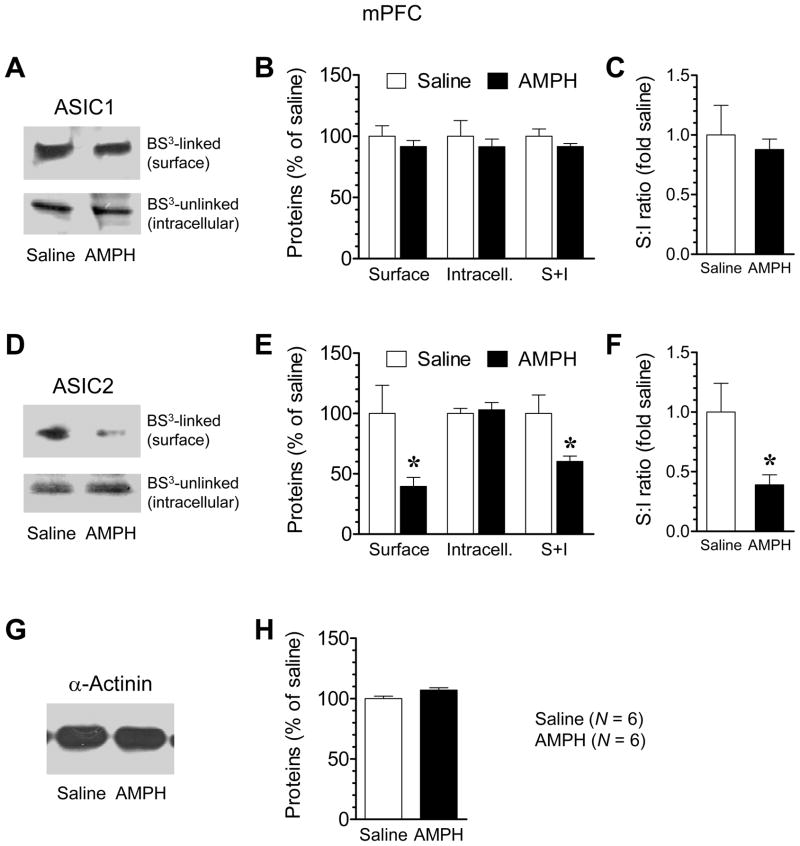
3.5. Effects of chronic AMPH administration on ASIC expression in the mPFC
The mPFC neurons express ASICs and participate in stimulant action (Biagini et al., 2001; Alvarez de la Rosa et al., 2003; Wemmie et al., 2003; Steketee, 2005; Goto and Grace, 2005). We therefore explored if ASIC expression in this region is subject to the modulation by chronic AMPH exposure. In contrast to the striatum (CPu and NAc) where ASIC1 expression was upregulated by AMPH, ASIC1 expression in the mPFC was stable in response to chronic AMPH administration. This was shown by an insignificant difference in ASIC1 levels in both surface and intracellular pools between AMPH- and saline-treated rats at a 14-day withdrawal period (Fig. 5A and 5B). As a result, there was no change in the surface:intracellular ratio of ASIC1 in this area (Fig. 5C). ASIC2 expression, on the other hand, was sensitive to AMPH. As shown in Fig. 5D and 5E, ASIC2 levels in the surface pool were markedly reduced in AMPH-treated rats compared to saline-treated rats. ASIC2 levels in the intracellular pool, however, remained unchanged (Fig. 5D and 5E). Due to the robust reduction of ASIC2 in the surface pool, total cellular ASIC2 proteins and the surface:intracellular ratio of ASIC2 were decreased (Fig. 5E and 5F). No change in α-actinin expression was observed in the mPFC between AMPH- and saline-treated animals (Fig. 5G and 5H). The data obtained here identify ASIC2 as a selective target of AMPH in the mPFC. Its expression in the distinct surface membrane was sensitive to AMPH for a negative modulation.
Fig. 5. Effects of repeated AMPH administration on ASIC1 and ASIC2 expression in the mPFC.
(A and B) Repeated AMPH administration had no effect on ASIC1 expression in surface (S) and intracellular (I) pools. (C) Repeated AMPH administration did not alter the S:I ratio of ASIC1. (D and E) Repeated AMPH administration reduced ASIC2 levels in the surface, but not the intracellular, pool. (F) Repeated AMPH administration reduced the S:I ratio of ASIC2. (G and H) Repeated AMPH administration had no effect on α-actinin expression. Rats were injected with saline or AMPH (once daily for 7 days; 1.25 mg/kg for the first and last injections and 4 mg/kg for the middle 5 daily injections). Animals were sacrificed at 14 days after the last injection. The mPFC tissue was dissected for the surface cross-linking experiments with BS3. Data are expressed in terms of means ± SEM (n = 6 per group). *p < 0.05 versus saline.
4. Discussion
This study is an effort initiated to investigate the effect of a psychostimulant on gene expression of ASICs in the rat limbic system in vivo. Animals were treated with repeated i.p. injections of saline or AMPH (1.25 mg/kg for day 1 and day 7 and 4 mg/kg for days 2–6). Changes in basal protein levels of ASIC1 and ASIC2 isolated from specific surface and intracellular pools through the cross-linking reagent BS3 were detected at a 14-day withdrawal period. We found that repeated AMPH treatments upregulated ASIC1 levels in both surface and intracellular pools of CPu neurons. AMPH, however, had no effect on the surface and intracellular ASIC2 expression in this region. Similar results were observed in the NAc. In the mPFC, repeated AMPH did not alter ASIC1 expression in surface and intracellular pools. In contrast, it reduced ASIC2 expression in the surface pool, although it did not in the intracellular pool. These results demonstrate that repeated AMPH administration is able to alter ASIC expression in a region-, pool-, and subunit-specific manner.
Various synaptic proteins (receptors, channels, enzymes, scaffolds, etc.) have been screened for their plastic changes at either mRNA or protein levels in response to repeated drug exposure in order to determine their roles in short- and especially long-term drug action (Kalivas et al., 2003). Documented striatal genes that showed enduring changes after repeated drug administration include glutamate receptors and their anchoring proteins (PSD-95 and Homer) (Mao and Wang, 2001; Szumlinski et al., 2004; Yao et al., 2004; Boudreau and Wolf, 2005), other transmitter receptors (Yuferov et al., 2005; Zhang et al., 2005), signaling and enzyme molecules (McClung et al., 2004; Svenningsson et al., 2005), and structural proteins (Albertson et al., 2004; Yuferov et al., 2005; Zhang et al., 2005). All these responsive genes are thought to work together in an orchestra of molecular adaptations to determine the development and expression of various forms of drug-induced synaptic and behavioral plasticity. In this study, a new responsive gene is identified. Following repeated AMPH administration, ASIC1 was upregulated in its expression in the striatum. This establishes the possibility for this channel to participate in the concert of neuroadaptations to control neuronal and behavioral responses to psychostimulants.
Several characteristics were noticed in the ASIC1 response to AMPH. First, the channel was upregulated in abundance in a region-specific manner. While ASIC1 is expressed in both mPFC and striatum (Biagini et al., 2001; Alvarez de la Rosa et al., 2003; Wemmie et al., 2003) and both regions are among the forebrain structures important for behavioral sensitization to repeated psychostimulant exposure (Steketee, 2005; Goto and Grace, 2005; Schmidt et al., 2005; Laviolette and Grace, 2006), only the striatum exhibited an increase in ASIC1 expression. It is unclear how AMPH altered ASIC1 expression in the striatum but not in the mPFC. The mesocortical dopaminergic pathway is believed to have unique neurochemical characteristics relative to the two other major dopaminergic pathways in the brain, the nigrostriatal and mesolimbic pathways (Bannon and Roth, 1983). AMPH produced a much less increase in dopamine in the mesocortical projection area, mPFC, versus the nigrostriatal projection areas, dorsal striatum (Pehek, 1999). It is maybe due to this difference and/or many other differences in neurochemical and physiological aspects between the two regions, AMPH caused differential changes in ASIC1 expression in these areas. Second, the increase in ASIC1 expression occurred in the absence of changes in ASIC2. This seems to indicate that ASIC1 rather than ASIC2 is the subtype targeted by AMPH. The insensitivity of ASIC2 to the drug suggests the high vulnerability of ASIC1 in the striatum to dopamine stimulation. Finally, ASIC1 in different subcellular compartments was similarly increased by AMPH. Using BS3 surface cross-linking assays, we were able to measure ASIC1 responses in distinct surface and intracellular pools. We found that AMPH induced changes in ASIC1 expression in the two defined pools. These changes were parallel in direction and magnitude. As a result, the total cellular level of ASIC1 was enhanced in striatal neurons.
In a recent study, we tested the effect of repeated cocaine administration on cellular levels of ASIC1 proteins in the mouse striatum and mPFC (Zhang et al., 2009). We found that cocaine increased ASIC1 expression in the striatum but not in the mPFC, which is consistent with the results observed with AMPH in this study. This probably reflects the fact that both cocaine and amphetamine increase synaptic availability of dopamine and thus share similarities in many biological actions, even though they increase synaptic dopamine by different mechanisms. Cocaine increases synaptic dopamine accumulation by blocking the dopamine transporter and thereby reducing the reuptake of released dopamine into dopamine nerve terminals whereas AMPH directly stimulates the nonexocytotic release of dopamine.
Another noticeable finding in this study is the reduction of ASIC2 expression in the mPFC. This reduction was also subunit-specific since ASIC1 was not found to be reduced in this region. Moreover, ASIC2 in the surface pool is particularly susceptible to AMPH. In AMPH-treated rats, ASIC2 in the surface pool was substantially reduced while ASIC2 in the intracellular pool was not. The pool-specific loss of ASIC2 reveals this subunit to be a sensitive target of AMPH in the mPFC.
The precise role of plastic ASIC adaptations in short- or long-term drug action is unknown. It is also unclear, to our knowledge, whether a systemic injection of AMPH can significantly affect brain pH. What is known at present is that ASIC1 is enriched at synaptic sites in the mammalian brain (Wemmie et al., 2002; 2003). This situates the channel well to modulate synaptic activity and plasticity. In fact, ASIC1 has been documented to modify synaptic plasticity relevant to learning and memory and pathogenesis of several long-lasting mental disorders (Wemmie et al., 2006, Xiong et al., 2008; Dwyer et al., 2009). Drug addiction is a persistent mental illness that is derived from long-lasting changes in synaptic plasticity. In the animal model of behavioral sensitization, plastic changes in excitatory synapses on striatal spiny neurons or mPFC neurons have been considered to constitute critical molecular mechanisms for motor sensitization. For instance, two types of synaptic plasticity (long-term potentiation or depression) in the striatum are subject to the modification in response to chronic stimulant exposure (Yao et al., 2004; Kalivas and Hu, 2006; Mao et al., 2009). These modifications are believed to be important steps in the remodeling of excitatory synapses, leading to enduring behavioral plasticity. Given the synapse-enriched distribution of ASIC1 and the high sensitivity of this channel to drug exposure, it is possible that ASIC1 contributes to the reshaping of excitatory synapses. In concert with other synaptic proteins, ASIC1 contributes to control synaptic and behavioral adaptations to AMPH. Future studies will need to elucidate the accurate role of ASIC1 as well as ASIC2 in striatal or cortical neurons in processing synaptic and behavioral adaptations to drugs.
Acknowledgments
This work was supported by the NIH grants R01DA010355-15 (J.Q.W.) and R01MH061469-10 (J.Q.W.) and by a research grant from St. Luke’s Hospital Foundation (Kansas City, MO).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPH
amphetamine
- ASIC
acid-sensing ion channel
- BS3
bis(sulfosuccinimidyl)suberate
- CPu
caudate putamen
- i.p.
intraperitoneal
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol (Lond) 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits from H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Babinski K, Avoli M, Marcinkiewcz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- Bonnon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988;50:346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron specific, proton-gated ion channel. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Miesch J, Johnson M, Root L, Zhu XM, Chen D, Simon RP, Xiong ZG. Proton-gated channels in PC12 cells. J Neurophysiol. 2002;87:2555–2561. doi: 10.1152/jn.00741.2001. [DOI] [PubMed] [Google Scholar]
- de Weille JR, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localization of a sustained human proton-gated cation channel. FEBS Lett. 1998;433:257–260. doi: 10.1016/s0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop J, Beyer CE. Acid-sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology. 2009;203:41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouzux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009;162:55–66. doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Toda S, Bowers MS, Baker DA, Ghasemzadeh MB. The temporal sequence of changes in gene expression by drugs of abuse. Methods Mol Med. 2003;79:3–11. doi: 10.1385/1-59259-358-5:03. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Review. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, Zhou HF, Xu M, Wang JQ. Activity-dependent modulation of limbic dopamine D3 receptor by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Differentially altered mGluR1 and mGluR5 mRNA expression in rat caudate nucleus and nucleus accumbens in the development and expression of behavioral sensitization to repeated amphetamine administration. Synapse. 2001;41:230–240. doi: 10.1002/syn.1080. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Pehek EA. Comparison of effects of haloperidol administration on amphetamine-stimulated dopamine release in the rat medial prefrontal cortex and dorsal striatum. J Pharm Exp Ther. 1999;289:14–23. [PubMed] [Google Scholar]
- Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviors and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353–360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hagemen AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006;209:59–68. doi: 10.1007/s00232-005-0840-x. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J Neurosci. 2004;24:10846–10857. doi: 10.1523/JNEUROSCI.2496-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuchle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SGN, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E, Kreek MJ. Microarray studies of psychostimulant-induced changes in gene expression. Addict Biol. 2005;10:101–118. doi: 10.1080/13556210412331308976. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Wang JQ, Chu XP. Upregulation of acid-sensing ion channel 1 protein expression by chronic administration of cocaine in the mouse striatum in vivo. Neurosci Lett. 2009;459:119–122. doi: 10.1016/j.neulet.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdale is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]