Abstract
Influenza prophylaxis would benefit from a vaccination method enabling simplified logistics and improved immunogenicity without the dangers posed by hypodermic needles. Here, we introduce dissolving microneedle patches for influenza vaccination using a simple patch-based system that targets delivery to skin’s antigen-presenting cells. Microneedles were fabricated using a biocompatible polymer encapsulating inactivated influenza virus vaccine for insertion and dissolution in the skin within minutes. Microneedle vaccination generated robust antibody and cellular immune responses in mice that provided complete protection against lethal challenge. Compared to conventional intramuscular injection, microneedle vaccination resulted in more efficient lung virus clearance and enhanced cellular recall responses after challenge. These results suggest that dissolving microneedle patches can provide a novel technology for simpler and safer vaccination with improved immunogenicity that could facilitate increased vaccination coverage.
Introduction
Effectiveness of influenza vaccination is limited by quality and breadth of the immune response and time required for vaccine delivery1. Traditional intramuscular (IM) injection requires hypodermic needles that cause needle phobia and generate biohazardous waste. An advantageous immunization scenario would involve transdermal delivery of the vaccine using a device that promises (i) increased vaccine immunogenicity, (ii) enhanced patient compliance via simple self-administration and mass immunization, and (iii) elimination of hypodermic needles and their associated biohazardous waste.
This study presents dissolving microneedle patches to increase vaccine immunogenicity by targeting antigen delivery to skin. Microneedles are micron-scale structures that painlessly pierce into the skin to administer vaccines in a minimally invasive and targeted manner2. Skin is a highly active immune organ containing a large population of resident antigen-presenting cells3. Human clinical studies have shown evidence for dose sparing of intradermal influenza vaccination compared to IM immunization, although some other studies have not4–7. Intradermal influenza vaccination at full dose (15 μg hemagglutinin (HA) antigen per strain) and reduced dose (9 μg HA per strain) have recently been licensed for human use in some countries (i.e., Intanza® and IDflu®, Sanofi Pasteur). Widespread use of intradermal immunization has been limited by traditional intradermal injections using the Mantoux technique, which requires highly trained personnel and is often unreliable8. Needle-free transdermal patches have been reported, but the skin’s outer layer (stratum corneum) must be disrupted for delivery of large vaccine molecules9. In contrast, microneedles are designed to reliably administer antigen at a specific skin depth that maximizes interaction with resident antigen presenting cells.
Previous studies show that non-dissolving metal and silicon microneedle patches can be painless10 and effectively administer vaccine in animals11,12 including influenza vaccine13–15. Water-soluble microneedles have been shown to encapsulate bioactive molecules and deliver their cargo into skin16–19, but vaccination using this approach has not been studied before.
In this study, we compare standard IM immunization to vaccination using polymer microneedles that dissolve within minutes and completely resorb in the skin, resulting in no biohazardous sharps. We show that a single vaccine dose with dissolving microneedles induces protective immune responses superior to those obtained with IM injection at the same dose, including increased lung viral clearance. Dissolving microneedles also offer additional patient and logistical benefits, including small storage and disposal size; inexpensive fabrication; and ease of use to enable self-administration at home.
Results
Design and fabrication of dissolving polymer microneedles
The polymer material, microneedle geometry and device fabrication process were designed to safely encapsulate influenza virus while preserving its antigenicity, insert into skin without mechanical failure, and rapidly dissolve in skin, leaving behind safe dissolution products. The resulting microneedles measured 650 μm tall with sharp tips tapering to a 10 μm radius of curvature (Fig. 1a) and were assembled into an array of 100 needles (Fig. 1c) that encapsulated 3 μg of inactivated influenza virus vaccine per patch.
Fig. 1. Dissolving polymer microneedle patches.
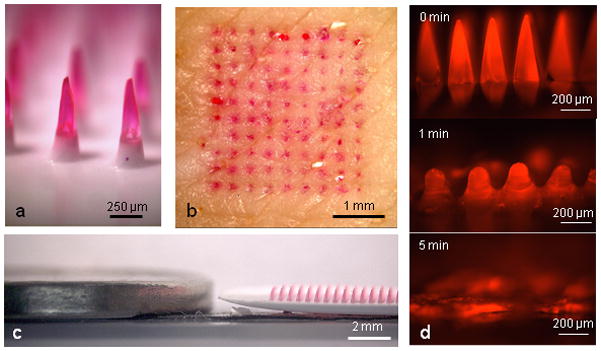
(a) Side view of dissolving polymer microneedles. (b) En face view of porcine skin after insertion and removal of microneedles, showing delivery of the encapsulated compound (sulforhodamine). (c) Relative height of microneedles next to a U.S. nickel coin. (d) Polymer microneedle dissolution in pig skin in vitro. Frame 1 = pre-insertion, frame 2 = after 1 min insertion in skin, frame 3 = after 5 min insertion in skin.
These microneedles were fabricated by room temperature photopolymerization of a liquid monomer (vinyl pyrrolidone) within a microneedle mold to form polyvinylpyrrolidone (PVP) microneedles that encapsulate the lyophilized vaccine. PVP was chosen as the structural material for the polymer microneedles used in this study, because it is biocompatible, mechanically strong and highly water soluble20. Moreover, PVP polymer microneedles were fabricated by a gentle, room-temperature photopolymerization process, which avoids need for organic solvents or elevated temperatures that can damage vaccine or other biomolecule stability.
Insertion and dissolution of microneedles in skin
The resulting microneedles were able to be inserted into skin with gentle force applied by thumb (Fig. 1b). We determined the fracture force of microneedles to be 0.13±0.03 N per needle, which provides a two-fold margin of safety over the force (0.058 N per needle) required for insertion into skin using microneedles of this geometry, according to previous measurements21. Upon insertion into porcine cadaver skin, microneedles penetrated to a depth of approximately 200 μm and deposited their encapsulated payload within epidermis and upper dermis (Fig. 2a-,b). This localization is likely to be similar in human skin, which has comparable thickness to porcine skin22.
Fig. 2. Delivery to skin using microneedles.
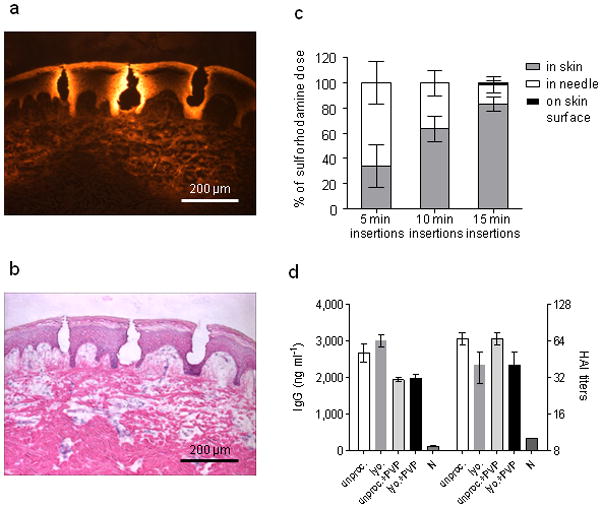
(a) Fluorescence micrograph of pig skin histological section after insertion of dissolving microneedles in vitro. (b) Brightfield micrograph of the same image with H&E staining. (c) Dissolving microneedle delivery efficiency to mice in vivo. Sulforhodamine was encapsulated within microneedles and administered to mice. The delivery efficiency was determined by measuring the amount of sulforhodamine left in microneedles after insertion as well as on the skin surface of the mouse. The remaining sulforhodamine was considered to be delivered to the skin. n = 5 for each time point. The delivery efficiencies for the three time points were statistically different from one another (Student’s t-test, P < 0.05). (d) mice (n = 3) were immunized IM with 20 μg inactivated influenza virus (A/PR/8/34) after different processing and formulation. Serum IgG antibody titers and HAI were measured 14 days after immunization. Antigen lyophilization, mixture with PVP and encapsulation in microneedles had no effect on IgG or HAI titers. Groups: unproc.: unprocessed inactivated influenza virus in PBS; lyo: lyophilized, re-dissolved in PBS inactivated influenza virus; encaps. + PVP: lyophilized inactivated influenza virus encapsulated in PVP; unproc. + PVP: unprocessed inactivated influenza virus in PBS mixed with PVP; N: naïve mice.
To characterize kinetics of dissolution in skin, microneedles were inserted into porcine skin and monitored over time. Significant dissolution occurred within 1 min, and after 5 min the microneedles were 89±3% (by mass) dissolved (Fig. 1d). Given the similarity of porcine and human skin, we expect that microneedle dissolution in human skin could also be complete within just a few minutes. Because vaccination experiments in this study used mouse skin, we also measured dissolution kinetics of dissolving microneedles encapsulating the viral antigen in mice. In this scenario, microneedle dissolution was slower, but nonetheless increased with time (P<0.05), depositing 34±17%, 63±10% and 83±6% in the skin after 5, 10 and 15 min, respectively, and leaving almost no residue on the skin surface (Fig. 2c).
Antigen stability
To assess stability of inactivated influenza vaccine in dissolving microneedles, we identified two steps during fabrication of PVP microneedles that might cause damage: initial lyophilization of vaccine and subsequent encapsulation within microneedles during polymerization.
To isolate effects of lyophilization and PVP, inactivated influenza virus was administered IM in mice (i) as the original vaccine solution, (ii) after lyophilization, (iii) as the original vaccine solution mixed with PVP, and (iv) after lyophilization and encapsulation within PVP microneedles. Compared to naïve animals, all four vaccinated groups showed elevated influenza-specific IgG titers and hemagglutination inhibition (HAI) titers (Fig. 2d, P<0.01). Among the four vaccinated groups, there was no significant effect of vaccine processing or formulation on IgG or HAI titers (P>0.05).
Humoral immune responses
The efficacy of skin immunization with dissolving microneedles was determined in BALB/c mice that received a single dose of 6 μg of whole encapsulated inactivated influenza virus. The microneedle patches were applied on the caudal dorsal area of skin for approximately 15 min, which was sufficient to dissolve the microneedles and deliver at least 80% of the antigen into skin. Induction of humoral immune responses using dissolving microneedles was compared at the same dose to those observed by IM immunization, which is the standard influenza vaccination method (Fig. 3a–d). Blood was collected on days 14 and 28 post-immunization to determine levels of anti-influenza-specific antibodies. Mice immunized with microneedles demonstrated a significant increase of anti-influenza IgG titers by day 14 (Fig. 3a, P<0.0003). Titers were at similar levels for both IM and microneedle groups at day 28 (p=0.9).
Fig. 3. Microneedle immunization studies.
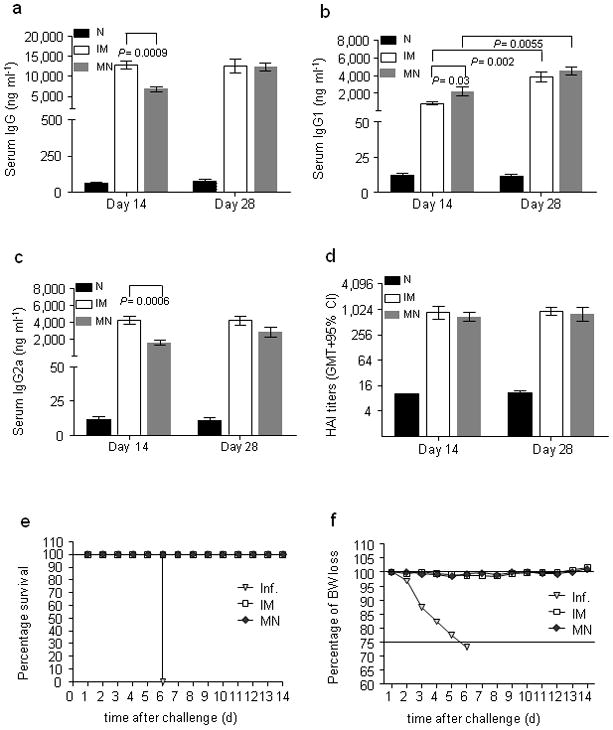
(a) 12 mice were immunized IM with inactivated influenza virus (A/PR/8/34) or using a microneedle patch encapsulating the same amount of virus. Sera were sampled 14 and 28 days after immunization to determine anti-influenza IgG titers. (b) IgG1 titers (c) IgG2a titers (d) HAI titers on days 14 and 28. (e) Survival rates of immunized and naïve mice upon lethal challenge with 5xLD50 of homologous influenza virus. (f) Percentage of body weight changes upon lethal challenge. Groups: N: naïve group; IM: intramuscularly immunized group; MN: microneedle immunized group; Inf.: unimmunized challenged group.
We also determined levels of influenza-specific isotypes, IgG1 and IgG2a, at 14 and 28 days after immunization. At day 14, IM-immunized mice showed significantly higher IgG2a responses than the microneedle group (p=0.0006), whereas the microneedle group had more pronounced IgG1 titers than the IM group (p=0.03). At day 28 there were no significant differences in the isotype levels between the groups. Thus, the IM group had Th1 biased responses early after immunization (IgG1/IgG2a=0.2) but isotype levels were similar one month later (IgG1/IgG2a=0.9). In contrast, the microneedle group showed a slight predominance of IgG1 production over time (IgG1/IgG2a in the range of 1.35–1.53) (Fig. 3b,c,4f).
Fig. 4. Long-lived immune responses.
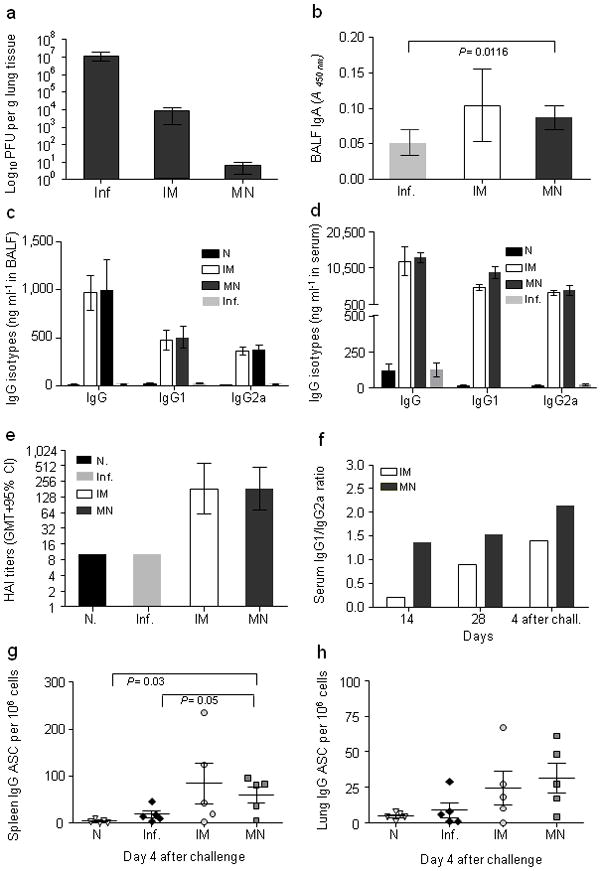
Mice immunized with influenza virus by microneedle or IM route of delivery were challenged with live homologous virus 90 days after immunization; Bronchoalveolar lavage fluid (BALF) and sera were collected at four days after challenge. (a) Lung virus titers determined by plaque assay. (b) Lung IgA titers determined by quantitative ELISA. (c) Lung IgG titers and their isotypes. (d) Serum IgG titers and their isotypes. (e) Serum HAI titers after infection determined as geometric mean titers with 95% confidence intervals. (f) Serum IgG2a/IgG1 ratio on days 14 and 28 after immunization and on day 4 after challenge. (g) Anti-influenza IgG antigen-secreting cells (ASC) from splenocytes re-stimulated with inactivated influenza virus. (h) Lung anti-influenza IgG ASC cells. Groups are as described in Fig. 3.
HAI activity is generally used as the serological measure for functional antibodies associated with protection. We observed high HAI titers after one immunization (Fig. 3d). HAI titers detected in the microneedle group were similar for the two time point bleedings and to IM group titers, demonstrating that a single microneedle immunization induced high levels of functional antibodies.
Protection against lethal viral challenge
To determine whether microneedle immunization can confer protective immunity, the immunized groups were challenged with 5xLD50 of mouse-adapted PR8 influenza virus 30 days after vaccination. All immunized animals survived challenge (Fig. 3e) and lost <5% body weight (Fig. 3f), showing that vaccine delivery with dissolving microneedles provided protection equal to the IM group. In contrast, the unimmunized group did not survive beyond 6 days post-challenge.
We then investigated the ability of challenged mice to clear influenza virus from the lung 90 days after vaccination to assess longevity and efficiency of recall responses. IM immunized mice showed a 103 decrease in lung viral titers compared to unimmunized infected mice, whereas microneedle-immunized mice showed a dramatic 106 decrease in lung viral titers (Fig. 4a). Because challenge of animals took place three months after vaccination, we observed that microneedle immunization induced more robust recall responses than IM vaccination as shown by faster virus clearance.
Recall immune responses
To evaluate induction of local immune responses, we measured influenza-specific IgG and IgA titers in lungs of challenged mice 90 days post-immunization. We found that sIgA levels were modestly increased in vaccinated groups and were similar among microneedle and IM groups (Fig. 4b). Lung IgG titers were also similar in microneedle and IM immunized mice, including IgG1 and IgG2a isotype profiles (Fig. 4c). Systemically, we observed that challenged mice had serum influenza-specific IgG titers similar to those observed 28 days after immunization, with no significant differences among immunized groups (Fig. 4d). Serum HAI titers also reached similar levels in all immunized challenged groups, consistent with total antibody levels (Fig. 4e). Although we noted an increase of IgG1 titers post-infection in vaccinated animals, microneedle-immunized mice had a higher IgG1/IgG2a ratio than the IM group as observed in pre-challenge samples (Fig. 4f). Thus, changes in antibody levels were consistent with protective responses in immunized mice. Overall, these data demonstrate that microneedle vaccination induced similar antibody recall responses compared to IM vaccination.
Antibody-secreting cells (ASC) are partly responsible for recall immune responses that confer protection against influenza infection. Mice challenged 90 days after immunization were examined for influenza IgG ASC in spleen and lungs on day 4 post-infection. In spleen, ASC numbers were elevated in both the microneedle and IM groups; despite lack of noticeable differences between groups, the microneedle group was the only one showing significantly higher numbers of ASC than naïve or infected mice (Fig. 4g, P<0.03). In lungs, we observed that the microneedle and IM groups had 3–5 times higher ASC numbers than unimmunized infected or naïve mice. These results suggest that a skin vaccination route using dissolving microneedles induced sustained humoral immune responses in lungs at least as strong as responses induced by IM immunization (Fig. 4h).
Induction of systemic cytokine responses
We next investigated induction of cellular immune responses systemically upon challenge 90 days post-immunization. We re-stimulated splenocytes isolated from challenged mice on day 4 with HA Class I (HA I) and II (HA II) restricted peptides or inactivated influenza virus for 48 h and 72 h to determine the contribution of CD4+ and CD8+ T lymphocytes secreting interleukin-4 (IL-4) and interferon-γ (IFN-γ) (Fig. 5a,b). IL-4 secretion was higher in the IM group in the presence of Class I or Class II peptides, although increases were more prominent with Class I, suggesting increased CD8+ T cell-derived response (Fig. 5a). In contrast, IFN-γ levels secreted by CD8+ or CD4+ cells were 2 to 3-fold higher in the microneedle group when compared to IM (Fig. 5b). Naïve mice did not show any differences in cytokine levels from unimmunized infected mice (data not shown). Elevated IFN-γ levels in microneedle-immunized mice suggest that microneedle immunization generates a stronger T cell helper type 1 and effector response, which are necessary to promote antibody production and support cytotoxic activity, events that are crucial for viral clearance23.
Fig. 5. Cellular immune responses after challenge.
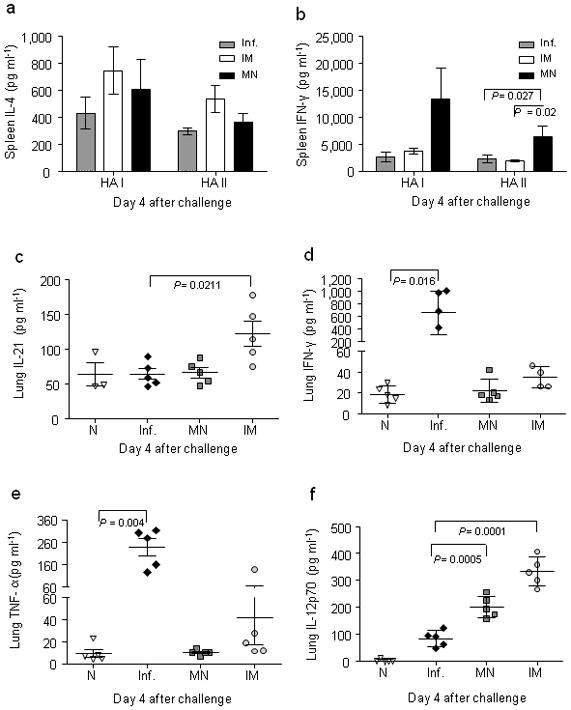
Cellular immune responses were determined in splenocyte cultures and lung suspensions. (a) IL-4 levels determined from 72-h splenocyte culture after isolation from immunized and unimmunized mice at four days after challenge and re-stimulation with HA Class I and II influenza peptides. (b) IFN-γ levels in 72-h splenocyte cultures. (c) Lung IL-21. (d) Lung IFN-γ. (e) Lung TNF-α. (f) Lung IL-12p70. Groups are as described in Fig. 3.
Assessment of cellular immune responses in lungs
To assess cellular immune responses elicited in the mucosal compartment, we re-stimulated lung cell suspensions in vitro with inactivated A/PR/8/34 virus and assessed levels of interleukin-21 (IL-21), IFN-γ, tumor necrosis factor-α (TNF-α), and interleukin-12p70 (IL-12p70). IL-21 is a pleiotropic cytokine known to upregulate genes associated with innate immunity and Th1 responses24 as well as regulating B cell isotype class switching25. It also augments IFN-γ production in vitro when combined with other cytokines26. We found that IL-21 level in lungs of IM-vaccinated mice was significantly higher than other groups (Fig. 5c, P=0.0211), with IFN-γ production correspondingly upregulated in the same group (Fig. 5d). Unimmunized infected mice showed highest IFN-γ and TNF-α levels (Fig. 5d,e), consistent with stronger inflammatory reaction in animals not protected by vaccination. Interestingly, both IM (P<0.0001) and microneedle (P<0.0005) groups had significantly higher IL-12p70 production than naïve or infected groups, which correlates with the high INF-γ which was more prominent in the IM group (Fig. 5f).
Levels of IFN-γ, IL-12p70 and IL-21 induced after polyclonal re-stimulation in lung were higher in the IM compared to microneedle group, which suggests stronger local Th1 response in the MN group upon challenge. In contrast, influenza virus MHC Class I and II restricted T cell responses were increased in spleen of microneedle-immunized groups, indicative of increased recall CD4+ and CD8+ T cell responses systemically. Increased IFN-γ production in the microneedle-immunized group may reflect enhanced generation and maintenance of memory T cells that are responsible for increased virus clearance observed in lungs when compared to the IM group. Overall, these data demonstrate that microneedle immunization can generate a robust cellular and humoral immune response similar to that observed with the conventional IM route, and suggest that microneedle immunization can establish a sustained and broader immune response.
Comparison of dissolving polymer microneedles and coated metal microneedles
As a final set of experiments, we compared dissolving polymer microneedles used in this study to coated metal microneedles used previously13–15 by vaccinating mice using each of these microneedle technologies and measuring humoral and cellular immune responses after two weeks (see Supplementary Study). Humoral immune responses were similar (Supplementary Fig. S1), but cellular responses differed (Supplementary Figs. S2 and S3), most notably shown through increased IL-4 and IFN-γ production from inguinal lymph node cells in response to inactivated influenza virus stimulation in mice vaccinated using dissolving polymer microneedles compared to coated metal microneedles. This result suggests that dissolving microneedles not only offer advantages over IM injection, but also represent an improvement over coated metal microneedles.
Discussion
This study aimed to evaluate use of a simple patch-based vaccination method designed to overcome limitations of hypodermic needle injection, both in terms of targeting skin antigen-presenting cells and avoiding hypodermic needles27,28. We therefore designed, fabricated and analyzed a novel dissolving microneedle patch for dermal vaccination. Because microneedles dissolve in skin’s interstitial fluid, there is no sharps waste, which makes dissolving microneedles impossible to reuse and thereby eliminates risks of biohazardous sharps.
This novel approach incorporates vaccine in a lyophilized form within the structural polymer material of the microneedle, thereby avoiding need for reconstitution before administration. These polymer microneedles dissolve in skin within minutes and are safely eliminated by the body, as evidenced by PVP’s historical use as a plasma expander29. Use of needles measuring just hundreds of microns in length not only eliminates pain10 and enables simple delivery using a thin patch, but also inherently targets antigen to the abundant antigen-presenting cells of skin’s epidermis and dermis3.
Using this approach, this study demonstrates that influenza vaccine delivery with dissolving microneedles can induce robust humoral and cellular immune responses after a single immunization with a low antigen dose that confers protective immunity against lethal viral challenge. Immunologic responses to microneedle vaccination were similar to IM injection by some measures and stronger by others. Overall, microneedle immunization showed improved IgA lung titers, enhanced recall cellular immune responses, increased numbers of antibody-secreting cells and, importantly, more efficient viral clearance.
Although it is possible that dissolving microneedles have strong immunogenicity because of an adjuvant effect caused by PVP, we believe this is unlikely because IM injection of inactivated virus with PVP did not enhance immune response compared to vaccination without PVP (Fig. 2d). It is also possible that skin flora could be drawn into skin during microneedle insertion and thereby serve as an adjuvant. We think this is also unlikely because skin was carefully cleaned before microneedle insertion and because hypodermic needle insertion for IM injection could similarly draw in skin flora.
Thus, dissolving microneedle patches may provide not only practical advantages compared to hypodermic needles, but may also provide better protective immunity. Similar reports in human studies have shown that intradermal immunization can induce primary immune responses that are equivalent or surpass IM delivery of seasonal influenza vaccine with possible dose-sparing effects4–7. Although this study did not assess dose-sparing, the most significant immunologic difference between vaccine delivery using dissolving microneedles versus IM immunization is the 1000-fold more efficient lung virus clearance after microneedle vaccination, which is expected to correlate with reduced morbidity and mortality. Notably this difference was observed upon challenge three months after immunization, suggesting that microneedle immunization induced more robust recall immune responses.
These results may be due to higher numbers of antibody-secreting cells found in spleen and lungs of microneedle-immunized mice as well as enhanced cellular memory responses in spleens, as shown by increased IFN-γ secretion found after in vitro re-stimulation. Cellular immune responses may promote rapid viral clearance from lung and thereby decrease morbidity, for example, via pre-existing CD8+ T cell-mediated immunity directed at peptides from conserved internal proteins of the influenza A virus30. Enhanced production of serum IgG2a antibodies after microneedle vaccination may also reflect the role of humoral immune responses that assist in effective virus clearance. These differences are most likely due to the route of immunization, although antigen formulation, slower release kinetics and other features of the dissolving microneedle delivery system may also play a role.
Immunization via skin may target innate dendritic cell populations directly through lymphatics from proximal draining lymph nodes and simultaneously by activating the rich dendritic cell network that resides in skin. It is well established that the innate immune system has a pivotal role in adaptive immune responses31 possibly accounting for differences we observe between dissolving microneedle patches and IM vaccination32,33. The observed early virus clearance from lungs may be the result of enhanced involvement and mobilization of innate and adaptive cell populations that induce broader humoral and cellular immune responses.
Overall, these results demonstrate that dissolving microneedle patches offer an attractive approach to administer influenza vaccine with improved safety, immunogenicity and logistical operations that may enable increased patient coverage of influenza vaccination. The dissolving microneedle vaccine patch developed in this study also provides a novel platform technology for simple administration of other vaccines and medicines to skin without the need for hypodermic needles.
Methods
Cells and virus stocks
Madin-Darby canine kidney (MDCK) cells (ATCC CCL 34, American Type Culture Collection) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech) containing 10% fetal bovine serum (Hyclone, ThermoFisher Scientific). Influenza virus stocks (A/PR8/34, H1N1) were prepared, purified and inactivated as previously described34. Inactivated influenza virus suspensions in PBS were lyophilized using settings based on a prior study35 and described in Supplementary Methods. Hemagglutination activity was determined using chicken red blood cells (LAMPIRE Biological Laboratories) as previously described36. The mouse-adapted A/PR/8/34 strain was obtained by eight serial passages in lungs of BALB/c mice. LD50 was calculated by the Reed-Muench formula37 and viral titer was determined by plaque assay34.
Polymer microneedle fabrication and encapsulation of influenza vaccine
Dissolving polymer microneedles were created via in situ polymerization of liquid monomer within a microneedle mold, as described previously19. Briefly, a microneedle master structure was created via a lens-based, lithographic microfabrication process. A reusable inverse mold was created by pouring polydimethylsiloxane (PDMS, 184 Dow Corning) over the master structure, allowing it to cure overnight, and carefully peeling the resulting mold off the master structure. We then applied 100 μl of vinylpyrrolidone monomer (VP, 99%, Sigma-Aldrich),, free radical initiator azobisisobutyronitrile (1.0 mol%, AIBN) and inactivated influenza virus (6 mg ml−1) to the mold surface and administered vacuum (−102 kPa) for 1–2 min to pull the solution into the microneedle mold and form the microneedles. Then, a second mixture of 100 μl of vinylpyrrolidone monomer and AIBN initiator (without vaccine) was applied to the surface of the mold to form the patch backing. Finally, the system was placed under a UV lamp (100 W, 300 nm, BLAK RAY) to initiate photopolymerization. After 30 min, the PVP microneedle patch was carefully removed from the mold and stored in a desiccator for up to 30 days.
Antigen stability study
Initial studies were conducted to test the stability of the processed antigen. Four different vaccine preparations were administered IM, as described below, to assess the effect of microneedle fabrication processes on antigen stability in comparison with naïve mice. For the first two groups, 100 μg untreated inactivated influenza virus was re-suspended either alone or in combination with 83 mg of PVP in 1.0 ml water. For the third group, 100 μg lyophilized inactivated influenza virus was re-suspended in 1.0 ml water. For the fourth group, 100 μg lyophilized inactivated influenza virus was encapsulated in a microneedle patch containing 83 mg PVP, which was dissolved in 1.0 ml water. Two weeks after immunization, sera were collected and tested for anti-influenza specific IgG titers, as described below.
Immunizations
Female BALB/c mice (Charles River Laboratory) (11 mice per group, 6–8 weeks old) received a single dose of vaccine by microneedle or IM immunization. For microneedle delivery, two days prior to immunization mice were anesthetized with a ketamine and xylazine cocktail and the dorsal caudal surface was prepared and hair was removed as previously described34. Microneedles were manually inserted into the caudal site of the dorsal surface of the skin, left in place for 15 min, and then removed. Immunization with 6 μg of vaccine was accomplished by inserting two arrays of microneedles at the same time, each encapsulating 3 μg of vaccine. Vaccine dose is reported as the mass of virus protein, which was composed of ~30% HA protein. IM immunization was carried out by injecting 6 μg of the vaccine suspended in 50 μl of PBS into the upper quadrant of the gluteal muscle. Animal studies were approved by the Emory University IACUC.
Challenge of mice with influenza virus
To determine post-challenge survival rates and immune responses, 6 mice per group were challenged 1 month after immunization by intranasal instillation of 50 μl (180 PFU) of live mouse-adapted A/PR/8/34 virus and monitored for 14 days. As a control group, we included 6 unimmunized challenged mice. A weight loss exceeding 25% was used as the experimental end-point, at which mice were euthanized. The challenged mice were monitored daily for signs of morbidity (body weight changes, fever and hunched posture) and mortality.
Characterization of immune response
As described in Supplementary Methods, blood was collected 14 and 28 days after immunization to determine humoral immune responses (total IgG, IgG isotypes and HAI titers). Four days after challenge, blood was collected to determine humoral immune responses; spleens were collected to assay antibody-secreting cells and cytokine expressions levels; and lungs were collected to determine lung virus titers, IgG and IgA titers, antibody-secreting cells and cytokine expression levels.
Supplementary Material
Acknowledgments
This study was carried out at the Emory Vaccine Center and the Georgia Tech Center for Drug Design, Development and Delivery and Institute for Bioengineering and Biosciences. The work was supported in part by U.S. National Institutes of Health grants R01-EB006369 and U01-AI0680003. Sean Sullivan was a trainee supported by a fellowship from the U.S. Department of Education GAANN program. Maria del Pilar Martin was a trainee supported by contract HHSN266200700006C from NIH/NIAID.
M.R.P. declares a competing interest. He serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This possible conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
S.P.S. D.G.K., M.P.M. and I.S. carried out most experimental studies; J.W.L and V.Z prepared microneedles and helped carry out the Supplementary Study; S.P.S, D.G.K., I.S. and M.R.P. designed the study and its analysis; S.P.S., I.S. and M.R.P. wrote the manuscript; and N.M, R.W.C, I.S. and M.R.P. supervised the project.
References
- 1.Influenza activity--United States and worldwide, 2007–08 season. MMWR Morb Mortal Wkly Rep. 2008;57:692–697. [PubMed] [Google Scholar]
- 2.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Top Microbiol Immunol. 2006;304:247–268. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 4.Belshe RB, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 5.Holland D, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme P, et al. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 7.Hickling J, Jones R. Intradermal Delivery of Vaccines: A review of the literature and the potential for development for use in low- and middle income countries. Program for Appropriate Technology in Health (PATH), Ferney Voltaire; France: 2009. [Google Scholar]
- 8.Flynn PM, et al. Influence of needle gauge in Mantoux skin testing. Chest. 1994;106:1463–1465. doi: 10.1378/chest.106.5.1463. [DOI] [PubMed] [Google Scholar]
- 9.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikszta JA, et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 12.Widera G, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Koutsonanos DG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Q, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci U S A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YC, et al. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyano T, et al. Sugar micro needles as transdermic drug delivery system. Biomed Microdevices. 2005;7:185–188. doi: 10.1007/s10544-005-3024-7. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Yoshimitsu J, Shiroyama K, Sugioka N, Takada K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J Drug Target. 2006;14:255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan SP, Murthy N, Prausnitz MR. Minimally invasive protein delivery with rapidly dissolving microneedles. Adv Mat. 2008;20:933–938. doi: 10.1002/adma.200701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson BV. PVP: a critical review of the kinetics and toxicology of polyvinylpurrolidone (povidone) Lewis Publishers; Chelsea, MI: 1990. [Google Scholar]
- 21.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Bronaugh RL, Stewart RF, Congdon ER. Methods for in vitro percutaneous absorption studies II. Animal models for human skin. Toxicol Appl Pharmacol. 1982;62:481–488. doi: 10.1016/0041-008x(82)90149-1. [DOI] [PubMed] [Google Scholar]
- 23.McGill J, Legge KL. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J Immunol. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 26.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MA, Pisani E. The cost of unsafe injections. Bull World Health Organ. 1999;77:808–811. [PMC free article] [PubMed] [Google Scholar]
- 28.Mitragotri S. Immunization without needles. Nat Rev Immun. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 29.Ravin HA, Seligman AM, Fine J. Polyvinyl pyrrolidone as a plasma expander; studies on its excretion, distribution and metabolism. N Engl J Med. 1952;247:921–929. doi: 10.1056/NEJM195212112472403. [DOI] [PubMed] [Google Scholar]
- 30.Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. J Clin Invest. 2008;118:3273–3275. doi: 10.1172/JCI37232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsikis PD, Schoenberger SP, Pulendran B. Probing the ‘labyrinth’ linking the innate and adaptive immune systems. Nat Immunol. 2007;8:899–901. doi: 10.1038/ni0907-899. [DOI] [PubMed] [Google Scholar]
- 32.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29:15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 34.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–6119. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Amorij JP, et al. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine. 2007;25:6447–6457. doi: 10.1016/j.vaccine.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 36.Compans RW. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J Virol. 1974;14:1307–1309. doi: 10.1128/jvi.14.5.1307-1309.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hygiene. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


