Abstract
Methamphetamine (METH) is a psychomotor stimulant strongly associated with increases in sexual drive and behavior in women and men. Even though men and women are equally as likely to be addicted to or use METH, studies of sexual behavior often focus on male users. The paucity in studies examining the effect of METH in women is of great concern, when one considers the high correlation with sexually transmitted diseases such as HIV/AIDS and unplanned pregnancies. In fact, why METH so profoundly increases sexual drive is unknown. We have demonstrated that repeated exposure to METH enhances both receptivity and proceptivity in hormonally-primed female rats. The current study examined whether a repeated exposure to METH enhanced female-initiated sexual behaviors in hormonally-primed rats. In a paced mating paradigm, METH treatment significantly decreased the female’s return latency following a mount (57%) and an ejaculation (44%), and the likelihood to leave the male following an intromission (37%) compared to controls. The METH-induced changes in paced mating behavior were accompanied by a 60% increase in spinophilin levels in the medial amygdala following hormonal priming and METH treatment. Taken together, these findings suggest that METH increases female sexual motivation and behavior in the rat potentially via changes in the neural substrate that require repeated exposure to the drug.
Keywords: sexual motivation, women’s health, addiction, psychostimulants, paced mating, spinophilin
Introduction
Methamphetamine (METH) is a highly addictive psychostimulant drug of abuse commonly associated with an increased sex drive and sexual activities including high-risk behaviors (Rawson et al., 2002; Semple et al., 2004a; Semple et al., 2004b; Mansergh et al., 2006). Nevertheless, women who use METH have been relatively understudied (Corsi and Booth, 2008). The few studies that have investigated the drug-sex connection in women report that the degree of METH use is directly related to a positive or pleasurable sexual experience among women who use the drug, suggesting a biological basis for the drug and sex association (Rawson et al., 2002; Semple et al., 2004a; Semple et al., 2004b; Corsi and Booth, 2008).
The mechanisms by which METH induces female sexual drive and behavior are relatively unexplored. While self-reporting surveys and retrospective studies are informative, they will not allow one to directly test the cellular and molecular events that may underlie METH’s influence on sexual behaviors. For this, rodent models are most appropriate as they permit insight into the physiological processes underlying predicted behaviors. We have previously reported that administration of METH to hormonally primed female rats enhances proceptive behaviors and lordosis responses (Holder et al., 2010). Perhaps more exciting is the elucidation of the potential neurocircuitry underlying METH-induced female sexual behaviors: the combined administration of ovarian hormones and METH increases the neuronal activation in the medial nucleus of the amygdala (MeA) and the ventromedial nucleus of the hypothalamus (VMN; Holder et al., 2010), nuclei implicated in the modulation of appetitive aspects of female behavior.
In light of the original findings, here we extend our studies to a paced mating paradigm, which allows for a more direct measure of female-initiated sexual behaviors (reviewed in Erskine, 1989; Pfaus et al., 2003). In a natural or semi-natural environment, the female controls the occurrence and rate of sexual interaction through a pattern of approach to and withdrawal from the male (referred to as “pacing;” McClintock and Adler, 1978; Erskine, 1989). This pattern of pacing behavior is thought to measure aspects of female sexual motivation (reviewed in Paredes and Vazquez, 1999; Pfaff and Ågmo, 2002; Pfaus et al., 2003; Kondo and Sakuma, 2005; Ågmo, 2007). In the laboratory, we can recreate pacing behavior by use of an arena containing barriers that the female, but not the male, can cross. Based on our previous finding that METH increased proceptive behaviors, we predicted that METH treatment would increase female-initiated contacts in the paced mating arena.
It is well established that repeated administrations of METH, similar to our established treatment paradigm (Holder et al., 2010), increase dendritic branching and spine density in medium spiny neurons (Robinson and Kolb, 2004; Jedynak et al., 2007) and induce expression of genes involved in synaptic connectivity (Ishikawa et al., 2006; Numachi et al., 2007) in brain nuclei comprising the mesolimbic reward pathway such as the nucleus accumbens, striatum and amygdala. However, it is unknown whether METH alone or in combination with ovarian hormones induces changes in spine density in nuclei involved in female sexual behavior. Here, we quantified spinophilin, a cytoskeleton-associated protein highly enriched in dendritic spines (Allen et al., 1997; Feng et al., 2000), in the in the MeA, the VMN and the cortex from female rats treated with METH or vehicle following paced mating behavior.
The data reported herein support a role for METH in the enhancement of female-initiated sexual behaviors. Moreover, these enhancements were associated with an increase in spinophilin levels in the MeA. Taken together, these data indicate that METH increases female sexual motivation, which may occur via modifications of the neuronal substrate due to repeated exposures of METH.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (250–300g) were purchased from Charles River Laboratories (Kingston, NY) and housed in the Laboratory Animal Facility of the Bressler Research Building at the University of Maryland School of Medicine under a reversed 12 h: 12 h dark: light cycle (lights off at 1000 h) with food and water available ad libitum. Animals were bilaterally ovariectomized (OVX) under isofluorane anesthesia. Following surgery, animals were allowed a 10 day recovery period. All procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Hormones and Methamphetamine Treatment
17-β-estradiol benzoate (EB) and progesterone (P) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The EB (10μg/0.1ml) and P (500μg/0.1ml) were dissolved in sesame oil for subcutaneous (SC) administration. METH (Sigma-Aldrich) was dissolved in sterile saline vehicle to a concentration of 5mg/ml for intraperitoneal (IP) injection. All injections were administered between 0900 and 1000 h. Forty-eight hours before the start of the behavioral testing, the animals were hormonally primed with 5μg EB followed by 10μg EB 24 h later (Figure 1). Four hours prior to testing, the rats were injected with P (500 μg); the treatment paradigm was used in our previous study (Holder et al., 2010). This hormone dosing regime mimics the gradual rise in hormones that occurs during the estrous cycle and reliably induces female receptivity (Todd et al., 2005; Schwarz and McCarthy, 2008; Hadjimarkou et al., 2008; Holder et al., 2010). During the three days of hormonal priming, rats received a daily injection of METH (5mg/kg/day) or saline vehicle in a repeated administration paradigm that mimics the self-administration pattern seen in METH users (Haile et al., 2009). This dose of METH was also previously demonstrated to facilitate proceptive and receptive sexual behaviors (Holder et al., 2010).
Figure 1.
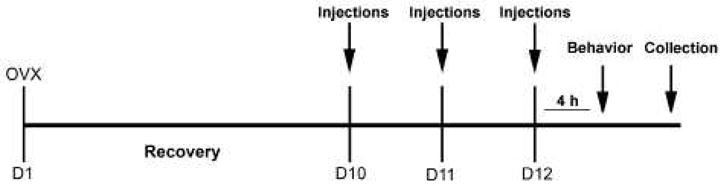
Experimental Timeline. Rats were ovariectomized (OVX; D1) and allowed to recover. On D10, rats were primed with 5 μg estradiol benzoate (EB). On D11, rats were primed with 10 μg EB. Four hours before the behavior on D12, animals received an injection of 500μg progesterone (P). METH (5mg/kg) or saline vehicle was co-administered on each day of hormonal priming. Immediately following the behavior on D12, collections for protein analysis via Western blotting were performed.
Paced Mating Behavioral Testing
The behavioral tests were conducted under dim red light in the dark phase of the light cycle between 1300 and 1600 h, approximately 4 h after the last METH administration. Each behavioral test was recorded by a video camera for analysis at a later time by an investigator blind to the treatment groups. During the tests, the investigator remained at a consistent location approximately 0.5 m away from the observation chambers.
Natural variations in the degree of paced mating behaviors are present in in-bred rat strains and typically correlate with the degree of receptivity (Erskine, 1989). To avoid any potential group biases and to ensure that there were no potential outliers, all animals were first tested for sexual receptivity as described previously (Holder et al., 2010). Only the rats that exhibited sexual receptivity, defined by a lordosis quotient (LQ) of greater than 80% were used in the paced mating paradigm. This receptivity testing revealed very little variation between the animals; only one animal failed to meet the receptivity criterion and was removed from the study.
The rats were then randomly assigned to one of two groups and treated with ovarian hormones and either METH (n=7) or saline vehicle (n=8) for three days and were tested for paced mating behavior on the third day of METH treatment. Additionally, six control animals were treated with oil vehicle and either METH (n=3) or saline vehicle (n=3) and tested for paced mating behavior. These oil-treated groups served as control tissue in the subsequent western blot analysis of spinophilin protein levels.
Paced mating behavior was observed in a clear Plexiglas arena (90 cm long × 45 cm wide × 24 cm high) with clean bedding covering the floor. The arena was divided into three compartments, the middle observational chamber (50 × 45 × 24) and two escape chambers (15 × 45 × 24 cm) on either side. Each escape chamber was demarcated by a partition with two openings (5.0 cm) on the right and left of center and separated by 17 cm.
Five minutes before the start of the mating test, the male rat was placed into the center compartment. At the start of the mating test, the experimental female rat was placed in one of the two outer chambers. Each behavioral test was recorded by a video camera and was completed when 25 min had elapsed. Paced mating tests were terminated if no intromissions were received within the first 15 min of the test. Any female that failed to demonstrate paced mating behavior (i.e., did not received 10 intromissions) initially was re-tested with a new male. All females received at least 10 intromissions.
Prior to the experimental session, all animals underwent three acclimations sessions: two without the male present and one with the male. The females during these sessions were allowed to explore the entire extent of the arena and obtain knowledge about escape routes and escape strategy. None of these sessions were incorporated into the results.
Quantification of Paced Mating Behavior
The contact-return latency and percentage of exits of the female in response to each mating stimulus (mounts, intromissions and ejaculations) from the male were calculated. Contact-return latency represented the time elapsed before the female reentered the male’s compartment following mating stimulation. Percentages of exits represented the frequency of withdrawals by the female from the male’s compartment following mating stimulation. Additionally, receptive and proceptive behaviors were scored as described previously (Holder et al., 2010) and below. The time spent with the male, the number of female solicitations (headwise orientations followed by a runaway), proceptive behaviors (hops, darts and ear wiggling), rejection behaviors (kicks and defensive postures), and arena crossings (sum of entries and exits from the male rat’s compartment) were also quantified. Finally, the quantitative (lordosis quotient) and qualitative (lordosis intensity score; LS) parameters of lordosis were scored as previously described (Holder et al., 2010).
Western blot immunoblotting
Immediately following paced mating behavior, animals were asphyxiated by CO2 (Figure 1). Brains were removed and immediately flash frozen in dry-ice-chilled isopentane and stored at −80°C until use. Starting at approximately −2.56 mm from Bregma, brains were cut into three consecutive 300 μm sections on a cryostat. The MeA, VMN and cortex were micropunched using the Palkovits micropunch procedure (Palkovits and Brownstein, 1988) using a punch approximately 1 mm in diameter. The punch targeted to the MeA used the optic tract as a medial boundary and the bottom of the brain as the ventral boundary. Punches targeted to the VMN and the cortex were consistent with established laboratory protocols (Blutstein et al., 2006).
The micropunched tissue was homogenized in buffer containing 1% NP-40 (Sigma), 0.5% Sodium Deoxycholate (Sigma), 0.1% SDS (Quality Biological, Inc, Gaithersburg, MD), 150mM NaCl, 50mM Tris HCl (pH8). At the time of homogenization, protease and phosphatase inhibitors (1:1000 each; Sigma) were added. Protein concentration was determined using a bicinchoninic acid assay kit (Pierce, Rockford, IL). Protein (10 μg) was loaded into a 10% Tris-glycine SDS-PAGE gel (Invitrogen, Carlsbad, CA). Electrophoresed proteins were blotted onto a polyvinyl difluoride membrane (Invitrogen).
The membranes were washed in 20 mM Tris-buffered saline solution with 0.05% Tween 20 (T-TBS) and then blocked for 1 hr in 5% powdered milk at room temperature. After the milk block, the membranes were incubated in spinophilin (1:5000; Millipore Corporation, Billerica, MA) antibody solution (2.5% powdered milk in T-TBS) overnight at 4°C. Following this incubation, membranes were washed three times in T-TBS and incubated for 1 hr at room temperature in rabbit secondary antibody solution (T-TBS, 1:2000). The Phototype-HRP chemiluminescent system (Cell Signaling Technology, Danvers, MA) was used for to detect the 120 kDa spinophilin immunoblot. The blots were exposed to Hyperfilm-ECL (Kodak, Rochester, NY). The films were then scanned into a computer at 1200 dpi, and the scanned images were analyzed using NIH Image software (http://rsb.info.nih.gov/nih-image). The optical densities were measured for each individual band. Membranes were then stained with Ponceau S solution (0.5% Ponceau in 1% glacial acetic acid made in dH2O), which appears at approximately 45 kDa for standardization to correct for any errors in sample loading (Olesen and Auger, 2005; Schwarz et al., 2008; Wright and McCarthy, 2009). The integrative grayscale pixel area densitometry captured with a CCD camera was quantified with NIH Image software as described above.
Statistical analyses
Results are expressed as means ± SEM. The distribution of data did not deviate significantly from normality. Student’s t-tests were used for two-group comparisons. A two-way ANOVA with hormone and drug treatment as independent measures, followed by Bonferroni t-test post hoc comparisons, was used to analyze spinophilin protein expression. All statistical tests were conducted using the GraphPad Prism program (San Diego, CA, USA) on a Macintosh Duo-core computer.
Results
Paced Mating Behavior
There was a significant effect of METH treatment on the contact return latency following a mount [t(13) = 1.930, p<0.05; Figure 2A], but there was no significant difference in the mean percent exits [t(13) = 0.545, p=0.24; Figure 2B]. METH treatment caused a reduction in the contact return latency following an intromission, but it did not reach statistical significance [t(13) = 1.477, p=0.08; Figure 2C]; however, METH treatment did significantly decrease the likelihood to exit the male’s chamber following an intromission [t(13) = 2.360, p<0.05; Figure 2D]. METH also significantly decreased the contact return latency following an ejaculation [t(13) = 1.887, p<0.05; Figure 2E], but had no effect on the likelihood to leave the male’s chamber. It should be noted that all animals left the male’s chamber following an ejaculation regardless of treatment group. Finally, animals treated with oil vehicle did not show any paced mating or sexual behavior and have, therefore, been excluded from these analyses.
Figure 2.
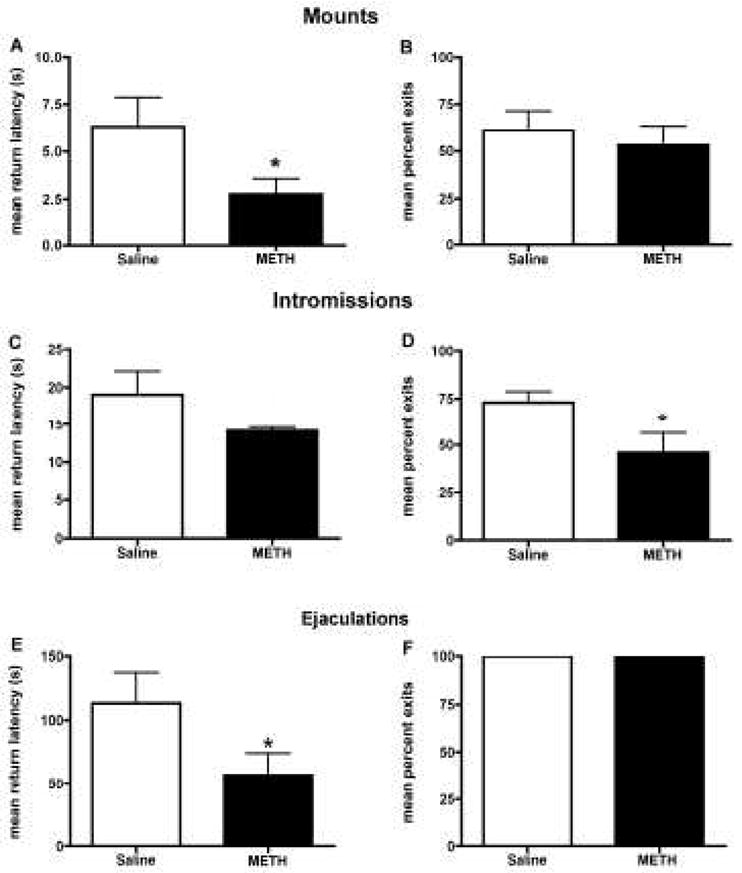
Effect of methamphetamine (METH) on paced mating behavior. Effects of METH on (A) contact return latency and (B) the percentage of exits following a mount. METH treatment significantly decreased the time for the female to return to the male following a mount (*p<0.05), but there was no effect on the likelihood of exiting the male’s chamber. Effects of METH on (C) contact return latency and (D) the percentage of exits following an intromission. There was no effect of METH on the time for the female to return to the male following an intromission. However, METH treatment did decrease the likelihood of exiting the male’s chamber following an intromission (*p<0.05). Effects of METH on (E) contact return latency and (F) the percentage of exits following an ejaculation. METH treatment significantly decreased the time for the female to return to the male following an ejaculation (*p<0.05), but there was no effect on the likelihood of exiting the male’s chamber. Student’s t-tests were performed for each analysis. Data are represented as means ± SEM (n = 7–8 animals in each group).
Appetitive Sexual Behavior during Paced Mating Behavior
Females treated with METH showed a significant increase in the number of solicitations [t(13) = 2.980, p<0.001; Figure 3A] and proceptive events [t(13) = 5.635, p<0.0001; Figure 3B]. Additionally, METH treatment caused a significant decrease in the number of rejections behaviors [t(13) = 3.457, p<0.01; Figure 3C]. METH treatment increased the time spent in the male’s chamber (733.7s ± 95.77), compared to saline control (562.0 ± 52.98), but it did not reach statistical significance [t(13) = 1.624, p=0.06].
Figure 3.
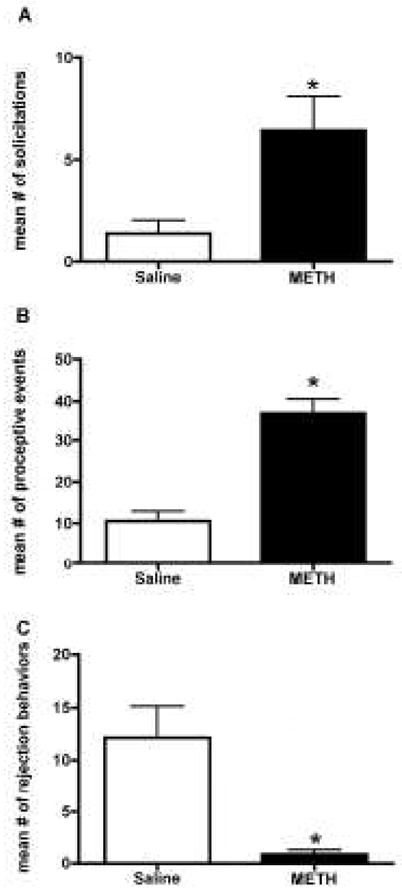
Effect of methamphetamine (METH) on appetitive sexual behaviors observed during paced mating behavior. METH treatment significantly increased the mean number of (A) solicitation and (B) proceptive behaviors compared to the saline-controls. A t-test indicated that METH treatment significantly decreased the mean number of (C) rejection behaviors compared to the saline-controls. Student’s t-tests were performed for each analysis. Data are represented as means ± SEM (n = 7–8 animals in each group).
Receptive Sexual Behavior during Paced Mating Behavior
METH treatment increased sexual receptivity as shown by an increase in both the LQ [t(13) = 3.293, p<0.001; Figure 4A] and LS [t(13) = 3.652, p<0.01; Figure 4B].
Figure 4.
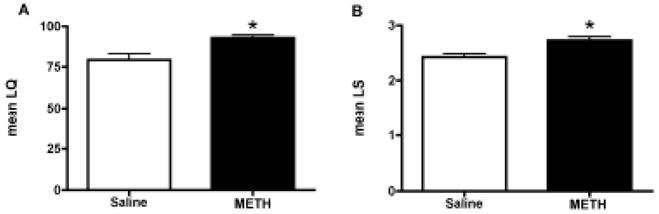
Effect of methamphetamine (METH) on receptive sexual behaviors observed during paced mating behavior. METH treatment also increased the lordosis response as quantified by the (A) Lordosis Quotient (LQ) and (B) Lordosis Intensity Score (LS) compared to the saline-treated controls. Student’s t-tests were performed for each analysis. Data are represented as means ± SEM (n = 7–8 animals in each group).
Arena Crossings during Paced Mating Behavior
To measure the locomotor activity during the paced mating behavior, we examined the arena crossings. There was no significant difference in the mean arena crossing between METH treated animals (51.29 ± 8.0) and saline-treated controls (51.25 ± 7.9).
Spinophilin Protein Levels
A two-way ANOVA revealed a significant main effect of ovarian hormones on spinophilin protein levels in the MeA [F(1,17) = 31.24, p<0.0001; Figure 5A, B]. A Bonferroni post hoc test revealed that the combination of ovarian hormones and METH significantly increased spinophilin protein levels by approximately 60% (p<0.05), compared to hormonally-primed, saline-treated controls. Additionally, ovarian hormones increased spinophilin, compared to the respective oil-treated controls (p<0.05). There was neither a significant interaction between ovarian hormones and METH [F(1,17) = 1.558, p=0.27], nor an effect of METH [F(1,17) = 2.043, p=0.17] on spinophilin protein levels in the MeA.
Figure 5.
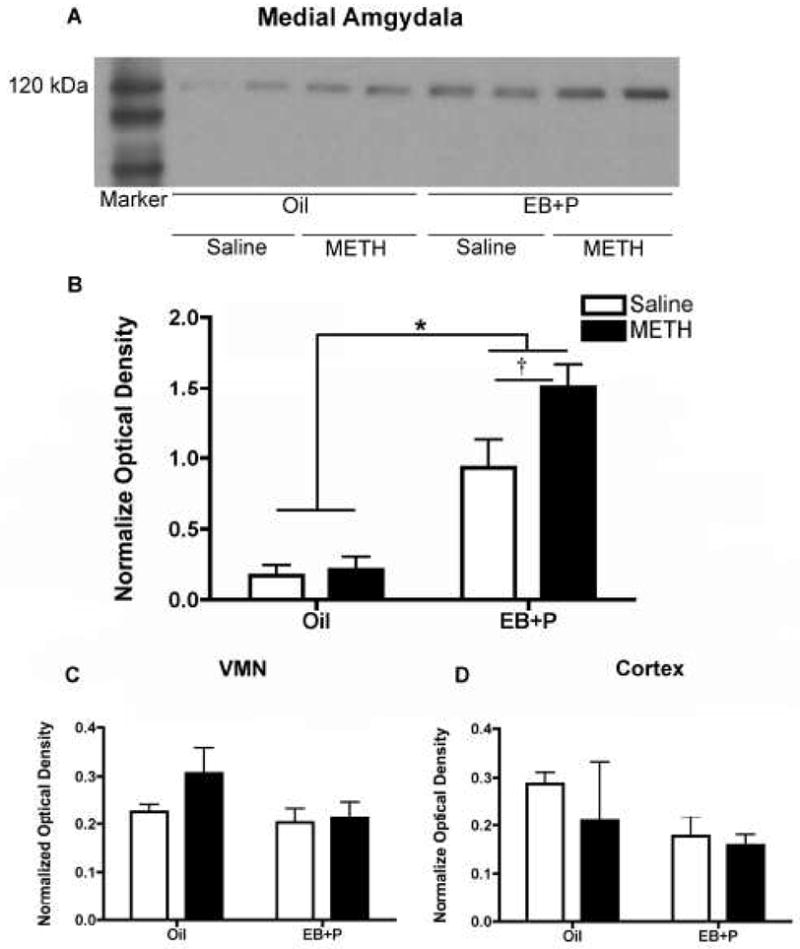
Effect of methamphetamine (METH) on spinophilin protein expression. (A) Representative spinophilin immunoblots of micropunched tissue taken from the Medial Amgydala (MeA) of animals previously tested for paced mating behavior. A total of 10 μg of total protein was loaded into each lane. The blots were probed with a rabbit polyclonal antibody against spinophilin that recognized a band a 120 kDa. Each lane represents tissue from one animal. (B) Quantification of MeA western blots. A two-way ANOVA revealed a significant main effect of hormones on spinophilin protein levels, compared to oil-controls (*p<0.05). A Bonferroni post hoc comparison revealed a significant difference between hormone-primed rats treated with METH and with saline (†p<0.05). (C) Quantification of Ventromedial Nucleus of the Hypothalamus (VMN) western blots. A two-way ANOVA revealed no significant effect of either ovarian hormones or METH treatment on spinophilin protein levels. (D) Quantification of Cortex western blots. A two-way ANOVA revealed no significant effect of either ovarian hormones or METH treatment on spinophilin protein levels. Data are represented as the mean ratio of the optical density of the spinophilin-immunoreactive band to that of the Ponceau stain band ± SEM (oil controls, n = 3 per group; EB+P-treated animals, n = 7–8 per group).
In the VMN, there was no significant interaction of ovarian hormones and METH [F(1,17) = 1.995, p=0.18; Figure 5C], nor was there an significant effect of either ovarian hormones [F(1,17) = 0.6508, p=0.43] or METH [F(1,17) = 1.049, p=0.32]. Similarly, there was no significant interaction of ovarian hormones and METH [F(1,17) = 0.3053, p=0.59; Figure 5D], nor was there an significant effect of either ovarian hormones [F(1,17) = 2.257, p=0.15] or METH [F(1,17) = 0.8154, p=0.38], in the cortex.
Discussion
Previously, we have demonstrated that administration of METH to hormonally-primed female rats increases proceptive and receptive behaviors (Holder et al., 2010) leading us to posit that METH may augment female sexual motivation. Here, using a paced mating paradigm, we found that METH increased female-initiated sexual behaviors. This change in behavior was correlated with an increase in spinophilin protein levels in the MeA. These findings suggest that METH-induced changes in paced mating and potentially female sexual motivation may result from modification of the synaptic connections in the MeA by ovarian hormones and METH.
A variety of behavioral paradigms have been used to test aspects of female sexual motivation such as (i) the partner preference test in which the female chooses between a preferred mate versus non-preferred mate (Rivas and Mir, 1990; Paredes and Vazquez, 1999), (ii) sexual incentive motivation in which the female’s intensity of approach to a stimulus animal is measured (Ågmo, 1999; Ellingsen and Agmo, 2004), and (iii) paced mating behavior in which the female controls the occurrence and rate of sexual interaction (McClintock and Adler, 1978; Erskine, 1989). In the present study, a paced mating paradigm was chosen for its ethological relevance and ease in creating a semi-natural environment to facilitate pacing.
Overall, females treated with METH were less likely to leave a male following a sexual contact (i.e., mounting or intromission) and when they did leave, they returned sooner. Moreover, METH treatment enhanced the solicitation and proceptive behaviors as well as the lordosis responses that occurred during the paced mating. Importantly, METH did not increase the mean number of arena crossings, suggesting that observed behaviors were not due to a generalized effect on locomotor activity. In general, a highly sexually motivated female rat will display a reduction in escapes and time to return to the male following all types of sexual contact (Ågmo, 2007). Thus the METH-induced reduction in the return latency and escapes (percent exits) may suggest that METH is specifically enhancing female-initiated behaviors. In further support, METH increases the number of sexual contacts by females in a partner preference paradigm suggesting enhanced sexual motivation (Guarraci, 2009).
Of note in the present study, METH treatment did not uniformly reduce the return latencies and percent exits following a sexual stimulation (Figure 2B and 2C, respectively). One possible explanation is variations in the intensity of the male stimulation. The pattern of paced mating behavior depends upon the level of sensory stimulation received from the male; the more intense the stimulation the greater the percent exits and return latency (Erskine, 1989; Paredes and Vazquez, 1999; Ågmo, 2007).
Previously we have reported that the combined administration of ovarian hormones and METH increases the neuronal activation, as measured by Fos-immunoreactivity, in the MeA and VMN of female rats over either one alone (Holder et al., 2010). This finding is unique to these nuclei as other key areas associated with reward (both natural and drug) such as the nucleus accumbens, and the basolateral and central amygdala were only stimulated by METH. Conversely, preoptic area and the bed nucleus of the stria terminalis were stimulated by hormones or unaffected, respectively (Holder et al., 2010). While the VMN is indisputable in the expression of female sexual behavior, MeA is uniquely situated to act as a convergence point for METH and hormone actions since (i) it receives direct catecholaminergic input from the ventral tegmental area (VTA) and locus coeruleus (Gray, 1999; Pitkänen, 2000) (ii) is a steroid concentrating region (Pfaff and Keiner, 1973; Simerly et al., 1990), (iii) its output targets several major hypothalamic nuclei essential for social and reproductive behavior (Kevetter and Winans, 1981), (iv) is implicated in modulation of female sexual behavior including but not limited to paced mating (reviewed in Erskine, 1989) and (v) lesions decrease the expression of proceptive behavior (Afonso et al., 2009). In fact the current finding that the combination of hormones and METH significantly increased spinophilin levels over hormones alone in the MeA, but not the VMN or cortex further supports the assumption that the MeA is a potential drug-sex nexus.
The METH-induced increase in spinophilin protein levels in the MeA also suggests a potential alteration in synaptic connectivity. Numerous studies have demonstrated that spinophilin expression consistently tracks changes in dendritic spine density (Zhou et al. 2010; Allen, 2004; Sarrouilhe et al., 2006; Todd et al., 2007; Prange-Kiel et al., 2009), supporting its role as a marker of structural plasticity (Sarrouilhe et al., 2006). Repeated administration of METH induces structural plasticity in nuclei traditionally associated with the mesolimbic reward pathway such as the nucleus accumbens, medial prefrontal cortex (Robinson and Kolb, 2004) and the dorsal striatum (Jedynak et al., 2007). Similarly, spine density in the MeA of the female rat is sensitive to changes in the ovarian hormonal milieu (de Castilhos et al., 2008). Interestingly, in the absence of ovarian hormones, METH did not affect spinophilin levels in the MeA, suggesting that METH may have a synergistic effect with ovarian hormones on synaptic connectivity. To the best of our knowledge, this is the first report of a synergistic action between ovarian hormones and METH on spine plasticity in the MeA.
It should be noted that the examination of spinophilin was conducted following paced mating behavioral testing. Thus, the possibility remains that the behavior, in addition to the hormonal priming and METH treatment, could have influenced the spinophilin levels in the MeA.
Our current data demonstrate that the combination of METH and ovarian hormones increased behaviors indicative of female sexual motivation. Coincident with the METH-induced change in behavior was an increase in spinophilin levels specific to the MeA. Based on these findings it is tempting to speculate that METH converges with ovarian hormone action in the MeA to increase the activity of the circuitry underlying sexual motivation and behavior.
Acknowledgments
This research was supported by NIH grant DA024943 awarded to Mary K Holder. The NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, Lehmann H, Tse M, Woehrling A, Pfaus JG. Estrogen and the neural mediation of female-male mounting in the rat. Behav Neurosci. 2009;123:369–81. doi: 10.1037/a0014121. [DOI] [PubMed] [Google Scholar]
- Ågmo A. Sexual motivation--an inquiry into events determining the occurrence of sexual behavior. Behav Brain Res. 1999;105:129–50. doi: 10.1016/s0166-4328(99)00088-1. [DOI] [PubMed] [Google Scholar]
- Ågmo A. Sexual and Dysfunctional Sexual Behavior. Elsevier Ltd; New York, NY: 2007. [Google Scholar]
- Allen PB. Functional plasticity in the organization of signaling complexes in the striatum. Parkinsonism Relat Disord. 2004;10:287–92. doi: 10.1016/j.parkreldis.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A. 1997;94:9956–61. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutstein T, Devidze N, Jasnow AM, Pfaff DW, Mong JA. Oestradiol up-regulates glutamine synthetase mRNA and protein expression in the hypothalamus and hippocampus: implications for a role of hormonally responsive glia in amino acid neurotransmission. J Neuroendocrinol. 2006;18:692–702. doi: 10.1111/j.1365-2826.2006.01466.x. [DOI] [PubMed] [Google Scholar]
- Corsi KF, Booth RE. HIV sex risk behaviors among heterosexual methamphetamine users: literature review from 2000 to present. Curr Drug Abuse Rev. 2008;1:292–6. doi: 10.2174/1874473710801030292. [DOI] [PubMed] [Google Scholar]
- de Castilhos J, Forti CD, Achaval M, Rasia-Filho AA. Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: a Golgi study. Brain Res. 2008;1240:73–81. doi: 10.1016/j.brainres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Ellingsen E, Agmo A. Sexual-incentive motivation and paced sexual behavior in female rats after treatment with drugs modifying dopaminergic neurotransmission. Pharmacology, Biochemistry, & Behavior. 2004;77:431–445. doi: 10.1016/j.pbb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: A review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–92. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS. Functional and anatomical relationships among the amygdala, basal forebrain, ventral striatum, and cortex. An integrative discussion. Ann N Y Acad Sci. 1999;877:439–44. doi: 10.1111/j.1749-6632.1999.tb09281.x. [DOI] [PubMed] [Google Scholar]
- Guarraci FA. “Sex, drugs and the brain”: The interaction between drugs of abuse and sexual behavior in the female rat. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–92. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. Am J Drug Alcohol Abuse. 2009;35:161–77. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder MK, Hadjimarkou MM, Zup SL, Blutstein T, Benham RS, McCarthy MM, Mong JA. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus. Psychoneuroendocrinology. 2010;35:197–208. doi: 10.1016/j.psyneuen.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Nitta A, Mizoguchi H, Mohri A, Murai R, Miyamoto Y, Noda Y, Kitaichi K, Yamada K, Nabeshima T. Effects of single and repeated administration of methamphetamine or morphine on neuroglycan C gene expression in the rat brain. Int J Neuropsychopharmacol. 2006;9:407–15. doi: 10.1017/S1461145705005870. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–53. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sakuma Y. The medial amygdala controls the coital access of female rats: a possible involvement of emotional responsiveness. Jpn J Physiol. 2005;55:345–53. doi: 10.2170/jjphysiol.RP001105. [DOI] [PubMed] [Google Scholar]
- Mansergh G, Purcell DM, Stall R, McFarlane M, Semann S, Valentine J, Valdiserri R. CDC consultation on methamphetamine use and sexual risk behavior for HIV/STD infection: Summary and suggestions. Public Health Rep. 2006;121:127–132. doi: 10.1177/003335490612100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock MK, Adler NT. Induction of persistent estrus by airborne chemical communication among female rats. Horm Behav. 1978;11:414–8. doi: 10.1016/0018-506x(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Yoshida S, Yamashita M, Fujiyama K, Toda S, Matsuoka H, Kajii Y, Nishikawa T. Altered EphA5 mRNA expression in rat brain with a single methamphetamine treatment. Neurosci Lett. 2007;424:116–21. doi: 10.1016/j.neulet.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. J Neuroendocrinol. 2005;17:255–61. doi: 10.1111/j.1365-2826.2005.01302.x. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain. Elsevier; New York, NY: 1988. [Google Scholar]
- Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav Brain Res. 1999;105:117–27. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Ågmo A. Reproductive motivation. In: Phashler H, Gallistel R, editors. Steven’s Handbook of Experimental Psychology. Learning, Motivation, and Emotion. Wiley; New York: 2002. pp. 709–736. [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila G. What Can Animal Models Tell Us About Human Sexual Response? Annu Rev Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. Oxford University Press; New York: 2000. pp. 31–115. [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Jarry H, Rune GM. Estrus cyclicity of spinogenesis: underlying mechanisms. J Neural Transm. 2009;116:1417–25. doi: 10.1007/s00702-009-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Washton A, Domier CP, Reiber C. Drugs and sexual effects: Role of drug type and gender. J Subst Abuse Treat. 2002;22:103–108. doi: 10.1016/s0740-5472(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Rivas FJ, Mir D. Effects of nucleus accumbens lesion on female rat sexual receptivity and proceptivity in a partner preference paradigm. Behav Brain Res. 1990;41:239–249. doi: 10.1016/0166-4328(90)90111-q. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88:1099–113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–98. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm Behav. 2008;54:662–8. doi: 10.1016/j.yhbeh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SJ, Grant I, Patterson TL. Female methamphetamine users: Social characteristics and sexual risk behavior. Women Health. 2004a;40:35–50. doi: 10.1300/j013v40n03_03. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. The context of sexual risk behavior among heterosexual methamphetamine users. Addict Behav. 2004b;29:807–10. doi: 10.1016/j.addbeh.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2005;48:512–21. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABA(A) receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev Neurobiol. 2007;67:304–15. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- Wright CL, McCarthy MM. Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling. J Neurosci. 2009;29:13274–82. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology. 2010;151:1153–60. doi: 10.1210/en.2009-0254. [DOI] [PubMed] [Google Scholar]


