Abstract
The hypothalamic neuropeptide orexin mediates arousal, sleep, and naturally rewarding behaviors, including food intake. Male sexual behavior is altered by orexin receptor-1 agonists or antagonists, suggesting a role for orexin-A in this naturally rewarding behavior. However, the specific role of endogenous orexin-A or B in different elements of male sexual behavior is currently unclear. Therefore, the current studies utilized markers for neural activation and orexin cell-specific lesions to test the hypothesis that orexin is critical for sexual motivation and performance in male rats. First, cFos expression in orexin neurons was demonstrated following presentation of a receptive or non-receptive female without further activation by different elements of mating. Next, the functional role of orexin was tested utilizing orexin-B conjugated saporin, resulting in orexin cell body lesions in the hypothalamus. Lesions were conducted in sexually naïve males and subsequent sexual behavior was recorded during four mating trials. Lesion males showed shortened latencies to mount and intromit during the first, but not subsequent mating trials, suggesting lesions facilitated initiation of sexual behavior in sexually naïve, but not experienced males. Likewise, lesions did not affect sexual motivation in experienced males, determined by runway tests. Finally, elevated plus maze tests demonstrated reduced anxiety-like behaviors in lesioned males, supporting a role for orexin in anxiety associated with initial exposure to the female in naïve animals. Overall, these findings show that orexin is not critical for male sexual performance or motivation, but may play a role in arousal and anxiety related to sexual behavior in naïve animals.
Keywords: orexin, hypocretin, sexual behavior, copulation, neural activation, motivation, hypothalamus, novelty, arousal, anxiety
Introduction
Orexin, also known as hypocretin, is a hypothalamic neuropeptide critical for feeding behavior, (de Lecea et al., 1998; Sakurai et al; 1998, Sakurai, 2006; Benoit et al., 2008) arousal and sleep (Chemelli et al., 1999; Lin et al., 1999, Sakurai, 2007; Furlong and Carrive, 2007; Furlong et al., 2009; Carter et al., 2009). Orexin neurons are localized to the lateral hypothalamic area (LHA) and perifornical dorsomedial hypothalamus (PFA-DMH) and produce two neuropeptides, orexin-A and B (de Lecea et al., 1998; Sakurai et al., 1998). Orexin neurons have been shown to project to brain structures involved in mediation of arousal including the locus coeruleus, tuberomammillary nucleus and peduculopontine tegmental nucleus (Peyron et al., 1998; Hagan et al., 1999; Horvath et al., 1999; Baldo et al., 2003). Orexin has also been implicated in reward and motivation, specifically related to food and drugs of abuse (Aston-Jones et al., 2009a; Aston-Jones et al., 2009b) and orexin neurons have been shown to project to reward related brain structures in the mesolimbic system including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Peyron et al., 1998; Fadel and Deutch, 2002; Martin et al., 2002; Baldo et al., 2003). Orexin neurons are activated by conditioned contextual cues associated with food and drug reward (Harris et al., 2005; de Lecea et al., 2006; Choi et al., 2010) and have been shown to play a role in reward-based feeding behavior (Choi et al., 2010). Moreover, intracerebroventricular (ICV) or intraperitoneal administration of an orexin receptor 1 (ORX1) antagonist results in reduced motivation for palatable food (Thorpe et al., 2005; Nair et al., 2008), whereas ICV orexin-A administration can reinstate this motivation (Boutrel et al., 2005).
The role of orexin in other rewarding behaviors is currently unclear, although several studies have implicated a role for orexin in control of sexual behavior in male rats. It has previously been shown that orexin neurons are activated by copulation in male rats (Muschamp et al., 2007). In addition, administration of orexin-A into the medial preoptic area (mPOA) resulted in enhanced sexual performance evidenced by reduced latencies to mount and intromit, and increased frequencies of mounts and intromission (Gulia et al., 2003). In contrast, ICV administration of orexin-A attenuated sexual motivation by reducing female preference, although only in highly sexually motivated males (Bai et al., 2009). Studies using ORX1 antagonists have also demonstrated contradictory data, as systemic administration of ORX1 antagonist slightly impaired sexual performance by increasing latency to intromit without affecting other parameters of sexual behavior (Muschamp et al., 2007), while ICV administration of ORX1 antagonist had no effect on sexual motivation (Bai et al., 2009). Together these studies suggest that administration of exogenous orexin-A affects sexual performance and motivation; however, endogenous orexin may not play an important role in mediating sexual behavior (Bai et al., 2009). Therefore, the goal of the present study was to determine if endogenous orexin is essential for male rat sexual motivation and performance.
First, it was determined when during sexual behavior orexin neurons are activated, testing the hypothesis that orexin neurons are activated upon introduction of the rewarding stimulus. Moreover, as it has been shown that sexual experience influences sexual performance (Dewsbury, 1969) and the rewarding properties of sexual behavior (Tenk et al., 2009), it was determined whether sexual experience influences orexin neuron activation during mating. Finally, it was tested whether orexin plays a critical role in sexual motivation and performance using cell body specific lesions of orexin neurons.
Materials and Methods
Adult male Sprague Dawley rats (200–250g) were obtained from Harlan (Indianapolis, IN) or Charles River Laboratories (Sherbrooke, Quebec, Canada) and housed individually or in pairs depending on the individual experiment (see below) in Plexiglas cages. The colony room was maintained on a 12/12 reversed light-dark cycle (lights off at 10 am) and food and water were available ad libitum except during behavioral testing. Female Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN) or Charles River Laboratories (Sherbrooke, Quebec, Canada) were bilaterally ovariectomized and implanted subcutaneously with 5% 17-β-estradiol benzoate silastic capsules. Sexual receptivity was induced by subcutaneous injections of progesterone (500 µg in 0.1ml of sesame oil) approximately 4 h prior to mating sessions. All procedures were approved by the Animal Care Committees at the University of Cincinnati and the University of Western Ontario and conform to guidelines outlined by the National Institute of Health and the Canadian Council on Animal Care. All behavioral testing was conducted during the first half of the dark phase under dim red illumination, except when noted otherwise.
Experimental Design
cFos expression studies
Male rats (n=48) were housed individually and half of the animals gained sexual experience in the home cage during 5 twice weekly mating sessions. Mating tests were performed in the home cage to eliminate arousal and cFos expression induced by exposure to a different mating arena and exposure to conditioned cues associated with prior mating (Balfour et al., 2004). A receptive female was introduced into the home cage and males were allowed to mate until one ejaculation or for 60 minutes. During each test sexual behavior was observed. The total number of mounts and intromissions, as well as the latencies to first mount and intromission (the time from presentation of the receptive female to the first mount or intromission), and ejaculation (the time from the first intromission to ejaculation), were recorded (Agmo, 1997). The remaining half of the animals remained sexually naïve. These animals were housed in the same room as the sexually experienced males, were handled and exposed to odors and sounds associated with mating, however did not mate. Naïve and experienced animals were each further subdivided into 6 experimental groups (n=4 per group). The 6 naïve and experienced groups included: Control males with no exposure to sexual behavior (Home Cage); males exposed to a non-receptive female in the home cage for 15 minutes (Anestrous Female). Males could investigate and interact, however did not mate due to lack of female receptivity; males exposed to the smells of a receptive female placed in a wire mesh box on top of the home cage for 15 minutes (Estrous Female); males that displayed mounts, but not intromissions or ejaculation with vaginally masked females (Mount); males that displayed mounts and intromissions only (Intromission); and males that mated to one ejaculation (Ejaculation). One hour after the end of the test, males were sacrificed to analyze cFos expression. Sexually experienced groups were matched on parameters of sexual behavior and there were no significant differences between groups prior to the final test. Moreover, there were no significant differences between naïve and experienced groups in numbers of mounts plus intromissions during the final test.
Perfusions: cFos expression
All males were deeply anesthetised with sodium pentobarbitol (270 mg/mL) and were transcardially-perfused with 4% paraformaldehyde (500 mL; PFA). Following perfusion brains were removed immediately and post-fixed for one hour in the same fixative then transferred to 20% sucrose solution for cryoprotection. Brains were sectioned on a freezing microtome (Microm, Walldorf, Germany) in coronal sections of 35 µm and collected in 4 parallel sections in cryoprotectant solution (30% sucrose in 0.1 M PB containing 30% ethylene glycol and 0.01% sodium azide) and stored at −20°C until further processing.
Immunohistochemistry
All incubations were performed at room temperature with gentle agitation. Free floating sections were washed extensively with 0.1M saline buffered sodium phosphate (PBS). Sections were blocked with 1% H2O2 (30% stock solution) in PBS for 10 minutes, then extensively rinsed again with PBS. Sections were incubated with an incubation solution (PBS containing 0.1% bovine serum albumin and 0.4% Triton X-100) for 1 hour. Primary antibody incubations were performed in the incubation solution overnight at room temperature. Following staining sections were rinsed in PBS, mounted onto plus charged glass slides and coverslipped with dibutyl phthalate xylene (DPX).
cFos/Orexin
One series of sections was immunoprocessed for cFos and orexin. Sections were incubated overnight with a rabbit raised antibody recognizing cFos (rabbit anti-cFos, sc-52; 1:10 000, Santa Cruz Biotechnology, Santa Cruz, CA) followed by 1 hour incubations with biotinylated goat anti-rabbit (1:500, Vector Laboratories, Burlingame, CA) and an avidin horseradish peroxidase complex (1:1000, ABC kit, Vector Laboratories, Burlingame, CA). Sections were incubated for 10 minutes in 0.02% diaminobenzidine (DAB) (Sigma, St. Louis, MO) in 0.1M phosphate buffer (PB) containing 0.012% hydrogen peroxide and 0.08% nickel sulfate, resulting in a blue-black reaction product. Sections were then incubated overnight with a rabbit raised antibody recognizing orexin-A (rabbit anti-orexin-A, H-003–30; 1:20 000, Phoenix Pharmaceuticals, Burlingame, CA) followed by a 1 hour incubation with biotinylated goat anti-rabbit and ABC, as described above. Finally, the sections were incubated for 10 minutes with 0.02% DAB in 0.1M PB containing 0.012% hydrogen peroxide, resulting in a reddish brown reaction product.
All antibodies have been characterized previously (Chen et al., 1999; Satoh et al., 2004; Solomon et al., 2007). Immunohistochemical controls included omission of primary antibodies, western blot analysis demonstrating single bands at appropriate weight (cFos), and loss of immunohistochemical orexin signal with orexin B-saporin lesions (orexin).
Data Analysis
cFos/Orexin
Neurons labelled for orexin or orexin and cFos were counted bilaterally in three representative sections per animal known to contain maximal numbers of the orexin neuronal population (Sakurai et al., 1998) spanning −2.3 mm to −3.6 mm from bregma (Paxinos and Watson, 1998) (Figure 1), using a drawing tube attached to a Leica microscope (Leica Microsystems; Wetzlar Germany), by an observed blinded to experimental groups. The PFA-DMH and LHA were delineated based on the location of the fornix (Figure 1a). Percentages of orexin neurons expressing cFos were calculated and averaged per hemisphere for each animal, and group means were calculated. Statistical significance between groups was determined using a two-way ANOVA with sexual experience and sexual behavior during final test as factors followed by Fisher’s LSD tests with a 95% confidence level.
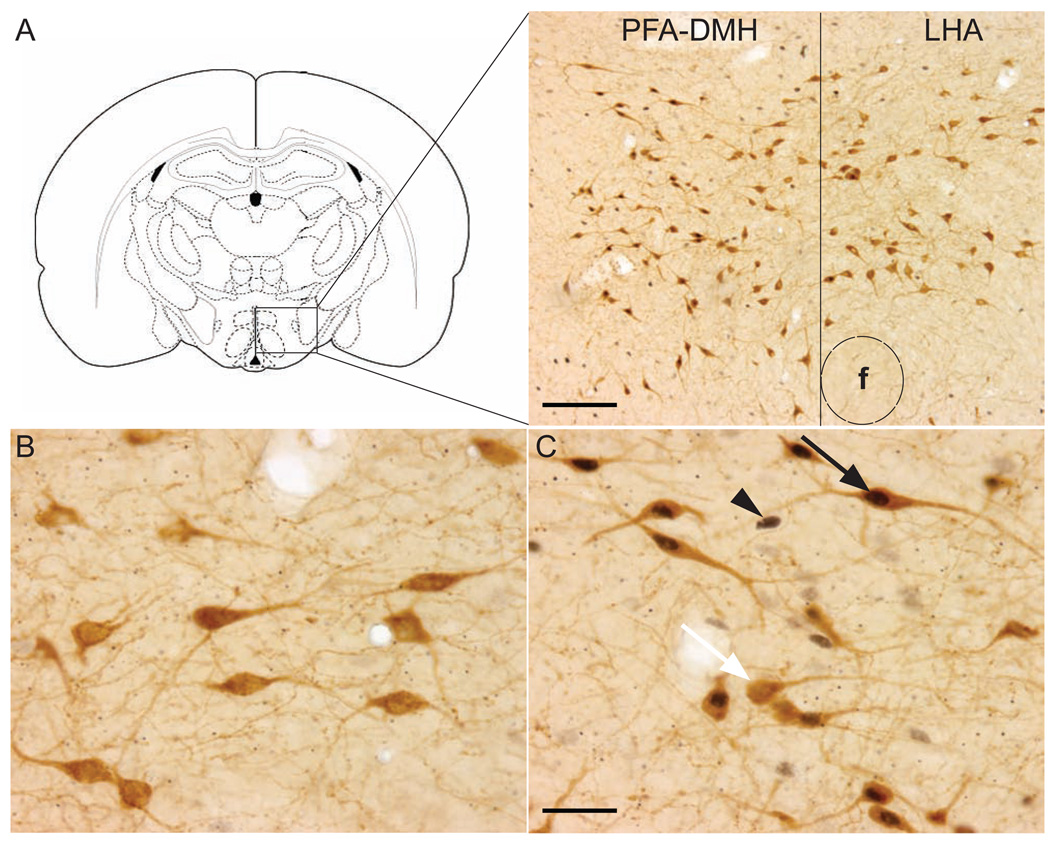
Figure 1.
Location of orexin neurons in the hypothalamus. (A) Anatomical location of orexin neurons in the hypothalamus. (Paxinos and Watson, 1998), Scale bar: 200 µm. (B) Single labelled orexin neurons in the PFA-DMH in an unmated control animal. (C) Orexin neurons in the PFA-DMH expressing cFos following mating. Scale bar: 50 µm. White arrow indicates a single labelled orexin neuron; arrowhead indicates a cFos positive neuron; black arrow indicates orexin neuron expressing cFos; Abbreviations: PFA-DMH, perifornical dorsomedial hypothalamus; LHA, lateral hypothalamic area; f, fornix.
Orexin Lesion Studies
Surgery
Males were housed individually and given one pre-test mating session with a receptive female prior to lesion and sham surgery. Sexual behavior was recorded as described above and groups were matched based on parameters of mating behavior. Male rats were anesthetized with isoflurane (Abbot Laboratories, St. Laurent, Quebec, Canada) using a Surgivet Isotec4 gas apparatus (Smiths Medical Vet Division, Markham, Ontario, Canada) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) with a gas mask covering the nose and mouth to maintain anaesthesia. An incision was made to expose the skull and lambda and bregma were found and determined to be level. A hole was drilled in the skull using a dremel drill (Dremel, Racine, WI) and glass micropipettes (40µm diameter, World Precision Instruments Inc, Sarasota, FL) filled with the a targeted toxin orexin-B saporin (IT-20, Advanced Targeting Systems, San Diego, CA; 200ng/µL in PBS); or unconjugated toxin BLANK-saporin (IT-21, Advanced Targeting Systems, San Diego, CA; 200ng/µL in PBS; sham controls) were lowered into the hypothalamus. This targeted toxin has been shown to bind with a high affinity to cells expressing orexin receptor 2 (ORX2) and with a significantly lower affinity for cells expressing ORX1 (Gerashchenko et al., 2001), and has been shown to specifically ablate orexin neurons in the hypothalamus (Frederick-Duus et al., 2007). Bilateral infusions of 1 µL (2 per hemisphere) were injected at the following coordinates: AP = −2.8 and −3.2; ML = 0.7 and 0.8; DV = −9.0 (Paxinos and Watson, 1998). After each infusion the needle was left in place for 3 minutes to allow diffusion. Needles were slowly removed and wounds were closed with wound clips. Two weeks following lesion surgery, all males were tested for sexual experience during four mating trials and were then subjected to the runway and/or elevated plus maze test (see below). Surgeries were performed in three different cohorts, separated by several weeks, to reach sufficient numbers of animals per group.
Sexual Behavior
All males were tested for sexual behavior during 4 mating sessions conducted every second day in the home cage. During each session, males mated with a receptive female to one ejaculation or for 60 minutes, whichever came first. Mating behavior was recorded as described above and copulation efficiency was also calculated [numbers of intromissions/(numbers of mounts + numbers of intromissions)]. Statistical differences in parameters of sexual performance were compared between lesion and sham groups for each trial using a one way ANOVA with lesion surgery as a factor and Fisher’s LSD test with a 95% confidence level, or when appropriate, non-parametric tests were run using a Kruskal-Wallis one-way ANOVA with lesion surgery as a factor and Dunn’s test with a 95% confidence level. In addition, data for each group were compared to the pre-surgery data using paired t-tests.
Sexual Motivation: Runway Test
Following testing of sexual behavior, a subgroup of the now sexually experienced males were tested for sexual motivation using a straight runway apparatus (MED Associates Inc., St. Albans, VT) (120 cm long; Lopez et al., 1999). Males habituated to the runway apparatus over two subsequent 10 minute trials conducted on the same day. Next, two test trials were conducted. During the first trial, a stimulus animal (estrous female, anestrous female or male) was placed in a goal box with perforated dividers at the end of the runway. A fan was used to blow the scents of the stimulus animals towards the male. Experimental males were placed in the start box, the door was opened to allow access to the runway, and time to reach the goal box was recorded. Once reaching the goal box, males were given 30 seconds to interact with the stimulus animal behind the screen. An identical second trial followed 1 hour later. Statistical significance between times to reach the goal box between trial 1 and trial 2 were analyzed using paired t-tests with a 95% confidence level. Statistical significance between groups was determined using a one-way ANOVA with lesion surgery as a factor followed by Fisher’s LSD tests with a 95% confidence level.
Anxiety-like Behavior: Elevated Plus Maze
A subgroup of the now sexually experienced males were tested for anxiety-like behavior to determine if effects of orexin lesions on sexual performance or motivation were due to changes in anxiety or arousal. Males were exposed to the elevated plus maze apparatus (EPM; MED Associates Inc., St. Albans, VT) in a brightly lit room during the end of the light phase. The EPM consisted of 4 arms each 50 cm in length extending from a central junction and was elevated 75 cm. Two arms of the maze were open to the outside environment and the other two were enclosed with dark siding 40 cm high. Animals were placed on the EPM and monitored for five minutes. Time spent in open and closed arms, and total numbers of entries into each arm were recorded using photobeam arrays. Statistical significance between groups was determined using a one-way ANOVA with lesion as a factor followed by Fisher’s LSD tests with a 95% confidence level.
Perfusions and mating-induced cFos
Following all behavioral testing, all males were deeply anesthetised with sodium pentobarbitol (270mg/mL) and were transcardially perfused with 500 mL of 4% PFA for lesion verification as described previously. In addition, to test the effects of orexin lesions on mating-induced cFos expression, groups of sham and lesion males mated until one ejaculation. One hour following ejaculation, males were transcardially perfused with 4% PFA as described above. Half of the males in this group were not introduced to a female and were perfused from the home cage to serve as unmated controls.
Immunohistochemistry
Brains were sectioned using a freezing microtome in 4 parallel series of 35 µm coronal sections and stored as described above. For lesion verification, one series of sections containing the hypothalamus from all lesion experiments was single labelled for orexin using the same rabbit anti-orexin-A and DAB protocol described above. One series of sections from the animals that mated was stained for cFos and orexin as described above.
Lesion Verification
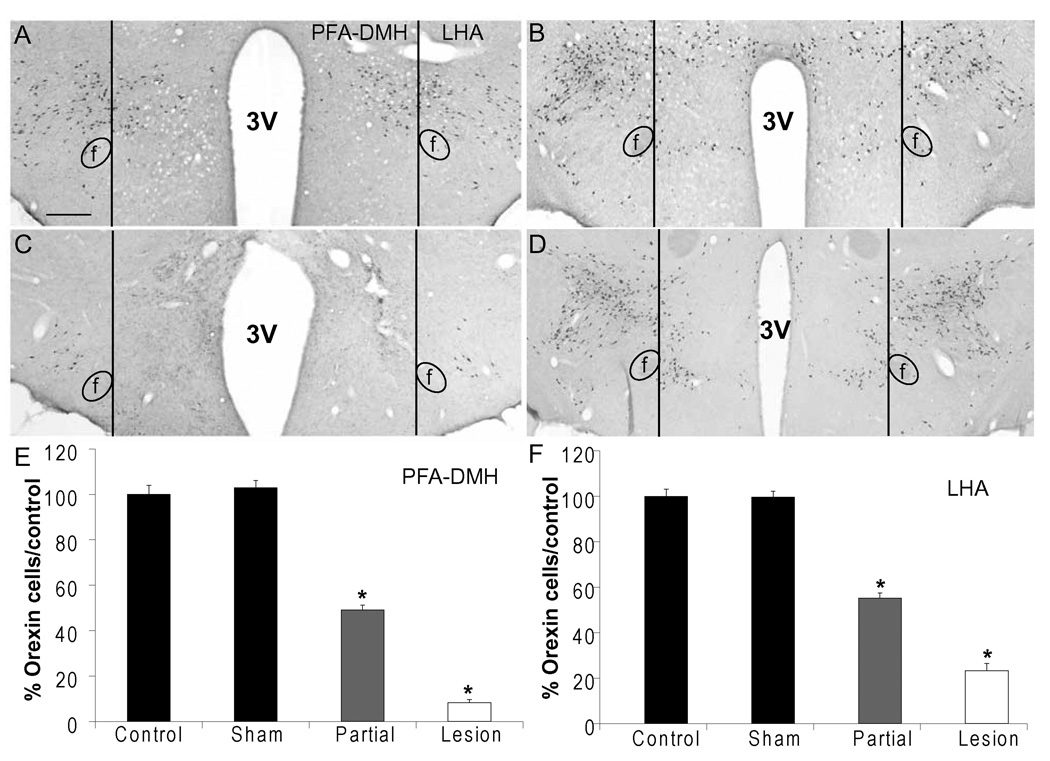
In each animal, numbers of orexin neurons immunoreactive for orexin were counted bilaterally in the PFA-DMH and LHA in 3 sections expressing maximal numbers of orexin cells in non-surgery controls, −2.3 mm to −3.6 mm from bregma as described above. Cells per hemisphere were averaged for each animal, and group means were calculated. Non-surgery control animals (from cFos experiments) were used to determine intact/baseline numbers of orexin neurons and data are expressed as percentages compared to the non-surgery control males (Figure 2). Males that had fewer than 20% orexin cells compared to non-surgery control animals were included in the lesion group. Animals with greater than 20%, but fewer than 80% were included in a partial lesion group. Sham controls did not have significant changes in numbers of orexin cells. Statistical significance between sham, partial and complete lesion animals was calculated using a one-way ANOVA and Fisher’s LSD test with a 95% confidence level.
Figure 2.
Lesion verification. Representative images showing orexin (A) and MCH (B) cells in a sham lesion animal injected with BLANK-saporin. Representative images showing loss of orexin cells (C), but intact MCH cells (D) in a lesion animal injected with orexin B-saporin. Scale bar: 400 µm; (E) Quantification of orexin neurons in the PFA-DMH (E) and LHA (F) of sham, partial and lesion males, expressed as percentages of non-surgery controls.* indicates significant difference from all other groups. (p<0.001). Sham: n=35; Partial Lesion: n=45; Lesion: n=19. Abbreviations: PFA-DMH: perifornical dorsomedial hypothalamus; LHA: lateral hypothalamic area; f: fornix.
Lesion Specificity
To verify that lesions were restricted to orexin neurons, one series of sections containing the hypothalamus from a subset of sham and lesion animals (n=20) was immunoprocessed for melanocyte concentrating hormone (MCH), a hypothalamic peptide that has overlapping location (but no colocalization) with orexin neurons (Broberger et al., 1998), using a rabbit-raised antibody recognizing MCH (rabbit anti-MCH, H-070-47; 1:150 000, Phoenix Pharmaceuticals, Burlingame, CA) and DAB as described previously. MCH neurons express ORX1 (Bäckberg et al., 2002) but not ORX2 (Volgin et al., 2004), and are not significantly reduced following orexin B-saporin treatment (Frederick-Duus et al., 2007). MCH immunoreactive cells were counted bilaterally in two sections per animal (sham: n= 7; lesion n=5), using alternate sections to those analyzed for orexin neurons. Lesions did not significantly reduce numbers of MCH neurons in either PFA-DMH or LHA (Table 1; Figure 2b, d; PFA-DMH: p=0.47; LHA: p=0.33). Furthermore, mating induced cFos expression was counted bilaterally in one representative section per animal (sham: n=4; lesion n=3), using alternate sections to those analyzed for orexin neurons. Lesions did not affect mating-induced cFos expression in the PFA-DMH or LHA (Table 1; PFA-DMH: p=0.53; LHA: p=0.82). Finally, representative sections used for orexin cell counts (animals: sham: n=6; lesion: n=6) were Nissl counterstained using cresyl violet (5 g cresyl violet acetate (C-5042, Sigma, St. Louis, MO), 0.5 g of sodium acetate trihydrate (S209, Thermo Fisher Scientific, Ottawa, Ontario, Canada), 1L double distilled water with glacial acetic acid (AX0073-6, EMD Chemicals, Mississauga, Ontario, Canada) at pH: 3.14). Counts of Nissl stained neurons were performed in standard areas of analysis (250 µm × 200 µm) in the general location of orexin neurons. Numbers of Nissl-stained neurons did not differ between sham and lesion groups (Table 1; PFA-DMH: p=0.23; LHA: p=0.33).
Table 1.
Verification of lesion specificity: Analysis of the numbers of neurons stained for Nissl, MCH or mating induced cFos demonstrated that there was no significant loss of neurons in general, MCH cells, or mating induced neural activation in the PFA-DMH or LHA following infusions of orexin B-saporin.
| Sham | Lesion | |||
|---|---|---|---|---|
| PFA-DMH | LHA | PFA-DMH | LHA | |
| Nissl | 93.3 ± 7.2 | 84.1 ± 7.4 | 82.8 ± 3.9 | 75.3 ± 4.6 |
| MCH | 101.3 ± 4.1 | 136.2 ± 8.1 | 88.2 ± 16.6 | 119.1 ± 13.5 |
|
Mating-induced
cFos |
45.5 ± 6.5 | 30.1 ± 2.7 | 40.0 ± 3.8 | 32.0 ± 5.0 |
Abbreviations: PFA-DMH, perifornical dorsomedial hypothalamus; LHA, lateral hypothalamic area; MCH, melanocyte concentrating hormone.
Since a deficiency in orexin has been show to contribute to narcolepsy in mice (Chemelli et al., 1999), dogs (Lin et al., 1999) and humans (Siegel, 1999; Nishino et al., 2000; Peyron et al., 2000; Thannickal et al., 2000) animals were observed to ensure the absence of a narcoleptic phenotype. Animals were observed for the duration of all behavioral tests reported in this study and did not display any characteristics of narcolepsy.
Mating-induced cFos expression in lesion animals
Numbers of cFos-immunoreactive cells were counted bilaterally in 3 sections per animals in standard areas of analysis in the ventral tegmental area (VTA; 900×900 µm), mPOA (400×600 µm); nucleus accumbens (NAc) core and shell (400×600 µm) and the prelimbic, infralimbic and anterior cingulate sub-regions of the medial prefrontal cortex (mPFC) (600×800 µm per sub-region) by an observed blinded to experimental groups. Counts were averaged for each animal, and group means were calculated. Statistical significance was calculated using a two-way ANOVA with sexual experience and lesion as factors followed by Fisher’s LSD test with a 95% confidence level.
Results
Orexin neuron activation during sexual behavior
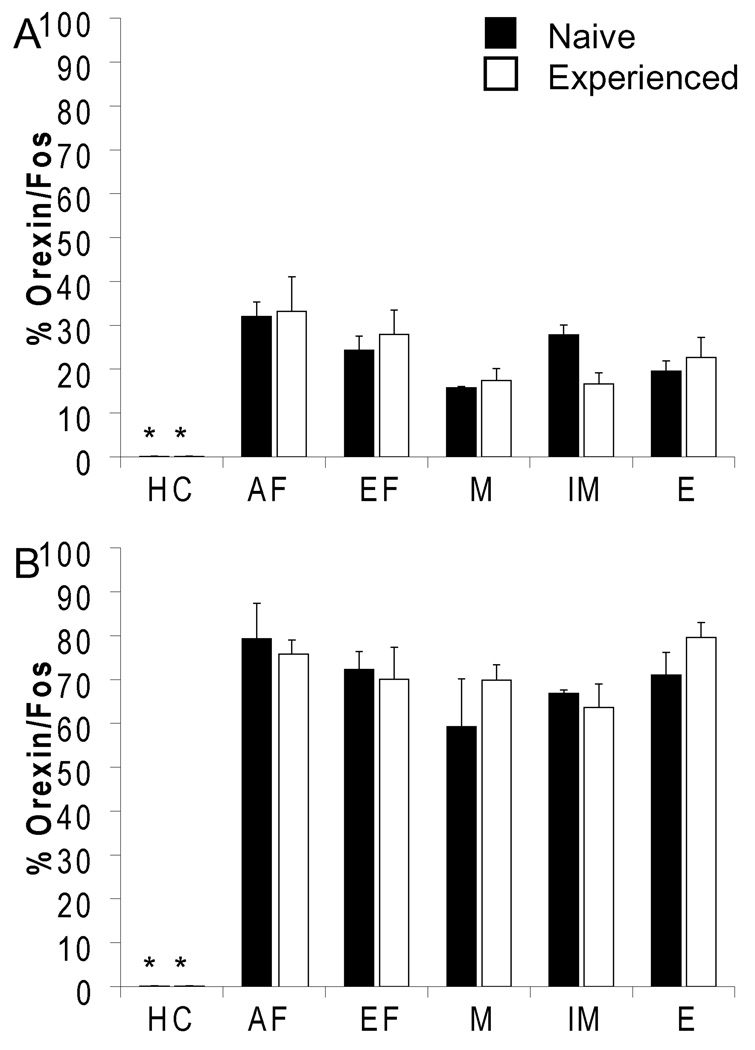
A significant increase in cFos expression in orexin neurons was observed following sexual behavior in both the PFA-DMH (F(5,31) 63.4; p<0.001; Figure 3a) and LHA (F(5,31) 10.4; p<0.001; Figure 3b), with no effect of sexual experience. Specifically, in both sexually naïve and experienced animals, all experimental groups of males displaying different parameters of sexual behavior (investigation of anestrous female, exposure to estrous female odors, display of mounting, intromissions, or ejaculation) showed equal induction of cFos compared to home cage controls with a higher percentage of orexin cells activated in the PFA-DMH (60–80%) versus the LHA (14–33%), without differences between the experimental groups. These results suggest that orexin neurons are activated following exposure to the stimulus female without further activation during sexual performance. Moreover, the activation is not dependent on incentive salience of the female stimulus as both non-receptive and receptive females induced activation in sexually experienced males.
Figure 3.
Orexin neurons in PFA-DMH (A) and LHA (B) expressed cFos following all parameters of mating behavior in naïve and experienced animals. Abbreviations: HC, home cage; AF: anestrous female; EF, estrous female; M, Mount; IM, Intromission; E, Ejaculation. * indicates significant difference from all other groups (p<0.001). n=4 per group.
Effects of orexin lesions
Sexual Behavior
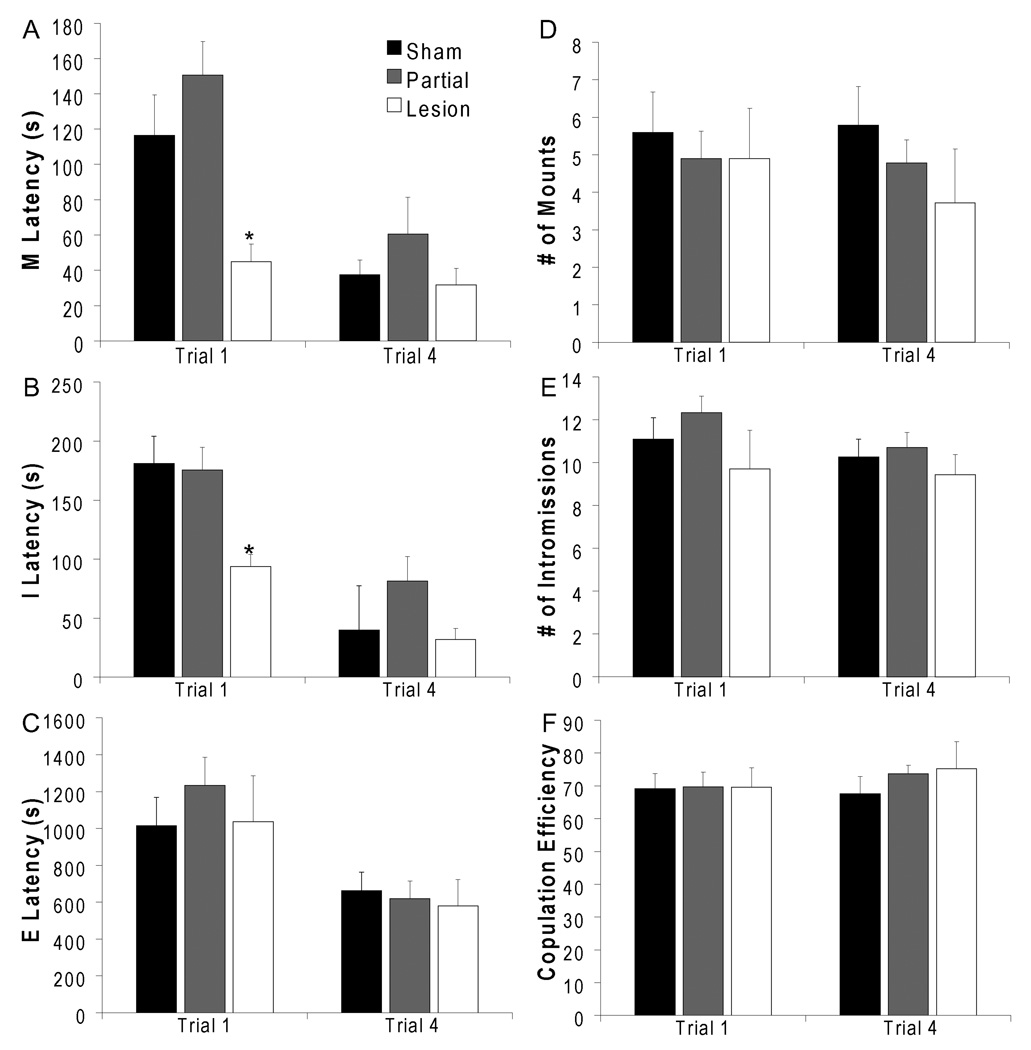
Orexin lesions resulted in facilitation of sexual behavior (mount latency: F(2,47) 3.962; p=0.034; intromission latency: H=9.104; p=0.011). During the first mating trial, lesion males showed shorter latencies to mount and intromission compared to sham animals (mount latency: p=0.03; intromission latency: p=0.01; Figure 4a–b) and compared to latencies during the pre-surgery mating trial (mount latency: p=0.02; intromission latency: p=0.03; Data not shown). Partial lesion males did not differ significantly from sham males, and neither group differed from the pre-surgery mating trial. Effects of lesions on mount and intromission latencies was attenuated with sexual experience, as there were no differences between groups, during any of the subsequent trials (trial 4 shown in Figure 4a–b). Ejaculation latencies (Figure 4c), numbers of mounts (Figure 4d) and intromissions (Figure 4e) as well as copulation efficiency (Figure 4f) did not significantly differ between groups during any of the trials or within each group between the first mating trial and the pre-surgery test.
Figure 4.
Orexin lesions shortened latencies to mount and intromission in sexually naive males during trial 1. Orexin lesions did not affect mating during trial 4, after males gained sexual experience. (A) Mount Latency. (B) Intromission Latency. (C) Ejaculation latency. (D) Number of Mounts. (E) Number of Intromissions. (F) Copulation Efficiency. * indicates significant difference from sham. Sham: n=19; Partial Lesion: n=23; Lesion: n=7.
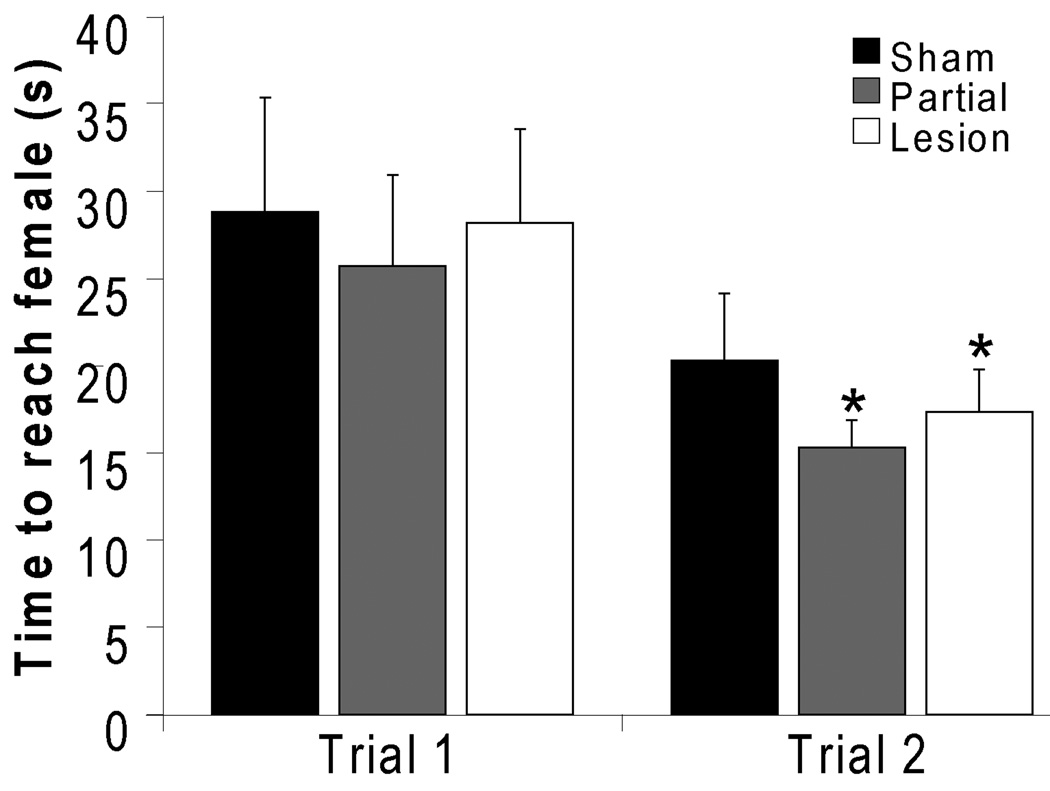
Runway test
Orexin lesions did not affect sexual motivation assessed in a straight runway test in sexually experienced males. Over the course of two test trials, lesion males ran significantly faster towards an estrous female in the second trial compared to the first trial (p=0.03; Figure 5). Such increased run time is indicative of sexual motivation (Lopez et al., 1999). Partial lesion and sham males also ran faster towards an estrous female during trial 2 (p=0.03), although this failed to reach significance in sham males (p=0.052). None of the groups showed increased speed to run towards an anestrous female or a male during trial 2. Moreover, no significant differences were observed between sham, partial and lesion males on speed to run towards any stimulus animal on neither trial 1 nor trial 2, demonstrating lack of differences in general activity on the runway.
Figure 5.
Orexin lesions did not affect sexual motivation in sexually experience males. Shown are times to reach an estrous female in the runway test during both trials 1 and 2. * indicates significant reduction in time to reach the female in trial 2 compared to trial 1. Sham: n=24; Partial Lesion: n=26; Lesion: n=12.
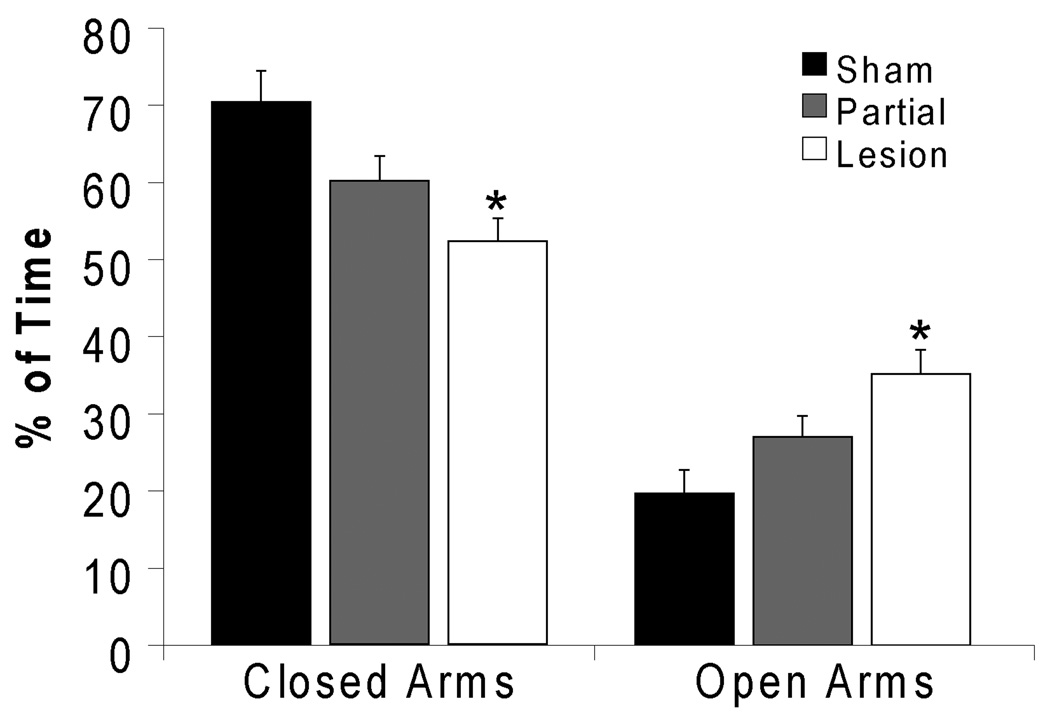
Anxiety-like behavior
Results thus far suggest that lesions may facilitate initiation of sexual behavior in naïve animals via a potential effect on responses to novelty and/or anxiety-like behaviors when the males encounter a novel female. In support, lesion males showed decreased anxiety-like behavior on the EPM, seen as a decreased percentage of time spent in the closed arms, (p=0.012; Figure 6) and an increased percentage of time on the open arms (p=0.023; Figure 6) compared to sham males. Partial lesions had no significant effect. These data further support that lesion decreased anxiety-like behavior.
Figure 6.
Orexin lesions decreased anxiety-like behavior on the elevated plus maze. Percentage of time spent in the closed arms (left) were decreased and percentage of time in open arms (right) were increased in lesioned males. * indicates significant difference from sham. Sham: n=27; Partial Lesion: n=36; Lesion: n=12.
cFos Expression
To assess whether endogenous orexin contributes to mating-induced neuronal activation in orexin-innervated brain regions, analysis of mating-induced cFos expression in the VTA, NAc core and shell, mPOA and the mPFC was conducted. In both lesion and sham males, mating significantly increased cFos in all of the analyzed brain areas compared to unmated controls (Table 2). Lesions did not affect neural activation, as sham and lesion animals did not differ in baseline or mating-induced cFos expression.
Table 2.
Mating induced cFos in sham, partial and lesion groups compared to non-mating controls of the same lesion status.
| Brain Area | Non Mating | Mating | ||||
|---|---|---|---|---|---|---|
| Sham | Partial | Lesion | Sham | Partial | Lesion | |
| VTA | 13.2 ± 9 | 5.8 ± 3.3 | 3.2 ± 2.2 | 83.3 ± 3.3 * | 79.4 ± 4.4 * | 63.3 ± 20 * |
| NAc Core | 7.9 ± 1.3 | 7.0 ± 3.0 | 2.7 ± 1.2 | 54.8 ± 3.4 * | 61.4 ± 5.3 * | 53.8 ± 24.2 * |
| NAc Shell | 5.3 ± 3.2 | 5.0 ± 3.9 | 2.1 ± 1.2 | 57.4 ± 2.7 * | 60.3 ± 5.2 * | 38.6 ± 13.7 * |
| mPOA | 5.9 ± 3.3 | 7.9 ± 3.3 | 8.3 ± 5.7 | 197.8 ± 21.4 * | 184.3 ± 11.7 * | 224.1 ± 22.4 * |
| mPFC | 28.0 ± 13.2 | 24.0 ± 11.3 | 8.6 ± 6.2 | 296.3 ± 79.9 * | 309.8 ± 44.3 * | 263.4 ± 98.2 * |
indicates significant difference from non-mating control (p<0.001 for all groups). No differences between groups were detected in either baseline or mating induced cFos expression.
Abbreviations: VTA: ventral tegmental area; NAc: nucleus accumbens, mPOA: medial preoptic area; mPFC: medial prefrontal cortex. There were no significant differences between cFos counts in any subregion of the mPFC, thus the combined expression of the three subregions is shown. Sham: n=8; Partial lesion: n=9; Lesion: n=6.
Discussion
These studies investigated the role of endogenous orexin in sexual performance and motivation in the male rat. It was found that orexin is not essential for sexual motivation or performance. Instead, orexin neurons are activated by the female stimulus, independent of the hormonal status of the female or sexual experience of the male. Moreover, removal of endogenous orexin by orexin cell-specific lesions decreased anxiety-like behaviors and facilitated initiation of sexual behavior in sexually naïve males. Thus, the results of this study support a role for orexin in arousal (de Lecea et al., 2006; Harris and Aston-Jones, 2006; Sakurai, 2007; Boutrel et al., 2009; Furlong and Carrive, 2009; Furlong et al., 2009) and anxiety (Suzuki et al., 2005; Davis et al, 2009; Li et al., 2010), but do not support a critical role for orexin in sexual motivation or performance.
The results of these studies further clarify the role of endogenous orexin and the apparent contrasting findings of the previous studies examining the role of orexin in male sexual behavior using pharmacological tools. Intra-mPOA infusions of exogenous orexin-A led to increased sexual arousal and improved sexual performance, suggesting that orexin may act in the mPOA to increase motivation and performance of sexual behavior (Gulia et al., 2003). However, in contrast, ICV infusions of orexin-A attenuated sexual motivation and arousal (Bai et al., 2009), while an orexin receptor antagonist had no effect on sexual arousal (Bai et al., 2009), indicating endogenous orexin may not play a role in sexual motivation. Finally, ORX1 blockade by systemic injections was shown to only slightly impair copulatory performance (Muschamp et al., 2007). From these conflicting studies, a few conclusions can be drawn. First, application of exogenous orexin-A may affect behavior, but ORX1 blockade is without major effects, suggesting a minor role for endogenous orexin in regulation of male sexual behavior (Bai et al., 2009). The current results support this possibility. The current studies using removal of orexin, by orexin cell-specific lesions indicate that endogenous orexin is not essential for sexual motivation or performance, in line with observations by Bai et al (2009). It is important, however to note that lack of effects of orexin lesions on sexual motivation in the runway may be due to the fact that animals had gained sexual experience prior to sexual motivation testing, therefore lack of effect in the runway test may have been due to the sexual experience of the males. Future experiments may address this caveat by testing the effects of orexin lesions on sexual motivation in naïve males.
It is also possible that the two orexin ligands and the two subtypes of orexin receptors (ORX1 and ORX2; Sakurai et al., 1998) may regulate sexual behavior in opposite directions. By utilizing orexin cell lesion techniques, the ligands for both subtypes of orexin receptors (orexin-A and B) were eliminated in the current study. The two receptor subtypes are expressed in different brain areas (Trivedi et al., 1998; Marcus et al., 2001) and have been shown to differentially regulate memory for cue induced cocaine-seeking (Smith et al., 2009). Previous studies on sexual behavior have primarily focussed on the role of orexin-A and ORX1 (see discussion above). The orexin receptor antagonist SB334867 used in the studies thus far specifically targets ORX1 which has a high affinity for orexin-A and significantly lower affinity for orexin-B (Sakurai et al., 1998). Likewise, orexin-A has been used as the exogenous orexin in previous studies (Gulia et al., 2003; Bai et al., 2009). Future studies are needed to investigate the role of orexin-B and ORX2 in regulation of male sexual behavior.
The current study tested the effects of long term loss of orexin. Muschamp et al. (2007) suggested that a long term reduction of orexin following castration may account for the loss of sexual motivation and performance. This hypothesis was contradicted by the current findings as orexin cell lesions did not reduce sexual motivation or performance. It is possible that the long term orexin loss in the current study may have resulted in compensatory mechanisms, although no changes in mating-induced neural activation within the circuit mediating sexual behavior were detected. Nonetheless, it is clear that reduced or lack of orexin does not prevent sexual behavior. Moreover, the results of the current study do not support a major role for orexin in induction of cFos expression by sexual behavior. It has been clearly established that orexin contributes to activation of neurons in the VTA (Korotkova et al., 2003; Borgland et al., 2006; Narita et al., 2006; Vittoz et al., 2008). However, orexin cell lesions did not block mating-induced neural activation in the VTA, or in any other reward-related brain regions analyzed, despite the presence of orexin-immunoreactive fibers in close proximity to the activated neurons in sham males. Thus, mating-induced neural activation in these brain regions does not appear to be dependent on orexin action.
A somewhat unexpected finding of the current study was the effect of orexin lesions on facilitation of the initiation of sexual behavior in sexual naïve, but not experienced animals. This was shown to be correlated with a reduction in anxiety-like behaviors. Therefore, the effects of orexin lesions on sexual motivation and performance may be secondary to its effects on anxiety and arousal. Indeed previous studies have suggested a role for orexin in anxiety as ICV infusion of orexin-A decreased time on the open arms of the EPM in mice (Suzuki et al., 2005). Infusion of orexin-A into the paraventricular nucleus of the thalamus of male rats decreased time spent in the center area of an open field chamber and decreased novel object exploration, indicating orexin may be involved in the generation of anxiety-like behavior (Li et al., 2010). In addition, dominant male rats that show increased risk taking on the EPM have increased levels of ORX1 mRNA in the mPFC (Davis et al., 2009). Orexin has also been shown to alter responses to stress (Ida et al., 1999; Ida et al., 2000), and stimulation of orexin receptors increases release of corticotrophin releasing factor (Al-Barazanji et al., 2001; Singareddy et al., 2006), corticosterone (Ida et al., 2000; Kuru et al., 2000) and adrenocorticotropic hormone (Kuru et al., 2000). Orexin antagonists are currently in clinical trials for treatment of insomnia, a disorder which is often comorbid with anxiety disorders (Sullivan and Neria, 2009), and it is hypothesized that orexin antagonists could potentially be used to treat anxiety disorders (Mathew et al., 2008). Given the growing body of evidence of a role for orexin in anxiety and arousal it appears orexin lesions may facilitate the initiation of sexual behavior in naive males by reducing anxiety-like responses associated with the introduction of a novel stimulus, i.e. the female.
Significant activation of orexin neurons was seen following sexual arousal and sexual behavior in both sexually naive and experienced animals in both the PFA-DMH and LHA, with 60–80% and 14–33% of orexin cells expressing cFos, respectively. There is a body of evidence supporting a dichotomy in orexin neuronal function within the orexin cell population, with the PFA-DMH being critically involved in arousal and the LHA being critical for reward-related behaviors (Harris et al., 2005; Harris and Aston-Jones, 2006, Aston-Jones, 2009a). Hence, activation of the PFA-DMH orexin cells by the female stimulus supports the hypothesis that orexin is activated by and is critical for arousal, including sexual arousal in naïve and experienced males, and anxiety associated with the novel female stimulus in naive males. However, PFA-DMH cells were activated to similar levels independent of the experience of the males and the hormonal status of the female, suggesting that the PFA-DMH cells were activated during general arousal and not specifically by sexual arousal. Moreover, our studies do not fully support the existence of a completely dichotomous orexin cell population as there was significant activation of the LHA following exposure to all parameters of sexual arousal and performance, regardless of whether the behaviors were associated with reward. Thus, experienced males exposed to an anestrous female showed equal levels of orexin cell activation in LHA compared to experienced males that copulated to ejaculation. However, only the latter group will form a conditioned place preference for mating (Tenk et al, 2009); suggesting that copulation to ejaculation is more rewarding than other elements of mating. The current study did not specifically test the role of orexin in sexual reward; hence future studies are needed to address that question.
In summary, the results of these studies demonstrate that orexin is not critical for sexual performance or motivation. Instead, orexin cell lesions were demonstrated to reduce anxiety, suggesting that endogenous orexin is involved in increasing anxiety. Moreover, removal of orexin resulted in facilitation of initiation of sexual behavior in sexually naïve males, suggesting that endogenous orexin may inhibit initiation of mating, possibly by increasing anxiety in response to the novel stimulus, i.e. the female. These findings further elucidate the neural circuitry involved in sexual performance and anxiety, and add to a growing body of literature on the role of orexin in mediation of arousal and anxiety.
Acknowledgements
This research was supported by grants from the National Institutes of Health (R01 DA014591), Canadian Institutes of Health Research (RN 014705), and National Sciences and Engineering Research Council of Canada (Discovery Grant (341710) to LMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A. Male rat sexual behavior. Brain Res Brain Res Protoc. 1997;1:203–209. doi: 10.1016/s1385-299x(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Al-Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J Neuroendocrinol. 2001;13:421–424. doi: 10.1046/j.1365-2826.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009a;56 Suppl 1:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2009b;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YJ, Li YH, Zheng XG, Han J, Yang XY, Sui N. Orexin A attenuates unconditioned sexual motivation in male rats. Pharmacol Biochem Behav. 2009;91:581–589. doi: 10.1016/j.pbb.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Bäckberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J. Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Tracy AL, Davis JF, Choi D, Clegg DJ. Novel functions of orexigenic hypothalamic peptides: from genes to behavior. Nutrition. 2008;24:843–847. doi: 10.1016/j.nut.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SASF, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 1999;260:161–164. doi: 10.1016/s0304-3940(98)00977-x. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Davis JF, Krause EG, Melhorn SJ, Sakai RR, Benoit SC. Dominant rats are natural risk takers and display increased motivation for food reward. Neuroscience. 2009;162:23–30. doi: 10.1016/j.neuroscience.2009.04.039. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J. Neurosci. 2006;26(41):10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA. Copulatory behaviour of rats (Rattus norvegicus) as a function of prior copulatory experience. Anim Behav. 1969;17:217–223. doi: 10.1016/0003-3472(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neuroscience. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128:107–119. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;8:1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia KK, Mallick HN, Kumar VM. Orexin A (hypocretin-1) application at the medial preoptic area potentiates male sexual behavior in rats. Neuroscience. 2003;116:921–923. doi: 10.1016/s0306-4522(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav. 2010;95:121–128. doi: 10.1016/j.pbb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Olster DH, Ettenberg A. Sexual motivation in the male rat: the role of primary incentives and copulatory experience. Horm Behav. 1999;36:176–185. doi: 10.1006/hbeh.1999.1535. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martin G, Fabre V, Siggins GR, de Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104:111–117. doi: 10.1016/s0167-0115(01)00354-8. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis. CNS Neurol Disord Drug Targets. 2006;5:313–325. doi: 10.2174/187152706777452218. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Fujioka A, Nakajima T, Kanbayashi T, Nishino S, Shigeyoshi Y, Yoneda H. FOS expression in orexin neurons following muscimol perfusion of preoptic area. Neuroreport. 2004;15:1127–1131. doi: 10.1097/00001756-200405190-00009. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singareddy R, Uhde T, Commissaris R. Differential effects of hypocretins on noise-alone versus potentiated startle responses. Physiol Behav. 2006;89:650–655. doi: 10.1016/j.physbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, De Fanti BA, Martinez JA. Peripheral ghrelin interacts with orexin neurons in glucostatic signalling. Regul Pept. 2007;144:17–24. doi: 10.1016/j.regpep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Neria Y. Pharmacotherapy in post-traumatic stress disorder: evidence from randomized controlled trials. Curr Opin Investig Drugs. 2009;10:35–45. [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgin DV, Swan J, Kubin L. Single cell RT-PCR gene expression profiling of acutely. dissociated and immunocytochemically identified central neurons. J. Neurosci Methods. 2004;136:229–236. doi: 10.1016/j.jneumeth.2004.01.013. [DOI] [PubMed] [Google Scholar]