Abstract
Rationale
Adhesive interactions between endothelial cells and leukocytes affect leukocyte trafficking in adipose tissue. The role of P-selectin glycoprotein ligand-1 (Psgl-1) in this process is unclear.
Objective
The goal of this study was to determine the effect of Psgl-1 deficiency on adhesive properties of the endothelium and on leukocyte recruitment into obese adipose depots.
Methods and Results
A genetic model of obesity was generated to study the effects of Psgl-1 deficiency on leukocyte trafficking. Leukocyte-endothelial interactions were increased in obese leptin receptor mutant mice (Lepr db/db,Psgl-1+/+), but not obese, Psgl-1 deficient mice (Lepr db/db,Psgl-1−/−), when compared to lean mice (Lepr +/+,Psgl-1+/+). This effect of Psgl-1 deficiency was due to indirect effects of Psgl-1, since Psgl-1+/+ adoptively transferred leukocytes did not exhibit enhanced rolling in Lepr db/db,Psgl-1−/− mice. Additionally, circulating levels of P-selectin, E-selectin, Monocyte chemoattractant protein-1, and macrophage content of visceral adipose tissue were reduced in Leprdb/db,Psgl-1−/− compared to Leprdb/db,Psgl-1+/+ mice. Reduced leukocyte-endothelial interactions and macrophage content of visceral adipose tissue due to Psgl-1 deficiency was also observed in a diet-induced obese mouse model. Psgl-1−/− mice were resistant to the endothelial effects of exogenous IL-1β, suggesting that defective cytokine signaling contributes to the effect of Psgl-1 deficiency on leukocyte-endothelial interactions. Mice deficient in the IL-1 receptor also had reduced levels of circulating P-selectin, similar to those observed in Psgl-1−/− mice.
Conclusions
Deficiency of Psgl-1 is associated with reduced IL-1 receptor-mediated adhesive properties of the endothelium and is protective against visceral fat inflammation in obese mice.
Keywords: inflammation, obesity, endothelium, leukocytes, cell adhesion molecules
INTRODUCTION
Obesity has been characterized as a chronic, low-grade inflammatory disease that is marked by an increase in macrophage content and macrophage activity in adipose tissue 1-4. Adhesive interactions between leukocytes and endothelial cells play a critical role in leukocyte trafficking during inflammatory diseases 5. Animal models of obesity have demonstrated an increase in leukocyte-endothelial interactions in visceral adipose tissue that is mediated by increased endothelial expression of P-selectin (P-sel) and E-selectin (E-sel) 6. The mechanism by which the endothelium is activated in obese adipose tissue is unclear.
P-selectin glycoprotein ligand-1 (Psgl-1) is the primary leukocyte ligand for P-sel 7 and an important ligand for E-sel 8. Leukocytes from Psgl-1−/− mice show reduced selectin-dependent rolling 9, 10 Thus, interactions between Psgl-1 and endothelial selectins may contribute to the influx of macrophages into adipose tissue, which may in turn trigger the chronic low grade inflammatory state associated with obesity 2, 4.
To determine the contribution and mechanism(s) of Psgl-1 towards leukocyte trafficking into adipose tissue, we analyzed leukocyte-endothelial interactions and fat inflammation in genetically and diet-induced obese mice with and without Psgl-1. Our findings indicate that Psgl-1 affects leukocyte trafficking, but that this occurs indirectly via Psgl-1-mediated regulation of endothelial adhesive properties.
MATERIALS AND METHODS
Animals and breeding
Psgl-1−/− mice were from Jackson Laboratory (Bar Harbor, ME) and backcrossed to the C57BL6/J strain for a total of 10 generations before use in crossbreedings. Leprdb/+ mice on the C57BL6/J strain background were purchased from Jackson Laboratory. To generate Leprdb/db,Psgl-1−/− mice, Leprdb/+ males were crossed to Psgl-1−/− females. Leprdb/+,Psgl-1+/− were then crossed to Psgl-1−/− mice to produce Leprdb/+,Psgl-1−/− offspring. These offspring were then intercrossed to generate Leprdb/db,Psgl-1−/− mice. Leprdb/db,Psgl-1+/+ mice were generated from an intercross between Leprdb/+,Psgl-1+/+ parents. IL-1R−/− and C57BL6/J control mice were from Jackson Laboratory.
Mice were fed either a standard laboratory rodent diet (#5001, TestDiet, Richmond, IN) or a high-fat, high-sucrose rodent diet (D12451, 45kcal% fat, Research Diets Inc, New Brunswick, NJ) in specific pathogen-free facilities. For the diet-induced obesity study, Psgl-1−/− and Psgl-1+/+ mice were started on a high-fat, high-sucrose diet at five weeks of age and remained on it for ten weeks. All procedures complied with the Principles of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Analysis of serum factors
Serum samples were collected by retro-orbital bleeding with non-heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA). Circulating levels of soluble P-selectin (sP-sel), soluble E-selectin (sE-sel), monocyte chemoattractant protein (MCP-1), and soluble Intercellular adhesion molecule-1 (sICAM-1) were assayed with murine ELISA kits (R&D systems, Minneapolis, MN) according to manufacturers' instructions. An Ultra Sensitive Rat Insulin Elisa kit (Crystal Chem Inc., Downers Grove, IL) was used to measure overnight fasted insulin levels, and overnight fasted glucose levels were determined using an Ascensia Contour Blood Glucose Meter and Ascensia Contour test strips (Bayer HealthCare LLC, Tarrytown, NY).
Weight and DEXA measurement
Body weight and percent body fat were measured using a Dual-Energy X-ray Absorptiometry (DEXA) scanner Lunar PIXImus2 (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) in diet-induced and genetically obese mice at 15 weeks of age.
Immunohistochemistry
For details, please see Supplemental Materials and Methods. Macrophages in adipose cross-sections were identified with a rat anti-mouse Mac-3 monoclonal antibody (1:100 dilution; BD Biosciences, San Jose, CA.
Real-Time Polymerase Chain Reaction (RTPCR)
For details, please see Supplemental Materials and Methods. P-sel, E-sel, MCP-1, and Tumor necrosis factor-alpha (TNFα) RTPCR primers were used. 7000 System SDS Software and the 2−ΔΔCT method 11 were used to analyze the results.
Intravital microscopy
For details, please see Supplemental Materials and Methods. For adoptive transfer experiments, leukocytes were isolated from whole blood of Psgl-1+/+ mice with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) according to manufacturers' instructions. The isolated leukocytes were resuspended in 1 mL of PBS and then incubated with 100 μl of 10X stock rhodamine 6G for 30 minutes. Leukocytes were centrifuged at 250 × g for 10 minutes. Cells were washed twice in PBS and ultimately resuspended in 1mL PBS. 1×106 leukocytes were then injected into Leprdb/db,Psgl-1−/− and Leprdb/db,Psgl-l+/+ mice.
To determine the type of leukocytes that were adoptively transferred into mice using this protocol, analysis of leukocytes was performed using a Bayer Advia 120 Hematology System.
Bone marrow transplantation
For details, please see Supplemental Materials and Methods. Bone marrow transplantation (BMT) was performed as previously described 12. 8-10 week-old Leprdb/db,Psgl-1+/+ mice were used as recipients for BMT from Psgl-1+/+, Psgl-1−/− or Psel-1−/− donors. Psgl-1−/− mice received marrow from Psgl-1+/+ donors, and Psgl-1+/+ mice received marrow from Psgl-1−/− donors. IL-1R +/+ mice received marrow from IL-1R−/− donors, and IL-1R −/− mice received marrow from IL-1R+/+ donors.
Recombinant IL-1β
For details, please see Supplemental Materials and Methods. Recombinant IL-1β (Peprotech, Rocky Hill, NJ) was injected to mice via tail vein. Mice were bled for serum 5 hours post IL-1β injection.
Immunoblotting
For details, please see Supplemental Materials and Methods. The antibodies for mouse IκBα, phospho-IκBα, and p38 were from Cell Signaling Technology (Danvers, MA).
Statistical analyses
Values are expressed as mean ± SEM. The statistical significance of differences between groups was determined by Student's t test. P values <0.05 were considered significant.
RESULTS
Metabolic profile of diet-induced and genetically obese Psgl-1 deficient mice
Mice deficient in leptin receptor signaling (Leprdb/db,Psgl-1+/+) develop severe obesity 13. No differences between Leprdb/db,Psgl-1+/+ mice and leptin receptor deficient mice without Psgl-1 (Leprdb/db,Psgl-1−/−) in body weight (49.6±0.9 (n=9) vs. 47.7±1 g (n=12), respectively, p=NS) or percent fat mass (56.9±1.1 vs. 56.5±0.5 %, respectively, p=NS) were observed at 15 weeks of age. Similarly, in diet-induced obesity, no differences between Psgl-1+/+ mice and Psgl-1−/− mice in body weight (31.4±1 (n=5) vs. 27.7±2.9 g (n=5), respectively, p=NS) or percent fat mass (30±3 vs. 34.2±4.6 %, respectively, p=NS) were observed at 15 weeks of age after 10 weeks on high-fat, high-sucrose diet.
Fasting glucose levels were significantly reduced in Leprdb/db, Psgl-1−/− mice (n=19) compared to Leprdb/db,Psgl-1+/+ mice (n=11) at 10 weeks of age (280.2±19.1 vs. 430±27.4 mg/dL, respectively, p<0.001), while fasting insulin levels were not different (5.8±0.9 (n=15) vs. 5.1±1 ng/ml (n=19), respectively, p=NS). In the diet-induced obesity model, glucose and insulin levels were not significantly different between Psgl-1+/+ (n=5) and Psgl-1−/− (n=4) mice at 10 weeks of age (glucose: 266±30.7 vs. 227.5±25.1 mg/dL, p=NS; insulin: 0.34±0.1 vs. 0.43±0.2 ng/ml, p=NS).
Effect of Psgl-1 deficiency on obesity-induced leukocyte-endothelial interactions in cremaster venules
Intravital microscopy was used to analyze leukocyte-endothelial interactions in cremaster venules of 15 week old Leprdb/db,Psgl-1+/+ mice. Leukocyte rolling was markedly increased in Leprdb/db,Psgl-1+/+ mice (n=4) compared to lean wild-type mice Psgl-1+/+ (n=4) (108.7±14.4 vs. 40.8±11.8 cells/mm, respectively, p<0.01). Leprdb/db,Psgl-1−/− mice (n=4) exhibited significantly reduced rolling compared to Leprdb/db,Psgl-1+/+ mice (9.1±2.2 vs. 108.7±14.4 cells/mm, respectively, p<0.001). No significant difference in firm attachment was observed in Leprdb/db,Psgl-1−/− mice compared to Leprdb/db,Psgl-1+/+ mice (17.2±2.9 vs. 30.6±6.9 cells/mm, respectively, p=0.1).
In diet-induced obesity, Psgl-1−/− mice exhibited significantly reduced rolling and firm attachment compared to Psgl-1+/+ mice (rolling: 5.3±1.2 vs. 35.5±8 cells/mm, respectively, p<0.01; firm attachment: 16.4±4.5 vs. 54.7±8.9 cells/mm, respectively, p<0.01).
Circulating levels of P-selectin, E-selectin, and MCP-1 in obese Psgl-1 deficient mice
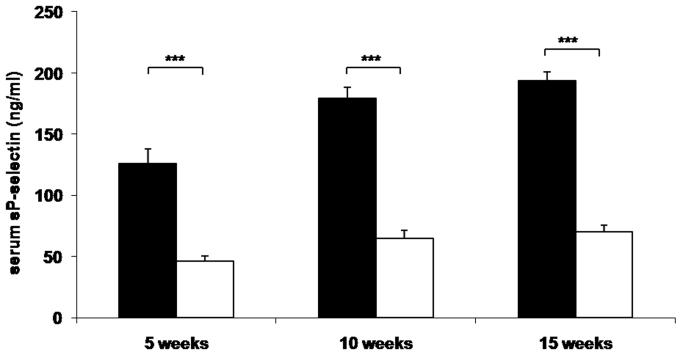
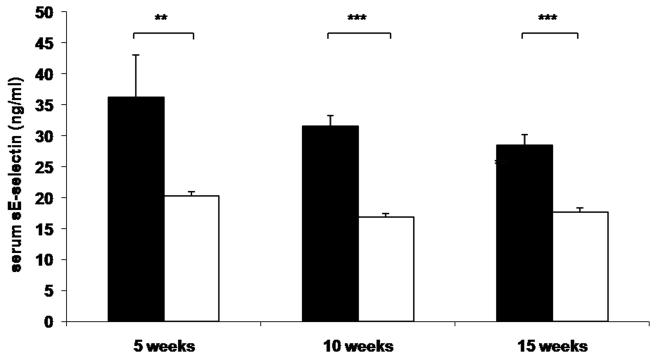
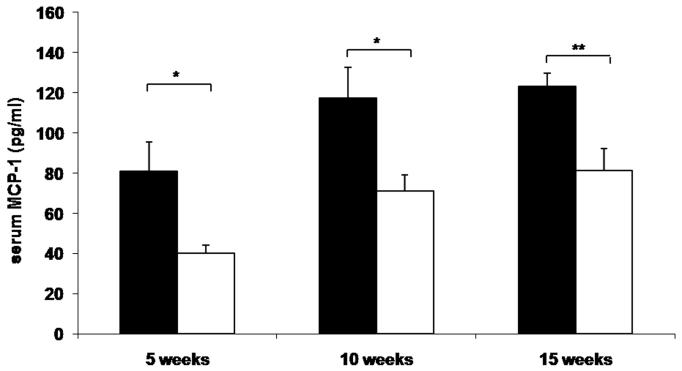
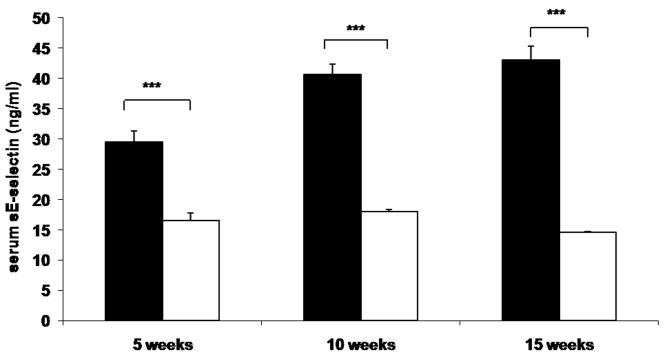
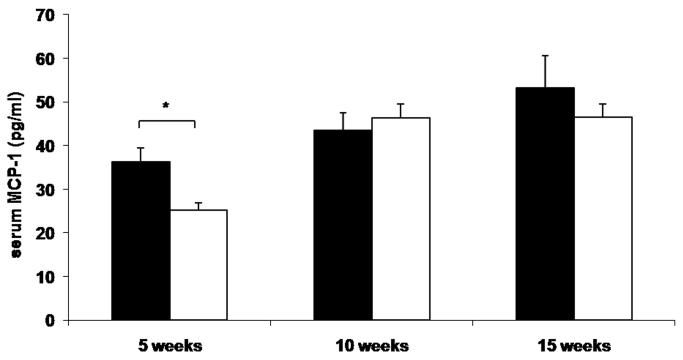
To determine whether reduced leukocyte-endothelial interactions in genetically and diet-induced Psgl-1 deficient mice were associated with reduced circulating levels of soluble adhesion molecules and MCP-1, sP-sel, sE-sel, and MCP-1 were measured from Leprdb/db,Psgl-1−/−, Leprdb/db,Psgl-1+/+ mice, and from Psgl-1−/− and Psgl-1+/+ mice on a high-fat, high-sucrose diet. At 5, 10, and 15 weeks of age, the levels of sP-sel, sE-sel, and MCP-1 were significantly reduced in Leprdb/db,Psgl-1−/− compared to Leprdb/db,Psgl-1+/+ mice (Figure 1A-C). Similarly, in diet-induced obesity at 5, 10, and 15 weeks of age, the levels of sP-sel and sE-sel were significantly reduced in Psgl-1−/− compared to Psgl-1+/+ mice (Figure 1D-E). MCP-1 was significantly different between Psgl-1−/− and Psgl-1+/+ mice at 5 weeks of age (Figure 1F).
Figure 1. Circulating sP-sel, sE-sel, and MCP-1 at 5, 10, and 15 weeks of age.
Leprdb/db,Psgl-1+/+ (black bars) and Leprdb/db,Psgl-1−/− mice (white bars). Five week time point for Leprdb/db,Psgl-1+/+ mice (n=5-7). All other time points for Leprdb/db,Psgl-1+/+ and Leprdb/db, Psgl-1−/− mice (n=7-13). A) sP-sel levels. B) sE-sel levels. C) MCP-1 levels. Psgl-1+/+ (black bars) (n=5) and Psgl-1−/− mice (white bars) (n=3-5) on high-fat, high-sucrose diet. D) sP-sel levels. E) sE-sel levels. F) MCP-1 levels. *p<0.05, **p<0.01, ***p<0.001.
Effect of Psgl-1 deficiency on leukocyte recruitment and inflammatory cytokine expression in adipose tissue in obese Psgl-1 deficient mice
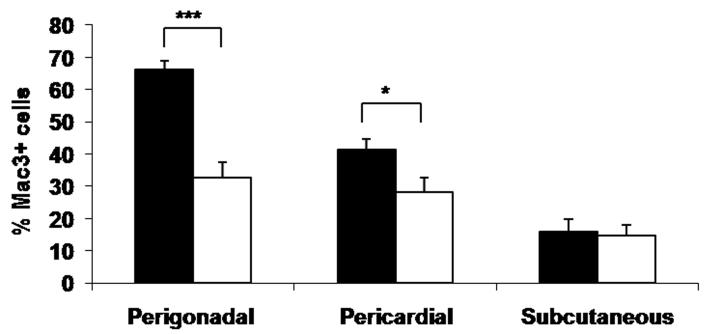
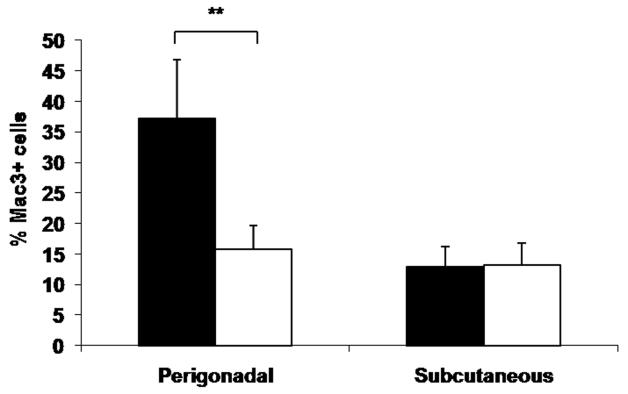
To determine if the decreased leukocyte-endothelial interactions observed in Leprdb/db,Psgl-1−/− mice compared to Leprdb/db,Psgl-1+/+ mice were associated with reduced adipose tissue macrophage content, macrophage immunostaining was performed on visceral (perigonadal and pericardial) and subcutaneous adipose depots retrieved from 15 week old obese Leprdb/db,Psgl-1−/− and Leprdb/db,Psgl-1+/+ mice. The macrophage content of perigonadal and pericardial fat was significantly reduced in Leprdb/db,Psgl-1−/− mice compared to Leprdb/db,Psgl-1+/+ mice (Figure 2A, Online Figure I A-F). No significant difference in macrophage content was observed in subcutaneous fat between Leprdb/db,Psgl-1−/− and Leprdb/db,Psgl-1+/+ mice (Figure 2A, Online Figure I A-F). Similarly, Psgl-1−/− mice on a high-fat, high-sucrose diet for 10 weeks had a significant reduction in macrophage content of perigonadal fat compared to Psgl-1+/+ control mice (Figure 2B-F). No significant difference in macrophage content was observed in subcutaneous fat between these mice (Figure 2B-F).
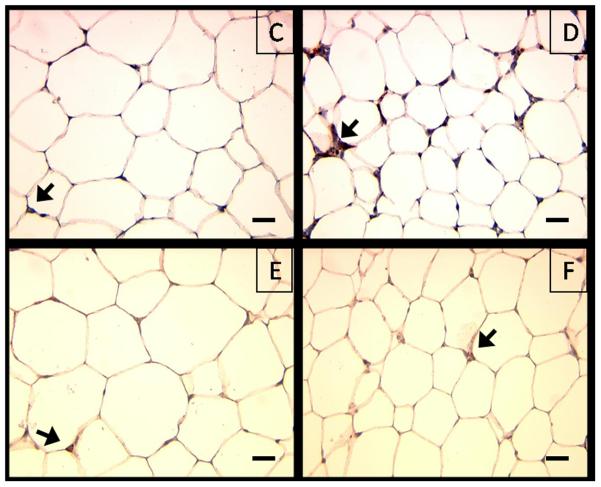
Figure 2. Macrophage content of perigonadal, pericardial, and subcutaneous adipose tissue.
A) Macrophage content of perigonadal (n=7-10), pericardial (n=7-8), and subcutaneous (n=5-8) adipose tissue in Leprdb/db,Psgl-1+/+ (black bars) and Leprdb/db,Psgl-1−/− mice (white bars) at 15 weeks of age. B) Macrophage content of perigonadal and subcutaneous adipose tissue in Psgl-1+/+ (black bars) (n=4) and Psgl-1−/− mice (white bars) (n=5) after 10 weeks of high-fat, high-sucrose diet at 15 weeks of age. Representative cross sections of perigonadal adipose tissue from C) Psgl-1−/− and D) Psgl-1+/+ mice, and subcutaneous adipose tissue from E) Psgl-1−/− and F) Psgl-1+/+ mice 10 weeks after high-fat, high-sucrose diet. Staining with Mac3 antibody, magnification 40X, scale bar 200 μm, arrows showing stained cells. *p<0.05, **p<0.01, ***p<0.001.
To determine the effect that Psgl-1 deficiency in obesity has on macrophage infiltration in different adipose depots compared to lean mice, macrophage content of subcutaneous and perigonadal fat in Leprdb/db,Psgl-1−/− and Leprdb/db,Psgl-1+/+ mice was compared to lean Psgl-1−/− and Psgl-1+/+ mice. There was no difference in macrophage content of perigonadal fat in Psgl-1−/− (n=5) versus Psgl-1+/+ (n=3) lean mice (14.9±1.0 vs. 16.4±0.8 % mac3+ cells, respectively, p=NS). There was also no difference in macrophage content of subcutaneous fat in Psgl-1−/− (n=4) versus Psgl-1+/+ (n=3) lean mice (9.7±0.6 vs. 8.9±0.1 % mac3+ cells, respectively, p=NS). Both lean Psgl-1−/− and Psgl-1+/+ had a significantly higher macrophage content in perigonadal fat compared to subcutaneous fat (Psgl-1−/− : 14.9±1.0 vs. 9.7±0.6 % mac3+ cells, respectively, p<0.01; Psgl-1+/+: 16.4±0.8 vs. 8.9±0.1 % mac3+ cells, respectively, p<0.001). There was no difference in macrophage content of subcutaneous fat in Psgl-1−/− compared to Leprdb/db,Psgl-1−/− mice (9.7±0.6 vs.14.8±3.2 % mac3+ cells, respectively, p=NS) or Psgl-1+/+ compared to Leprdb/db,Psgl-1+/+ mice (8.9±0.1 vs. 15.8±3.9 % mac3+ cells, respectively, p=NS). There was a significantly higher macrophage content in perigonadal fat in Leprdb/db,Psgl-1−/− compared to Psgl-1−/− mice (32.8±4.7 vs. 14.9±1.0 % mac3+ cells, respectively, p<0.05) and in Leprdb/db,Psgl-1+/+ compared to Psgl-1+/+ mice (66.1±2.7 vs. 16.4±0.8 % mac3+ cells, respectively, p<0.001); however, the rise in macrophage infiltration into perigonadal fat with obesity was significantly higher between Leprdb/db,Psgl-1+/+ and Psgl-1+/+ mice compared to Leprdb/db,Psgl-1−/− and Psgl-1−/− mice (49.8±2.7 vs. 17.8±4.7 % increase in mac3+ cells, respectively, p<0.001).
The expression of MCP-1 and TNFα was measured in perigonadal adipose tissue retrieved from 15 week old obese Leprdb/db,Psgl-1−/− (n=14) and Leprdb/db,Psgl-1+/+ (n=13) mice. Leprdb/db,Psgl-1−/− mice had reduced expression of MCP-1 and TNFα in perigonadal adipose tissue compared to Leprdb/db,Psgl-1+/+ controls (MCP-1: 2.33±0.3 vs. 4.05±0.7 relative expression units, respectively, p<0.05; TNFα: 3.4±0.4 vs. 5.84±0.6 relative expression units, respectively, p<0.01).
Effect of adoptive transfer of wild-type leukocytes into obese Psgl-1 deficient mice
To determine whether the effect of Psgl-1 on leukocyte rolling was due to lack of direct, acute Psgl-1 interactions with endothelial selectins or due to an indirect, more chronic effect of Psgl-1 on adhesive properties of the endothelium, adoptive transfer of rhodamine-labeled leukocytes from Psgl-1+/+ donors into Leprdb/db,Psgl-1−/− (n=3) and Leprdb/db,Psgl-1+/+ (n=5) recipients was performed. Transferred leukocytes consisted of 91.5±3.3% lymphocytes, 0.65±0.5% monocytes, and 5.5±3% neutrophils (n=3).
Control adoptive transfer experiments showed that rhodamine-labeled Psgl-1+/+ donor leukocytes infused into Leprdb/db,Psgl-1+/+ mice exhibited similar leukocyte rolling characteristics as endogenous rhodamine-labeled Leprdb/db,Psgl-1+/+ leukocytes (96.8±12.9 vs. 108.7±14.4 cells/mm, respectively, p=NS). When rhodamine-labeled Psgl-1+/+ donor leukocytes were infused into Leprdb/db,Psgl-1−/− mice, the donor leukocyte rolling was markedly less than that observed when Psgl-1+/+ donor leukocytes were infused into Leprdb/db,Psgl-1+/+ mice (2.1±0.7 vs. 96.8±12.9 cells/mm, respectively, p<0.001). The donor leukocyte firm attachment was also less in Leprdb/db,Psgl-1−/− mice compared to Leprdb/db, Psgl-1+/+ mice (8.8±2.7 vs. 18±2.9 cells/mm, respectively, p<0.05).
Effect of bone marrow derived Psgl-1 on sP-sel levels and leukocyte-endothelial interactions in obese mice
Since the adoptive transfer experiments suggested that Psgl-1 deficiency affects the activation state of the endothelium, BMT experiments were performed to identify the potential source of Psgl-1 responsible for regulating the endothelium. The BMT involved transplanting Psgl-1+/+ or Psgl-1−/− bone marrow into Leprdb/db,Psgl-1+/+ recipients. To identify the contribution of the hematopoietic Psgl-1 pool on the generation of sP-sel in obesity, sP-sel was measured four weeks after BMT. sP-sel was markedly reduced in Leprdb/db,Psgl-1+/+ mice receiving Psgl-1−/− marrow (n=6) compared to Leprdb/db,Psgl-1+/+ mice receiving Psgl-1+/+ marrow (n=3) (45.7±3.8 vs. 166.5±5.7 ng/ml, respectively, p<0.001).
Ten weeks post-BMT, the extent of leukocyte-endothelial interactions was measured in these mice using intravital microscopy. Leukocyte rolling and firm attachment was markedly reduced in Leprdb/db,Psgl-1+/+ mice receiving Psgl-1−/− marrow (n=5) compared to Leprdb/db,Psgl-1+/+ mice receiving Psgl-1+/+ marrow (n=4) (rolling: 3.6±1.1 vs. 87.0±7.7 cells/mm, respectively, p<0.001; firm attachment: 9.0±2.2 vs. 36.0±6.3 cells/mm, respectively, p<0.001). These results implicate the hematopoietic Psgl-1 in regulating the activation state of the endothelium.
Since both platelets and endothelial cells are rich sources of P-sel, bone marrow transplants were performed from wild-type (Psel+/+) or P-sel deficient (Psel−/−) donors into Leprdb/db,Psgl-1+/+ recipients to determine the predominant cellular source of sP-sel. Circulating sP-sel levels in obese mice receiving Psel−/− bone marrow (n=6) were not different from mice receiving Psel+/+ marrow (153.2±3.7 vs. 166.5±5.7 ng/ml, respectively, p=NS), implicating the endothelium as the major source of sP-sel in obese mice.
Psgl-1 regulates response to recombinant IL-1β
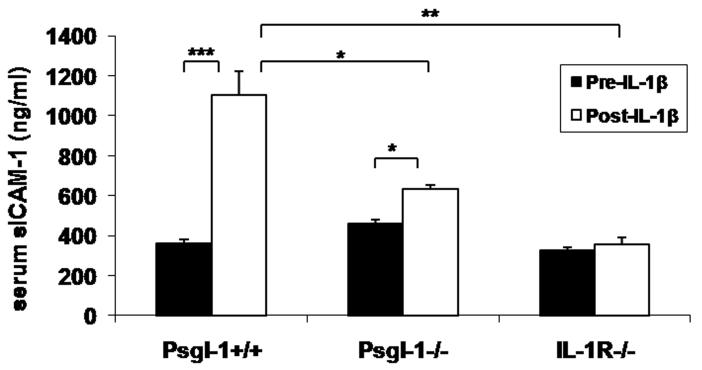
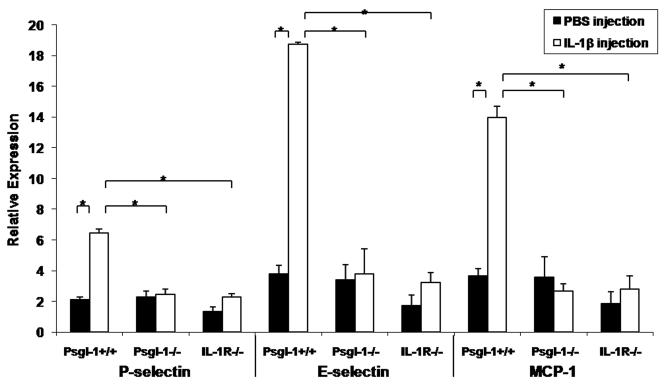
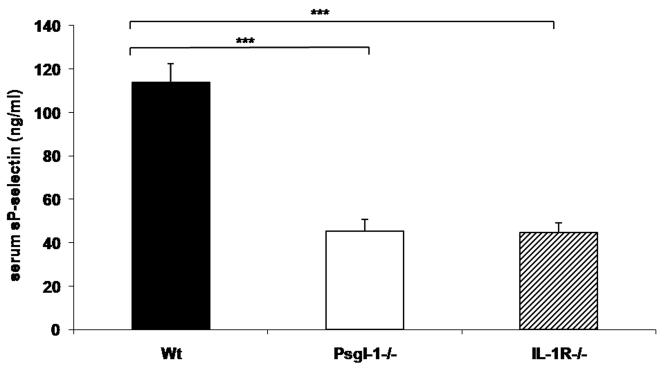
Since IL-1β has previously been shown to induce selectin-dependent leukocyte rolling 14, we explored the possibility that leukocyte Psgl-1 might affect endothelial expression of selectins and MCP-1 in response to IL-1β stimulation. Therefore, either Psgl-1+/+ or Psgl-1−/− mice were stimulated with recombinant IL-1β. As a control, mice deficient in the IL-1 receptor (IL-1R−/−) were also analyzed for potential non-specific effects of the recombinant IL-1β preparation. Five hours after an intravenous injection of recombinant IL-1β (500 ng), sP-sel, MCP-1, sE-sel, and sICAM-1 levels were measured from serum. IL-1β-induced serum levels of sP-sel, sE-sel, MCP-1, and sICAM-1 were completely blocked in IL-1R−/− mice, as expected (Figure 3A-D). Compared to serum from Psgl-1+/+ mice, IL-1β-induced changes in Psgl-1−/− mice were either completely blocked (sP-sel, MCP-1) or reduced (sE-sel and sICAM-1) (Figure 3A-D). Surprisingly, basal levels of sP-sel were reduced in IL-1R−/− mice similar to that observed in the Psgl-1−/− mice, indicating that even basal sP-sel levels are regulated via the IL-1 receptor (IL-1R) (Figure 4).
Figure 3. Circulating sP-sel, sE-sel, MCP-1, and sICAM-1 and expression of P-sel, E-sel, and MCP-1 in lung and perigonadal fat 5 hours following IL-1β injection.
Psgl-1+/+ (n=4), Psgl-1−/− (n=3), and IL-lR−/− mice (n=3). A) sP-sel levels, B) sE-sel levels, C) MCP-1 levels, and D) sICAM-1 levels in Psgl-1+/+, Psgl-1−/−, and IL-lR−/− mice before IL-1β challenge (black bar) and after IL-1β challenge (white bar). Expression of P-sel, E-sel, and MCP-1 in Psgl-1+/+, Psgl-1−/−, and IL-lR−/− in E) lung and in F) perigonadal fat following PBS injection (black bar) and following IL-1β challenge (white bar). * p<0.05, **p<0.01, ***p<0.001.
Figure 4. Circulating levels of sP-sel in mice at baseline.
Wild-type (Wt) (black bar) (n=11), Psgl-1−/− (white bar) (n=10), and IL-1R−/− mice (diagonal striped bar) (n=15) ***p<0.001.
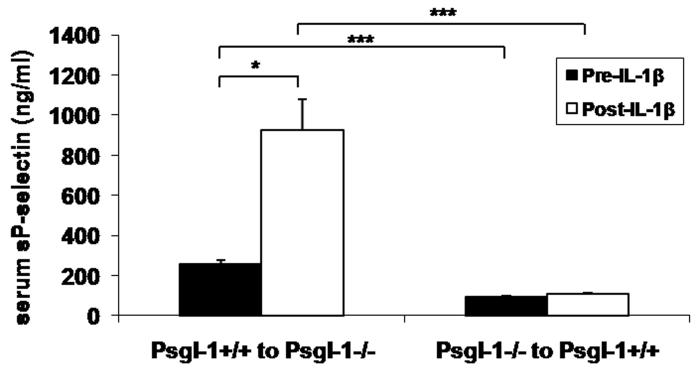
To confirm that Psgl-1 deficiency in the setting of obesity was also associated with resistance to IL-1β, Leprdb/db,Psgl-1−/− mice (n=4) and Leprdb/db,Psgl-1+/+ mice (n=3) were given 500 ng of recombinant IL-1β. Similar to lean mice, there was no increase in sP-sel in Leprdb/db,Psgl-1−/− mice after IL-1β injection (83.3±14.1 pre-IL-1β vs. 83.9±3.5 ng/ml post-IL-1β, p=NS), while there was a marked increase in sP-sel in Leprdb/db,Psgl-1+/+ mice (183.2±3.5 pre-IL-1β vs. 533.1±38.3 ng/ml post-IL-1β, p<0.001).
To determine the effect of Psgl-1 deficiency on transcriptional regulation of endothelial selectins (P-sel and E-sel) and MCP-1 following stimulation with recombinant IL-1β, lung tissue (as a rich source of endothelial cells) and perigonadal fat were removed for RNA isolation five hours following intravenous IL-1β challenge.
Psgl-1+/+ mice injected with IL-1β had a higher expression of P-sel, E-sel, and MCP-1 in the lung compared to Psgl-1+/+ mice injected with PBS (Figure 3E). There was no difference in P-sel, E-sel, and MCP-1 expression in the lung between Psgl-1−/− and IL-1R−/− mice injected with IL-1β compared to Psgl-1−/− and IL-1R−/− mice injected with PBS (Figure 3E). Psgl-1+/+ mice injected with IL-1β also had a higher expression of P-sel and MCP-1 and a trend toward higher expression of E-sel in perigonadal fat compared to Psgl-1+/+ mice injected with PBS (Figure 3F). There was no difference in P-sel, E-sel, and MCP-1 expression in perigonadal fat among Psgl-1−/− and IL-1R−/− mice injected with IL-1β compared to Psgl-1−/− and IL-1R−/− mice injected with PBS (Figure 3F).
Psgl-1−/− mice were also resistant to the effects of recombinant IL-1β on leukocyte rolling compared to Psgl-1+/+ mice (2.8±1 vs. 88.5±10.5 cells/mm, respectively, p<0.001).
Relevant IL-1R pools for regulation of endothelial selectins
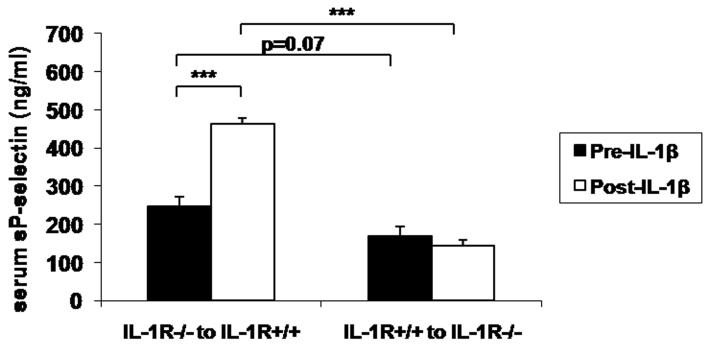
To further characterize the cellular pool of IL-1R relevant for the response to IL-1β, chimeric IL-1R−/− mice were generated by BMT. Following IL-1β challenge, sP-sel levels increased in IL-1R+/+ mice with IL-1R−/− marrow and did not change in IL-1R−/− mice with IL-1R+/+ marrow, indicating that P-sel, E-sel, and MCP-1 expression is regulated via non-hematopoietic IL-1R signaling (Figure 5A).
Figure 5. Relevant source of IL-1R and Psgl-1 for endothelial selectin regulation.
A) sP-sel levels in IL-1R+/+ mice that had received IL-1R−/− bone marrow (n=4) and IL-1R−/− mice that had received IL-1R+/+ bone marrow (n=6) before IL-1β injection (black bars) and 5 hours following IL-1β injection (white bars). B) sP-sel levels in Psgl-1−/− mice that had received Psgl-1+/+ marrow (n=3) and Psgl-1+/+ mice that had received Psgl-1−/− marrow (n=6) before IL-1β injection (black bars) and 5 hours following IL-1β injection (white bars). *p<0.05. ***p<0.001.
To address the cellular Psgl-1 pools relevant for the response to IL-1β, chimeric Psgl-1−/− mice were also generated by BMT. Following IL-1β challenge, sP-sel levels increased in Psgl-1−/− mice with Psgl-1+/+ marrow and did not change in Psgl-1+/+ mice with Psgl-1−/− marrow, indicating that hematopoietic Psgl-1 is regulating the endothelial response to IL-1β via endothelial IL-1R signaling (Figure 5B).
Psgl-1 effects on NFκB activation
IκBα retains NFκB dimers in the cytoplasm. Upon stimulation IκBα is rapidly phosphorylated, a signal that promotes its degradation by the proteasome, thus allowing NFκB dimers to shuttle to the nucleus and initiate gene transcription events 15. Therefore, phosphorylation and degradation of IκBα indicates activation of the NFκB signaling pathway.
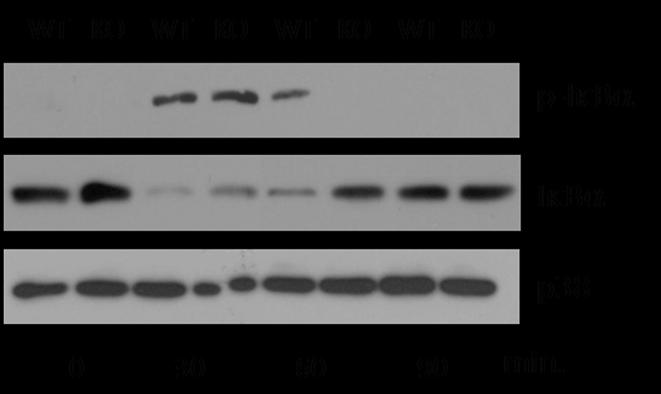
To determine if resistance to the endothelial effects of exogenous IL-1β in Psgl-1−/− mice was associated with reduced signaling via NFkB activation pathways, immunoblotting of lung tissue lysate was performed at various time points following IL-1β administration. Lung lysates from Psgl-1−/− mice exhibited less phosphorylation of IκBα 60 minutes following IL-1β challenge, while lysates from Psgl-1+/+ mice exhibited more degradation of IκBα 30 and 60 minutes following IL-1β challenge, indicating reduced activation of the NFκB signaling pathway in Psgl-1−/− mice (Figure 6). p38 was measured as a loading control. Taken together, Psgl-1 deficiency is associated with reduced activation of NFκB in response to IL-1β and reduced expression of MCP-1, P-sel, and E-sel.
Figure 6. Activation of NFκB in Psgl-1+/+ and Psgl-1−/− mice following IL-1β challenge.
Extracts from total lung homogenates from Psgl-1+/+ (WT) and Psgl-1−/− (KO) mice were probed with antibodies that detect total and phosphorylated IκBα or p38.
DISCUSSION
Adipose tissue inflammation may contribute to comorbidities associated with obesity 2, 4. Leukocyte-endothelial interactions are increased in visceral adipose tissue depots in obese mice 6. These increased leukocyte-endothelial interactions are associated with increased expression of P- and E-sel in endothelial cells from visceral adipose depots 6. Furthermore, antibody blockade of P- and E-sel reduced leukocyte rolling and firm attachment in obese visceral adipose tissue 6. Psgl-1 is the primary ligand for P-sel 7 and a major ligand for E-sel 8. Leukocytes from Psgl-1−/− mice show reduced selectin-dependent rolling 9, 10
To further explore the implications of selectin-ligand interactions in adipose tissue inflammation, we studied Psgl-1 deficiency in genetic and diet-induced mouse models of obesity. Leprdb/db mice lack leptin receptor signaling and become obese and hyperglycemic at an early age 13. Obese mice lacking leptin or a functional leptin receptor have been shown to develop increased macrophage fat infiltration compared to lean mice 2. Thus, this model is suitable for studying factors involved in obesity-related adipose tissue macrophage trafficking 6. In the current study, Leprdb/db,Psgl-1−/− mice and Psgl-1−/− mice on a high-fat, high-sucrose diet developed normally up to 15 weeks of age, with a similar gain in body weight and fat mass, compared to Leprdb/db,Psgl-1+/+ mice and Psgl-1+/+ mice on a high-fat, high-sucrose diet, respectively. To determine if deficiency of Psgl-1 affected leukocyte-endothelial interactions in obesity, we first needed to establish a model where interactions between leukocytes and endothelial cells were increased in the setting of obesity. Nishimura et al. 6 previously demonstrated that leukocyte-endothelial interactions were increased locally within visceral adipose depots in obese mice. In the current study, we found that obesity also induces marked increases in leukocyte rolling in cremaster venules of obese Leprdb/db,Psgl+/+ mice at 15 weeks of age compared to lean Lepr+/+,Psgl-1+/+ mice. Thus, the effect of obesity on leukocyte-endothelial interactions may be more systemic than previously suggested, although we cannot rule out a local effect of the adipose tissue surrounding the cremasteric vessels. Irrespective of the mechanism, the cremaster vessels appear to be suitable to study leukocyte-endothelial interactions triggered by obesity in mice and are more suitable for analysis using standard intravital microscopy techniques. We found that the effect of genetic and diet-induced obesity on leukocyte-endothelial rolling was neutralized in the setting of Psgl-1 deficiency suggesting that interactions between Psgl-1 and endothelial selectins are important for obesity-induced leukocyte rolling.
To determine if the decreased leukocyte-endothelial interactions observed in genetically or diet-induced obese Psgl-1−/− mice were associated with reduced adipose tissue macrophage content, macrophage immunostaining was performed on visceral and subcutaneous adipose tissue from those mice. Psgl-1 deficiency in both models of obesity resulted in reduced macrophage content in visceral adipose tissue, while no differences were observed in subcutaneous adipose tissue. Macrophage content was also analyzed in visceral and subcutaneous adipose tissue from lean Psgl-1−/− and Psgl-1+/+ mice. Psgl-1 deficiency was found to decrease the obesity induced rise in macrophage infiltration found in visceral fat. Obesity did not result in an increase in macrophage infiltration into subcutaneous fat, and Psgl-1 deficiency had no effect on macrophage content in subcutaneous fat in lean or obese mice, indicating differential regulation of adipose tissue macrophage infiltration between different adipose depots.
MCP-1 and TNFα expression has been shown to increase in visceral fat from obese mice 16, 17. Psgl-1 deficiency in obese mice was associated with reduced MCP-1 and TNFα expression in visceral fat, perhaps due to decreased macrophage content in visceral fat.
Since sP-sel levels are positively regulated by leukocyte Psgl-1 in the basal state 18, we determined if sP- and sE-sel levels in both genetic and diet-induced obesity were also reduced in the setting of Psgl-1 deficiency. In this study, sP-sel and sE-sel were lower in Leprdb/db,Psgl-1−/− mice and Psgl-1−/− mice on a high-fat, high-sucrose diet compared to Leprdb/db,Psgl-1+/+ mice and Psgl-1+/+ mice on a high-fat, high-sucrose diet, respectively. MCP-1 was also lower in Leprdb/db,Psgl-1−/− mice compared to Leprdb/db,Psgl-1+/+ mice. Collectively, these data indicate a regulatory role of Psgl-1 towards the generation of these soluble molecules in obesity.
To determine whether the effects of Psgl-1 on leukocyte-endothelial interactions in obesity were due to direct, acute interactions with endothelial selectins or to other, more chronic, effects of Psgl-1 on endothelial adhesive characteristics, adoptive transfer of rhodamine-labeled leukocytes was performed from Psgl-1+/+ mice into obese Leprdb/db,Psgl-1+/+ and Leprdb/db,Psgl-1−/− mice. Unexpectedly, donor Psgl-1+/+ leukocyte rolling was markedly reduced in the Leprdb/db,Psgl-1−/− recipients compared to the Leprdb/db,Psgl-1+/+ recipients. One explanation for these findings is that leukocyte Psgl-1 chronically upregulates endothelial adhesive molecules by an unclear mechanism.
BMT was performed to determine the cellular source of Psgl-1 in obese mice responsible for altering the adhesive properties of the endothelium. Genetically obese mice receiving bone marrow from Psgl-1−/− mice displayed reduced sP-sel levels in addition to reduced rolling and firm attachment compared to genetically obese mice receiving wild-type bone marrow, indicating that hematopoietic Psgl-1 is responsible for chronically altering the adhesive characteristics of the endothelium. BMT with P-sel deficient donors indicated the predominant tissue source of sP-sel in the obese state was non-hematopoietic.
The findings above suggested that hematopoietic Psgl-1 plays a major role in regulating adhesive characteristics of the endothelium in obesity. Since cytokines, such as IL-1β, have been shown to induce selectin dependent rolling 14, 19, we tested the hypothesis that Psgl-1 would positively regulate the endothelial response to IL-1β, while mice deficient in Psgl-1 would be resistant to IL-1β stimulation. Consistent with this hypothesis, sP-sel and MCP-1 levels in wild-type mice markedly increased following administration of recombinant IL-1β, while sP-sel and MCP-1 levels in Psgl-1−/− mice were unchanged following IL-1β stimulation. The rises in sE-sel and sICAM-1 were also attenuated in Psgl-1−/− mice following IL-1β treatment. The reduced circulating levels of these proteins were associated with corresponding reduced gene transcription in lung and perigonadal adipose tissue. The cell source of the transcriptional differences were attributed to the endothelial cells within lung and perigonadal adipose tissue because the factors measured were either exclusively or primarily expressed by the endothelium 18, 20-23. Lung tissue was used for transcriptional studies because it is a rich source of endothelial cells, and inflammation in the lung is regulated by IL-1β 24. Overall, the response of Psgl-1−/− mice to IL-1β was similar to that of mice with complete deficiency of the IL-1R. To further confirm the critical role of IL-1R signaling for regulation of sP-sel under basal, unstimulated conditions, sP-sel levels were markedly reduced in IL-1R−/− mice.
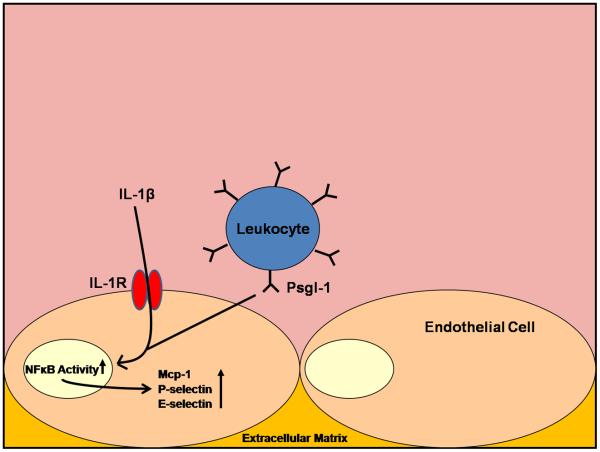
Although several cell types have been shown to express Psgl-1 and the IL-1R, the BMT experiments between Psgl-1−/− mice and Psgl-1+/+ mice and between IL-1R−/− and IL-1R+/+ mice indicate that hematopoietic Psgl-1 (i.e.leukocyte) is regulating the non-hematopoietic IL-1R signaling (i.e. endothelium). The precise cellular source and further downstream mechanism(s) by which Psgl-1 regulates the adhesive properties of the endothelium will require additional study. However, it appears that the presence of Psgl-1 facilitates or the deficiency of Psgl-1 induces a block in pathways leading to NFκB activation, which are likely responsible for the subsequent changes in adhesion molecule expression (Figure 7).
Figure 7.
Hematopoietic Psgl-1 regulates endothelial response to IL-1β
The effect of Psgl-1 on glucoregulation in genetically obese mice may be secondary to the reduced macrophage content of visceral adipose depots as preclinical studies have demonstrated that inflammatory fat leads to insulin resistance 25. This effect on glucose was not observed in the diet-induced obese mice suggesting a more severe state of diabetes may be required to reveal this protective effect of Psgl-1 deficiency on hyperglycemia. Other potential mechanisms for improved glucoregulation may involve IL-1R signaling effects on pancreatic beta cell insulin secretion 26. Of clinical interest, trials with IL-1R inhibitors are associated with improved glucoregulation in humans 27.
Taken together these results indicate an important role of hematopoietic Psgl-1 deficiency in the regulation of the adhesive properties of the endothelium via attenuation of endothelial signaling pathways. As a result, Psgl-1 deficiency is protective against visceral adipose tissue inflammation in obesity. Further elucidation of mechanisms by which leukocytes regulate the endothelial response to cytokines may lead to new treatments aimed at reducing the chronic inflammatory state associated with obesity.
NOVELTY AND SIGNIFICANCE.
What Is Known?
P-selectin glycoprotein ligand-1(Psgl-1) is a ligand on white blood cells that mediates binding to adhesion molecules on endothelial cells and other cell types
Deficiency of Psgl-1 reduces inflammation in some disease states
Obesity is associated with inflammation in visceral fat depots
What New Information Does This Article Contribute?
Deficiency of Psgl-1 leads to reduced visceral fat inflammation in obesity
Psgl-1 plays an important role in controlling adhesive interactions between blood cells and endothelial cells through an indirect mechanism
Psgl-1 deficiency leads to reduced endothelial activation in response to cytokine stimulation
Both Psgl-1 deficient mice and mice deficient in the IL-1 receptor have similarly low levels of circulating P-selectin in the basal state
Inflammation in obese visceral fat may contribute to the adverse consequences of obesity. Identification of the factors responsible for fat inflammation may lead to new treatments aimed at reducing the risks associated with obesity. This study indicates that a leukocyte ligand, Psgl-1, plays an important role in visceral fat inflammation. Furthermore, the absence of Psgl-1 on blood cells leads to reduced activation of the endothelial cells following cytokine stimulation. This is associated with reduced transcription of some adhesion molecules and reduced leukocyte rolling on endothelial cells. Therapeutic antagonism of Psgl-1 may be beneficial in reducing the chronic inflammatory state associated with obesity. In addition, elucidation of the precise mechanism by which Psgl-1 affects adhesive properties of endothelial cells may lead to new targets for the treatment of inflammatory disease states.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This study was supported by NIH grants HL57346 and HL073150 (D. Eitzman); by a Fellowship from the Arthritis Foundation (L. Franchi).
Non-standard Abbreviations and Acronyms
- DEXA
dual energy x-ray absorptiometry
- Psgl-1
P-selectin glycoprotein ligand-1
- MCP-1
Monocyte chemoattractant protein-1
- TNFα
Tumor necrosis factor-alpha
- IL-1β
Interleukin-1 beta
- IL-1R
Interleukin-1 receptor
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-Related Upregulation of Monocyte Chemotactic Factors in Adipocytes: Involvement of Nuclear Factor-{kappa}B and c-Jun NH2-Terminal Kinase Pathways. Diabetes. 2009;58:104–115. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullard DC. Adhesion molecules in inflammatory diseases: insights from knockout mice. Immunol Res. 2002;26:27–33. doi: 10.1385/IR:26:1-3:027. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–103. [PubMed] [Google Scholar]
- 8.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore K, Patel K, Bruehl R, Li F, Johnson D, Lichenstein H, Cummings R, Bainton D, McEver R. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J. Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman K, Moore K, McEver R, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand- 1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 14.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles is P-selectin dependent. Am J Physiol. 1997;272:H1725–1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, Ghosh S. Shared Principles in NF-°B Signaling. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, II, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte Death, Adipose Tissue Remodeling, and Obesity Complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 17.Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14:1353–1362. doi: 10.1038/oby.2006.153. [DOI] [PubMed] [Google Scholar]
- 18.Bodary PF, Homeister JW, Vargas FB, Wickenheiser KJ, Cudney SS, Bahrou KL, Ohman M, Rabbani AB, Eitzman DT. Generation of soluble P- and E-selectins in vivo is dependent on expression of P-selectin glycoprotein ligand-1. J Thromb Haemost. 2007;5:599–603. doi: 10.1111/j.1538-7836.2007.02388.x. [DOI] [PubMed] [Google Scholar]
- 19.Harari OA, McHale JF, Marshall D, Ahmed S, Brown D, Askenase PW, Haskard DO. Endothelial Cell E- and P-Selectin Up-Regulation in Murine Contact Sensitivity Is Prolonged by Distinct Mechanisms Occurring in Sequence. J Immunol. 1999;163:6860–6866. [PubMed] [Google Scholar]
- 20.Bevilacqua M, Stengelin S, Gimbrone M, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 21.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. The Journal of Clinical Investigation. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Monocyte Chemoattractant Protein-1 Deficiency Protects Against Visceral Fat-Induced Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1151–1158. doi: 10.1161/ATVBAHA.110.205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. The Journal of Cell Biology. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1Î2 induces acute lung injury and chronic repair leading to pulmonary fibrosis. The Journal of Clinical Investigation. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.