Abstract
An ethanolic extract of Antiaris toxicaria trunk bark showed potent in vitro cardiotonic effect on isolated guinea pig atria. Bioassay-guided fractionation of the extract led to identification of 9 new cardiac glycosides (1–9, named antiarosides A-I), antiarotoxinin A (10), and 18 known compounds. Their structures were established using MS and NMR spectroscopic studies, including homonuclear and heteronuclear correlation experiments. The ability of these cardiotonic compounds to produce positive inotropic action and their safety indexes were examined in comparison with those of ouabain, a classical inhibitor of Na+/K+-ATPase. Malayoside (23) was nearly equipotent and had a similar safety index to ouabain in guinea pig atria. However, the maximal positive inotropic effect and safety index of 23 in papillary muscle were better than those of ouabain. An electrophysiological recording showed that 23 inhibited sodium pump current in a concentration-dependent manner.
Cardenolides are a group of C23 steroids produced in nature by several plant families and some species of toads. Because a major site of their biological action is the heart, cardenolide glycosides are also known as cardiac glycosides. These compounds share common features of a steroidal aglycone linked at the 3β-OH group to one or more sugar moieties. Some cardiac glycosides are highly toxic to humans and animals. Despite their toxicity, certain glycosides have therapeutic effects and, at appropriate doses, have been used in treatment of congestive heart failure. The only receptor for these compounds is the integral membrane protein Na+/K+-ATPase. Cardiac glycosides inhibit Na+/K+-ATPase, resulting in a positive inotropic effect at therapeutic doses, but also in cardiac arrhythmias and death at toxic doses. Apart from their very widely known cardiotonic effects, cardiac glycosides may also inhibit cancer cell replication. Cardiac glycosides also act directly on the gastrointestinal tract, causing hemorrhagic enteritis and diarrhea.
Antiaris toxicaria (Pers.) Lesch. (Moraceae), commonly known as upas tree, is a well known toxic plant that is widely distributed throughout Malaysian forests. The latex of A. toxicaria, called “Jianxiefenghou” in Chinese, has an unwarranted reputation for killing people who fall asleep beneath it. It has been known for centuries that most poisoned darts used by indigenous people of Southeast Asia are prepared by concentration of latex harvested from A. toxicaria. Prey wounded by such an arrow can rarely move more than 100 meters. These poisons act as powerful muscle relaxants to paralyze the prey, but have no effect when the meat is eaten.1 Bisset reported that animals shot with poisoned darts “died with tetanic convulsions,” indicating that A. toxicaria-derived poisons function through the bloodstream.2 The notoriety of these materials prompted investigations of their constituents, and they were found to be a good source of cardenolide cardiac glycosides.3–7 The active principles were studied by Robinson and Ling, who observed cardiac irregularities and death when extracts of A. toxicaria were injected into cats.8 Fujimoto et al. first found that cardiac glycosides inhibited the activity of Na+/K+-ATPase.9
In the course of a drug-screening project on medicinal plants, an extract from the trunk bark of A. toxicaria was found to have a strong cardiotonic effect. Bioassay-guided fractionation led to the isolation and characterization of twenty-eight cardiac glycosides/aglycones, including new compounds 1–10, designated as antiarosides A (1), B (2), C (3), D (4), E (5), F (6), G (7), H (8), and I (9) and antiarotoxinin A (10). This study also evaluated the positive inotropic effect and safety index of these compounds in guinea pig heart muscle. The goals were to (a) find an effective and safe inotropic drug for improving hemodynamics in patients with heart failure and (b) establish structure-activity relationships of cardiac glycosides. Herein, we report the maximal positive inotropic effect and safety index of new compounds 1–10, as well as of known compounds 11–14 and 16–28, in guinea pig heart muscle. The positive control was ouabain, a classical inhibitor of Na+/K+-ATPase.
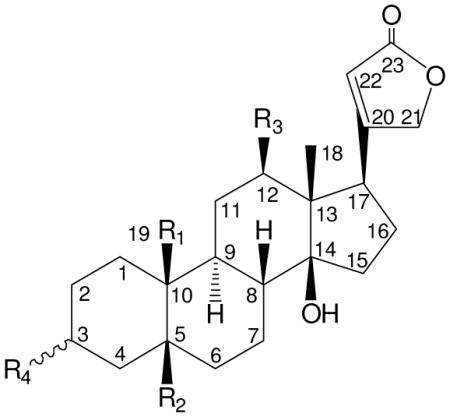 | ||||
|---|---|---|---|---|
| Compd | R1 | R2 | R3 | R4 |
| 1 | CH3 | OH | H | β-O-β-D-antiarose |
| 2 | CH2OH | H | H | β-O-α-L-rhamnosyl-(4→1)-β-D-glucose |
| 3 | CHO | OH | H | α-O-α-L-rhamnose |
| 4 | CHO | OH | H | α-O-α-L-rhamnosyl-(4→1)-β-D-glucose |
| 5 | COOH | H | H | β-O-α-L-rhamnose |
| 6 | COOglc | H | H | β-O-α-L-rhamnose |
| 7 | COOH | OH | OH | β-O-α-L-rhamnose |
| 8 | COOH | OH | OH | β-O-β-D-antiarose |
| 9 | H | OH | H | β-O-α-L-rhamnose |
| 23 | CHO | H | H | β-O-α-L-rhamnose |
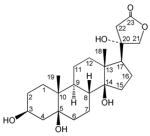 10 | ||||
Results and Discussion
Fresh trunk bark of A. toxicaria was extracted with ethanol. The residue obtained after evaporation of the ethanol extract was partitioned between CHCl3, n-BuOH, and H2O. The CHCl3, n-BuOH and H2O soluble fractions were concentrated and the extracts were individually subjected to column chromatography (CC) over silica gel and Diaion HP-20, respectively. The subfractions obtained were examined by H2SO4 test solution spray on TLC for cardiac glycosides, which show as green spots. The enriched cardiac glycoside fractions were subjected to a series of column chromatographic steps (silica gel, semipreparative reversed-phase HPLC, Sephadex LH-20) in order to obtain pure cardenolides 1–28, which were characterized by analysis of their spectroscopic data.
Compound 1 had molecular formula C29H44O9, as established from HRFABMS ([M + Na]+ m/z 559.3063) and by the presence of 29 signals in the 13C NMR spectrum. The NMR signals were due to three methyl, ten methylene, ten methine and six quaternary carbons as determined using DEPT 135 spectroscopy (Table 1). Compound 1 showed a UV absorption maximum at 213 nm and IR absorption at 1738 cm−1 (γ-lactonic carbonyl), which were indicative of a butenolactone system.10 The 1H NMR spectrum of 1 (Table 2) showed characteristic signals of a butenolactone ring at δ 5.01 and 5.28 (each 1H, dd, J = 18.0, 1.3 Hz, H-21a and b) and δ 6.12 (s, H-22), and methyl singlets at δ 1.03 and 1.08 (each 3H, s, H-18 and 19), indicating 1 to be a cardenolide with a C-19 methyl group. A doublet anomeric proton at δ 5.37 (J = 8.1 Hz, H-1′), four oxymethine protons between δ 4.10 and 4.71 and a methyl signal at δ 1.56 (3H, d, J = 6.4 Hz), together with the fragment ion at m/z 373 in FABMS, pointed to the presence of a β-linked deoxyhexose unit (Table 1). The deoxyhexose was concluded to be β-antiarose on the basis of 1H and 13C NMR data (Tables 1 and 2) and analyses of ROESY, COSY and HMQC experiments.7,11 The proton resonating at δ 4.45 (H-2′), a double doublet (J = 8.1, 2.9 Hz), indicated an axial-axial relationship to H-1′ and an axial-equatorial relationship to H-3′ (d, J = 2.9 Hz). Based on the strong NOE effects between H-1′ and H-5′, both protons are in axial positions. Furthermore, the measured JC1′-H1′ (157.6 Hz) for the anomeric axial proton is consistent with β-D chemistry of the sugar moiety.12 Thus, based on the above data, the sugar moiety was elucidated as β-D-antiarose. The aglycone was identified as periplogenin13 by analyses of COSY, HMQC, HMBC and ROESY experiments and comparison to literature data. The sugar unit was placed at C-3 on the basis of the glycosylation shifts of C-2 (δ 26.9), C-3 (δ 74.4), and C-4 (δ 34.5), and HMBC correlation between H-1′ of the antiarose unit and C-3 of the aglycone moiety. The β-orientation of antiarose at C-3 was deduced from the W1/2 constant of H-3 (br. s, W1/2 = 10.7 Hz). Thus, 1 was identified as periplogenin 3-O-β-antiaropyranoside and was named antiaroside A.
Table 1.
13C NMR Data of Compounds Isolated from A. toxicaria.
| Carbon | 1 | 2 | 3‡ | 4 | 5 | 6 | 7‡ | 8‡ | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26.2 | 24.9 | 25.8 | 25.8 | 21.8 | 21.8 | 21.9 | 21.8 | 22.2 | 25.9 |
| 2 | 26.9 | 26.9 | 30.7 | 30.7 | 26.8 | 26.3 | 26.5 | 27.0 | 29.2 | 28.6 |
| 3 | 74.4 | 72.7 | 72.5 | 72.6 | 71.2 | 71.6 | 72.9 | 73.4 | 71.6 | 67.8 |
| 4 | 34.5 | 27.4 | 35.5 | 35.5 | 29.1 | 26.5 | 36.2 | 36.0 | 31.9 | 35.9 |
| 5 | 73.8 | 30.2 | 71.3 | 72.7 | 32.8 | 32.4 | 73.9 | 73.7 | 72.2 | 74.5 |
| 6 | 36.2 | 30.5 | 42.8 | 42.9 | 29.7 | 30.1 | 36.6 | 37.5 | 32.0 | 37.9 |
| 7 | 24.6 | 21.8 | 22.3 | 22.3 | 26.5 | 22.2 | 24.5 | 24.8 | 27.1 | 24.4 |
| 8 | 41.1 | 42.0 | 42.9 | 42.9 | 42.3 | 41.9 | 40.4 | 40.4 | 41.5 | 39.7 |
| 9 | 39.4 | 35.9 | 41.9 | 41.9 | 35.7 | 36.0 | 36.3 | 36.4 | 38.4 | 39.1 |
| 10 | 41.3 | 39.4 | 56.2 | 56.2 | 49.7 | 50.4 | 54.9 | 55.1 | 37.4 | 41.4 |
| 11 | 22.0 | 22.0 | 22.0 | 22.0 | 22.5 | 28.7 | 33.3 | 33.0 | 20.9 | 21.4 |
| 12 | 40.1 | 40.5 | 39.6 | 39.7 | 40.2 | 40.1 | 74.6 | 74.7 | 39.8 | 40.4 |
| 13 | 50.0 | 50.3 | 50.0 | 50.0 | 50.5 | 50.0 | 57.0 | 57.0 | 50.2 | 49.2 |
| 14 | 84.8 | 85.0 | 84.4 | 84.4 | 84.9 | 84.9 | 85.3 | 85.3 | 84.5 | 85.1 |
| 15 | 33.3 | 33.1 | 32.8 | 32.8 | 33.0 | 32.9 | 32.9 | 33.2 | 33.1 | 44.2 |
| 16 | 27.4 | 27.2 | 27.2 | 27.2 | 27.4 | 27.3 | 28.0 | 28.1 | 27.4 | 23.7 |
| 17 | 51.5 | 51.6 | 51.4 | 51.4 | 51.5 | 51.4 | 46.6 | 46.6 | 51.5 | 59.0 |
| 18 | 16.2 | 16.3 | 16.0 | 16.0 | 16.4 | 16.2 | 10.5 | 10.5 | 16.2 | 16.6 |
| 19 | 17.2 | 65.6 | 208.3 | 208.3 | 179.5 | 175.7 | 176.9 | 176.8 | 17.4 | |
| 20 | 175.7 | 175.9 | 175.7 | 175.6 | 176.4 | 176.0 | 176.7 | 177.2 | 176.2 | 79.5 |
| 21 | 73.7 | 73.8 | 73.7 | 73.7 | 73.9 | 73.7 | 74.1 | 74.1 | 73.8 | 81.1 |
| 22 | 117.8 | 117.7 | 117.8 | 117.8 | 117.7 | 117.7 | 117.5 | 117.5 | 117.6 | 32.3 |
| 23 | 174.3 | 174.5 | 174.4 | 174.4 | 174.7 | 174.5 | 174.8 | 174.9 | 174.6 | 176.3 |
| 1′ | 100.0 | 99.8 | 99.9 | 99.7 | 99.7 | 100.0 | 100.5 | 99.8 | 99.9 | |
| 2′ | 69.5 | 72.4 | 72.8 | 71.7 | 73.0 | 72.8 | 73.0 | 69.6 | 72.9 | |
| 3′ | 73.7 | 72.8 | 72.8 | 72.3 | 72.9 | 72.8 | 72.6 | 73.7 | 72.9 | |
| 4′ | 73.3 | 85.5 | 74.2 | 85.1 | 74.1 | 74.2 | 73.9 | 73.7 | 74.2 | |
| 5′ | 69.9 | 68.4 | 69.9 | 68.2 | 70.3 | 70.0 | 70.7 | 70.0 | 70.2 | |
| 6′ | 16.9 | 18.5 | 18.6 | 18.4 | 18.8 | 18.6 | 18.7 | 17.1 | 18.7 | |
| 1″ | 106.9 | 106.7 | 95.7 | |||||||
| 2″ | 76.5 | 76.4 | 74.1 | |||||||
| 3″ | 78.6 | 78.6 | 79.1 | |||||||
| 4″ | 71.7 | 21.3 | 71.3 | |||||||
| 5″ | 78.4 | 78.4 | 79.4 | |||||||
| 6″ | 62.8 | 62.8 | 62.5 |
δ value in pyridine-d5 (100 MHz).
δ value in pyridine-d5 (75 MHz).
Table 2.
1H NMR Data of Compounds Isolated from A. toxicaria.
| Proton | 1 | 2 |
|---|---|---|
| 1a/b | 1.44 (m)/2.19 (dd, 14.2, 2.9) | 1.66 (br. t, 4.1)/2.33 (ddd, 13.5, 13.5, 3.1) |
| 2a/b | 1.79 (m)/2.02 (br. d, 13.0) | 1.72 (m)/1.89 (m) |
| 3 | 4.51 (br. s, 10.7) † | 4.15 (br. s, 13.6) † |
| 4a/b | 1.88 (m)/2.15 (dd, 15.2, 2.9) | 1.25 (br. d, 12.8)/2.01 (m) |
| 5 | 2.55 (br. d, 13.3) | |
| 6a/b | 1.54 (m)/1.95 (m) | 1.57 (m)/1.89 (m) |
| 7a/b | 1.33 (m)/2.23 (br. d, 11.7) | 1.43 (m)/2.14 (m) |
| 8 | 1.83 (m) | 2.01 (m) |
| 9 | 1.67 (br. t, 11.4) | 1.89 (m) |
| 10 | ||
| 11a/b | 1.38 (br. d, 10.0)/1.44 (m) | 1.43 (m) |
| 12a/b | 1.44 (m) | 1.48 (m) |
| 15a/b | 1.88 (m)/2.08 (m) | 1.89 (m)/2.17 (br. dd, 10.4, 10.4) |
| 16a/b | 1.95 (m)/2.08 (m) | 2.01 (m)/2.14 (m) |
| 17 | 2.81 (dd, 8.8, 5.0) | 2.81 (dd, 9.0, 5.1) |
| 18 | 1.03 (s) | 1.04 (s) |
| 19a/b | 1.08 (s) | 3.77 (dd, 10.9, 5.5)/4.07 (d, 10.9) |
| 21a/b | 5.01 (dd, 18.0, 1.3)/5.28 (dd, 18.0, 1.3) | 5.01 (d, 18.0)/5.29 (d, 18.0) |
| 22 | 6.12 (s) | 6.12 (s) |
| 1′ | 5.37 (d, 8.1) | 5.37 (br. s) |
| 2′ | 4.45 (dd, 8.1, 2.9) | 4.49 (br. s) |
| 3′ | 4.71 (d, 2.9) | 4.62 (dd, 9.1, 2.1) |
| 4′ | 4.10 (br. s) | 4.34 (d, 9.1) |
| 5′ | 4.58 (q, 6.4) | 4.29 (dq, 9.1, 6.0) |
| 6′ | 1.56 (d, 6.4) | 1.70 (d, 6.0) |
| 1″ | 5.18 (d, 7.7) | |
| 2″ | 4.05 (d, 7.7) | |
| 3″ | 4.18 (d, 9.1) | |
| 4″ | 4.21 (d, 9.1) | |
| 5″ | 3.79 (m) | |
| 6″a/b | 4.39 (m)/4.43 (m) | |
| Proton | 3 | 4 |
| 1a/b | 2.07 (m)/2.34 (dd, 10.1, 4.2) | 2.03 (m)/2.32 (m) |
| 2a/b | 1.61 (m)/2.29 (m) | 1.57 (ddd, 13.4, 13.4, 4.3)/2.28 (m) |
| 3 | 4.66 (dddd, 11.2, 11.2, 5.0, 4.3) | 4.60 (dddd, 10.4, 10.4, 5.1, 5.1) |
| 4a/b | 1.78 (br. d, 13.6)/2.42 (dd, 13.6, 4.3) | 1.72 (m)/2.39 (dd, 13.5, 5.1) |
| 5 | ||
| 6a/b | 1.68 (m)/2.22 (m) | 1.63 (m)/1.77 (m) |
| 7a/b | 1.68 (m)/2.29 (m) | 2.11 (br. dd, 8.0, 3.7)/2.28 (m) |
| 8 | 2.22 (m) | 2.16 (ddd, 13.7, 13.7, 4.3) |
| 9 | 1.40 (m) | 2.23 (m) |
| 10 | ||
| 11a/b | 1.40 (m)/1.68 (m) | 1.43 (m)/1.68 (br. d, 10.2) |
| 12a/b | 1.40 (m) | 1.41 (m)/1.27 (dd, 14.3, 3.1) |
| 15a/b | 1.29 (dd, 13.6, 3.0)/1.83 (m) | 1.41 (m)/1.85 (m) |
| 16a/b | 2.13 (m)/1.91 (m) | 2.05 (ddd, 13.5, 3.5, 3.4)/1.90 (ddd, 13.5, 9.0, 4.8) |
| 17 | 2.02 (dd, 8.8, 4.8) | 1.99 (m) |
| 18 | 2.73 (dd, 9.3, 4.8) | 2.74 (ddd, 9.0, 4.8) |
| 19a/b | 0.95 (s)/10.12 (s) | 0.96 (s)/0.13 (s) |
| 21a/b | 4.98 (dd, 18.0, 1.3)/5.25 (dd, 18.0, 1.3) | 4.97 (dd, 18.0, 1.4)/5.25 (dd, 18.0, 1.4) |
| 22 | 6.09 (s) | 6.09 (s) |
| 1′ | 5.36 (br. s) | 5.30 (br. s) |
| 2′ | 4.43 (br. s) | 4.40 (br. s) |
| 3′ | 4.44 (dd, 8.6, 3.3) | 4.54 (d, 10.1) |
| 4′ | 4.21 (m) | 4.32 (dd, 10.1, 9.3) |
| 5′ | 4.26 (dq, 8.6, 5.8) | 4.18 (m) |
| 6′ | 1.59 (d, 5.8) | 1.63 (d, 6.1) |
| 1″ | 5.20 (d, 7.7) | |
| 2″ | 4.08 (dd, 8.1, 7.7) | |
| 3″ | 4.18 (m) | |
| 4″ | 4.18 (m) | |
| 5″ | 3.78 (ddd, 8.4, 4.5, 4.5) | |
| 6″a/b | 4.34 (m)/4.42 (m) | |
| Proton | 5 | 6 |
| 1a/b | 1.60 (ddd, 12.6, 12.6, 3.1)/2.51 (m) | 1.55 (ddd, 14.0, 10.0, 2.6)/2.37 (ddd, 12.9, 12.9, 3.8) |
| 2a/b | 1.99 (m)/2.54 (m) | 1.64 (m)/1.91 (m) |
| 3 | 4.52 (br. s, 12.4) † | 4.17 (br. s, 10.7) † |
| 4a/b | 1.38 (m)/1.99 (m) | 2.00 (m)/2.64 (dd, 14.0, 3.3) |
| 5 | 2.92 (br. d, 12.6) | 2.88 (br. d, 11.9) |
| 6a/b | 1.70 (m)/1.97 (m) | 1.64 (m)/1.86 (m) |
| 7a/b | 1.67 (m)/1.91 (m) | 1.41 (m)/2.07 (m) |
| 8 | 2.59 (m) | 2.52 (ddd, 11.7, 11.7, 2.4) |
| 9 | 1.91 (m) | 1.91 (m) |
| 10 | ||
| 11a/b | 1.42 (dd, 129, 12.9)/2.17 (m) | 1.20 (m)/2.00 (m) |
| 12a/b | 1.48 (m) | 1.41 (m) |
| 15a/b | 1.87 (m)/2.11 (m) | 1.86 (m)/2.12 (m) |
| 16a/b | 2.06 (m) | 2.00 (m)/2.12 (m) |
| 17 | 2.84 (dd, 8.3, 4.2) | 2.79 (br. dd, 8.4, 5.2) |
| 18 | 1.20 (s) | 1.17 (s) |
| 19 | ||
| 21a/b | 5.06 (d, 18.0)/5.35 (d, 18.0) | 5.00 (d, 18.1)/5.28 (d, 18.1) |
| 22 | 6.14 (s) | 6.10 (s) |
| 1′ | 5.48 (br. s) | 5.39 (br. s) |
| 2′ | 4.57 (br. s) | 4.46 (m) |
| 3′ | 4.52 (d, 8.2) | 4.46 (m) |
| 4′ | 4.32 (m) | 4.22 (m) |
| 5′ | 4.32 (m) | 4.30 (dq, 6.0, 6.0) |
| 6′ | 1.67 (d, 5.1) | 1.65 (d, 6.0) |
| 1″ | 6.29 (d, 8.0) | |
| 2″ | 4.13 (dd, 8.0, 7.7) | |
| 3″ | 4.22 (m) | |
| 4″ | 4.22 (m) | |
| 5″ | 4.01 (br. dd, 7.1, 4.0) | |
| 6″a/b | 4.31 (dd, 5.9, 3.4)/4.38 (d, 11.3) | |
| Proton | 7‡ | 8‡ |
| 1a/b | 2.27 (br. d, 14.7)/2.97 (br. d, 14.7) | 2.28 (d, 16.4)/3.16 (dd, 14.7, 14.7) |
| 2a/b | 1.78 (br. d, 14.1)/1.98 (m) | 1.87 (m)/2.16 (m) |
| 3 | 4.35 (br. s, 9.3) † | 4.51 (br. s, 12.1) † |
| 4a/b | 1.87 (br. d, 14.5)/1.98 (m) | 2.00 (m)/2.16 (m) |
| 5 | ||
| 6a/b | 1.65 (m)/3.13 (br. d, 13.1) | 1.69 (br. d, 12.6)/3.11 (m) |
| 7a/b | 1.48 (m)/2.49 (d, 12.7) | 1.47 (br. d, 13.4)/2.48 (br. d, 11.9) |
| 8 | 3.06 (br. d, 10.1) | 3.04 (m) |
| 9 | 2.19 (m) | 2.00 (m) |
| 10 | ||
| 11a/b | 1.98 (m)/2.39 (dd, 7.4, 3.7) | 2.00 (m) |
| 12a/b | -/3.81 (br. d, 10.8) | -/3.77 (br. d, 6.9) |
| 15a/b | 1.98 (m)/1.98 (m) | 2.00 (m)/2.35 (m) |
| 16a | 2.16 (m) | 2.16 (m) |
| 17 | 3.75 (t, 7.5) | 3.75 (t, 7.9) |
| 18 | 1.26 (s) | 1.26 (s) |
| 19 | ||
| 21a/b | 5.13 (d, 18.1)/5.29 (d, 18.1) | 5.12 (d, 18.1)/5.28 (d, 18.1) |
| 22 | 6.24 (s) | 6.25 (s) |
| 1′ | 5.54 (br. s) | 5.44 (d, 8.1) |
| 2′ | 4.53 (br. s) | 4.49 (dd, 8.1, 2.6) |
| 3′ | 4.46 (br. d, 4.7) | 4.76 (br. s) |
| 4′ | 4.29 (m) | 4.14 (d, 3.0) |
| 5′ | 4.27 (br. q, 3.7) | 4.62 (q, 6.2) |
| 6′ | 1.65 (d, 3.7) | 1.56 (d, 6.2) |
| 1″ | ||
| 2″ | ||
| 3″ | ||
| 4″ | ||
| 5″ | ||
| 6″a/b | ||
| Proton | 9 | 10 |
| 1a/b | 1.38 (br. d, 13.8)/2.19 (br. d, 17.0) | 1.48 (m)/2.24 (m) |
| 2a/b | 1.65 (m)/2.00 (m) | 1.82 (m) |
| 3 | 4.22 (br. s, 9.1) † | 4.44 (br. s, 12.8) † |
| 4a/b | 1.79 (m)/2.10 (m) | 1.78 (d, 10.1)/2.29 (d, 14.6) |
| 6a/b | 1.79 (m)/2.10 (m) | 1.62 (br. d, 13.0)/1.96 (dt, 9.1, 4.0) |
| 7a/b | 1.26 (m)/2.41 (br. d, 7.9) | 2.24 (m) |
| 8 | 2.43 (br. d, 11.5) | 1.97 (dt, 9.1, 4.0) |
| 9 | 2.31 (br. d, 12.5) | 1.70 (br. t, 10.7) |
| 10 | 1.83 (d, 12.5) | |
| 11a/b | 1.06 (br. d, 12.0)/1.90 (m) | 1.45 (ddd, 10.6, 9.6, 4.0) |
| 12a/b | 1.44 (m) | 1.36 (dt, 13.3, 10.6)/1.48 (dt, 13.3, 9.6) |
| 15a/b | 1.90 (m)/2.10 (m) | 1.84 (m)/2.04 (m) |
| 16 | 2.00 (m) | 2.04 (m) |
| 17 | 2.10 (m) | 2.14 (dd, 8.2, 5.7) |
| 18 | 2.79 (br. d, 7.8) | 1.27 (s) |
| 19 | 1.06 (s) | 1.17 (s) |
| 21a/b | 5.03 (d, 18.1)/5.33 (d, 18.1) | 4.40 (d, 9.4)/4.78 (d, 9.4) |
| 22 | 6.12 (s) | 2.82 (d, 16.8)/2.86 (d, 16.8) |
| 1′ | 5.44 (br. s) | |
| 2′ | 4.50 (m) | |
| 3′ | 4.50 (m) | |
| 4′ | 4.29 (m) | |
| 5′ | 4.29 (m) | |
| 6′ | 1.65 (d, 3.8) | |
| 1″ | ||
| 2″ | ||
| 3″ | ||
| 4″ | ||
| 5″ | ||
| 6″a/b |
δ value in pyridine-d5 (400 MHz), coupling constants in Hz are given in parentheses.
W1/2 (Hz): width of 1/2 peak high.
δ value in pyridine-d5 (300 MHz), coupling constants in Hz are given in parentheses.
The HRFAB mass spectrum of 2 showed a molecular ion-related [M + K]+ peak at m/z 737.8260, corresponding to the molecular formula C35H54O14. The 1H and 13C NMR data (Tables 1 and 2) and fragment ions at m/z 519 and 356 in FABMS indicated 2 was a cardenolide disaccharide with two hexose sugar units. Analyses of COSY, HMQC and HMBC spectra indicated that the sugars were α-rhamnose and β-glucose with a (4-1) linkage. The downfield shift of C-4′ of the rhamnose unit to δ 85.5, and C-1″ of the glucose unit at δ 106.9, and an HMBC correlation between H-4′ and C-1″ confirmed the (4-1) linkage between them. The 13C NMR spectrum (Table 2), combined with DEPT 135, HMQC and HMBC experiments, of 2 indicated that the aglycone was cannogenol (15), which was isolated from the CHCl3-soluble fraction. Location of the sugar unit at C-3 was suggested by the downfield shift of C-3 from δ 66.1 in 15 to δ 72.7 in 2 and a HMBC correlation between H-1′ and C-3. The β-orientation of the C-3 disaccharide unit was deduced from the W1/2 constant of H-3 (br s, W1/2 = 13.6 Hz). Compound 2 was thus assigned to be 3 β-[(O-β-glucopyranosyl(1-4)-α-rhamnopyranosyl)oxy]cannogenol, and it was named antiaroside B.
Compound 3 showed a pseudo molecular ion peak at m/z 551.2855 in its HRFABMS and had the same molecular formula as convallatoxin (17), C29H42O10. UV, IR, 1H, 13C NMR, and MS spectroscopic analyses indicated that 3 was a stereoisomer of convallatoxin (17). Downfield shifts of C-1, C-2, and C-6 from δ 18.7, 25.4, and 36.9 in 17 to δ 25.8, 26.0, and 42.8 in 3 indicated that the orientation of C-3 was different from that of 17. The α-orientation of C-3 was deduced from the coupling constant values of H-3 (dddd, J = 11.2, 11.2, 5.0, 5.0 Hz). This assignment was supported by downfield shifts of H-2b, H-3, and H-4 from δ 2.00 (m), 4.32 (br. s, W1/2 = 8.3 Hz), 2.13 (d, J = 15.3 Hz), and 1.72 (m) in 17 to δ 2.29 (m), 4.66 (dddd, J = 11.2, 11.2, 5.0, 5.0 Hz), 2.42 (dd, J = 13.6, 4.3 Hz), and 1.78 (br. d, J = 17.1 Hz) in 3. HMBC correlation of H-1′ with C-3 [ δC 72.5/δH 5.36 (br. s)], and NOE correlation between H-3 and H-1′ inferred that the rhamnose unit was linked to C-3. Hence, structure 3 was established for antiaroside C.
Compound 4 was assigned molecular formula C35H52O15 by HRFABMS. Comparison of the 1H and 13C NMR spectra of 4 with those of 3 showed that the two structures were very similar, except for one additional sugar unit in 4. Based on its larger [M + K]+ ion at 751, 162 mass units more than that of 3, and appropriate sugar proton and carbon signals in the NMR spectra, 4 has one glucosyl unit in addition to a rhamnosyl moiety. The H-1″ signal appeared at δ 5.20 and showed HMBC correlation with the downfield shifted C-4′ (δ 85.1), as well as NOE with H-4′. These data determined the interglycosydic linkage of the two sugar moieties as α-rhamnosyl (4′-1′)-β-glucoside. A HMBC correlation between H-1′ and C-3 (δ 72.5) suggested that the sugar unit was attached at C-3, and α-orientation was deduced from the coupling type and constant values of H-3 (δ 4.69, dddd, J = 10.4, 10.4, 5.1, 5.1 Hz). Thus, the structure of 4 was deduced as 4′-O-β-glucopyranosyl antiaroside C and was named antiaroside D.
Compound 5 had the same molecular formula as 17 (C29H42O10). Comparison of the 1H/13C NMR spectra of 5 and 17 showed that the two structures were very similar, except for the absence of signals for both an aldehyde and one oxygenated carbon in the former. A strong carbonyl absorption in the IR spectrum at 1738 cm−1 and a carbon signal at δ 179.5 in the 13C NMR spectrum suggested that a carboxylic acid rather than aldehyde group was present at C-19. A proton signal at δ 2.92 (br. d, J = 12.6 Hz) in the 1H NMR was assignable to H-5, since it coupled with H-4 and -6 in the COSY spectrum. Absence of a carbon signal at δ 73.9 (C-5 in 17) and presence of a carbon signal at δ 32.8 (C-5 in 5) suggested that the OH group on C-5 in 17 was not present in 5. This postulate was supported by upfield shifts of C-4 and C-6 from δ 35.5 to δ 29.1 and δ 36.9 to δ 29.7, together with HMBC correlations of H-3, 4b, 6b, and 7b to C-5. In a ROESY experiment, a correlation between H-5 and H-1b (δ 3.29) determined β-orientation of H-5. An anomeric proton signal at δ 5.48 (br. s) and carbon signals at δ 99.7, 73.0, 72.9, 74.1, 70.3, and 18.8 indicated the presence of a rhamnosyl moiety. The HMBC correlation between H-3 and C-1′ placed the rhamnosyl unit on C-3, and a W1/2 coupling constant of 12.4 Hz for H-3 indicated α-orientation. Therefore, the structure 5 was established for antiaroside E.
Compound 6 (C35H52O15) had fragment ions at m/z 550 and 534 in the FABMS, and two anomeric signals in the 1H/13C NMR spectra indicated that 6 was a diglycoside with β-glucopyranose and α-rhamnose sugar units. The 1H and 13C NMR data of 6 were very close to those of 5, except for the added signals of a glucopyranose moiety and the position of C-19 (Tables 1 and 2). An anomeric proton signal at δ 6.29 (d, J = 8.0 Hz) in the 1H NMR spectrum and signals at δ 95.7, 74.1, 79.1, 71.3, 79.4, and 62.5 in the 13C NMR spectrum suggested the presence of β-glucose. The upfield shift of C-19 from δ 179.5 to δ 175.7, the downfield shift of the anomeric proton to δ 6.29, and a 3J HMBC correlation from H-1″ to C-19 suggested that the glucose unit was attached to C-19. Thus, the structure of 6 was determined as 19-O-β-glucopyranosyl antiaroside E, and it was named antiaroside F.
Compound 7 (C29H42O12) was 16 mass units larger than β-antiarin (28),13 isolated from the CHCl3 soluble fraction. The 1H and 13C NMR spectra of 7 were quite similar data to those of 28, except for absence the aldehyde signal in 7. Thus, 7 was likely a 19-nor-β-antiarin derivative. The major differences were that the proton signal at δ 10.37 (s) and the carbonyl signal at δ 208.5 in 28 disappeared in 7, and instead one carboxyl carbon signal appeared at δ 176.8. Thus, the aldehyde group of 28 was replaced by a carboxylic acid group in 7. This conclusion was supported by strong IR absorption at 1726 cm−1. The β-orientations of OH groups at C-3 and -12 were deduced from the coupling constants of H-3 (δ 4.35, br. s, W1/2 = 9.3 Hz), and H-12 (br. d, J = 10.8 Hz). On the basis of the above data, the structure of 7 was established, and it was named antiaroside G.
Compound 8 had a molecular formula the same as that of 7 (C29H42O12). The UV, IR and NMR data strongly resembled those of 7, consistent with a general structure containing a central cardenolide moiety trioxygenated at C-3, C-5, and C-12 and a carboxylic acid group in the 19-position. The sole significant differences observed were in signals of the glycosidic part of the molecules (Tables 1 and 2). The sugar proton signals of 8 indicated the presence of a β-antiarosyl moiety. These data were in agreement with the replacement of the rhamnosyl unit in 7 by a antiarosyl unit in 8. The β-orientation of C-3 was deduced by the W1/2 of H-3 (br. s, 12.1 Hz). Thus, structure of 8 was assigned, and it was named antiaroside H.
A molecular formula of C28H42O9 was deduced for compound 9, 14 mass units less than that of periplorhamnoside (11), which was isolated from the same extract. Comparison of its 1H and 13C NMR data with those of 11 showed that they were similar except for the absence of C-19 methyl group, the presence of one methine (-CH) at δ 1.83 (d, 12.5), and an upfield shift of C-10 from δ 41.2 to δ 37.4 in 9. Thus, 9 was determined as demethylperiplorhamnoside and it was named antiaroside I.
Compound 10 (C23H36O6) had 23 signals in the 13C NMR spectrum corresponding to two methyl, eleven methylene, four methine, and six quaternary carbon atoms. The 1H and 13C NMR spectra of 10 displayed signals characteristic of the steroid core of a cardenolide. However, the absence of typical signals for the olefinic group of the butenolactone ring and the downfield resonance of C-23 to 176.3, suggested that the carbonyl group of the five-membered lactone in 10 was not conjugated with a double bond. This was confirmed by the carbonyl absorption at 1761 cm−1 in the IR spectrum, the presence of two methylene groups at δC 81.1/δH 4.78 and 4.40 (each 1H, d, J = 16.8 Hz, H-21) and δC 32.3/δH 2.86 and 2.82 (each 1H, d, J = 16.8 Hz, H-22) and the absence of any significant UV absorption. Three OH groups were on the steroid skeleton (C-3, C-5, C-14) and a fourth OH group was placed at C-20; this latter carbon resonated at δ 79.5 and no extra oxygenated signal, other than aforementioned, was observed in the spectrum of 10. HMBC correlations from H-16, H-21b, and H-22 to C-20 and from H-21b to C-22 confirmed the OH group at C-20. Orientation of the C-3 OH was also determined by the W1/2 of H-3 (br s, 12.1 Hz). The above analysis established the structure of 10 as shown, and the compound was named antiarotoxinin A.
Periplorhamnoside (11),13 cheiranthoside VII (12),11 strophanthidol (13),14 convallatoxol (14),5 cannogenol (15),15 strophanthidin (16),13 convallatoxin (17),5 strophathojavoside (18),16 desglucocheirotoxin (19),14 strophalloside (20),13 convalloside (21),17 glucostrophalloside (22),18 malayoside (23),5 antiarigenin (24),13 α-antiarin (25),5 antialloside (26),16 toxicarioside B (27),7 and β-antiarin (28)13 were also isolated from A. toxicaria trunk bark. These known compounds were identified by comparison of their physical and spectroscopic properties with those reported in the literature.
Minimal effective concentrations (PIECmin) to increase contraction of rat left atria were 0.05, 0.25, 1 and 7.5 μM for ouabain, 23, 16 and 8, respectively. In right ventricular muscle, the PIECmin for the positive inotropic action of different cardiac glycosides varied from 0.075 μM to 8.33 μM (Table 3). Compounds with lower PIECmin may have stronger binding affinity to Na+/K+-ATPase of cardiac muscle.
Table 3.
Minimal Positive Inotropic Effectctive Concentration (PIECmin) of Cardiac Glycosides in Atria and Ventricular Strips.
| Left atria strips | Right ventricular strips | ||
|---|---|---|---|
| Cmpd | PIECmin (μM) | Cmpd | PIECmin (μM) |
| 1 | 0.10±0.07 | 1 | 0.083±0.03 |
| 2 | 0.38±0.13 | 2 | 0.075±0.05 |
| 3 | 2.75±0.25 | 3 | 4±1 |
| 4 | 2.75±0.25 | 4 | 2.75±0.25 |
| 5 | 0.42±0.08 | 5 | 1.33±0.6 |
| 6 | 1.42±0.58 | 6 | 3.17±0.93 |
| 7 | 3.75±1.25 | 7 | 3.67±1.33 |
| 8 | 7.50±2.50 | 8 | 8.33±1.67 |
| 9 | 0.83±0.17 | 9 | 0.67±0.17 |
| 10 | 0.1 | 10 | - |
| 11 | 0.05±0.01 | 11 | 0.25±0.1 |
| 12 | 0.1 | 12 | 0.25 |
| 13 | 1.25±0.75 | 13 | 0.30±0.20 |
| 14 | 0.1 | 14 | 0.25 |
| 16 | 1 | 16 | 4.8±1.5 |
| 17 | 0.1 | 17 | 0.125 |
| 18 | 0.25 | 18 | 1 |
| 19 | 0.05 | 19 | 0.5 |
| 20 | 0.5 | 20 | 0.5 |
| 21 | 0.25 | 21 | 1 |
| 22 | 0.25 | 22 | 1 |
| 23 | 0.25 | 23 | 0.25 |
| 24 | 0.05 | 24 | - |
| 25 | 0.5 | 25 | 2 |
| 26 | 0.25 | 26 | - |
| 27 | 0.1 | 27 | - |
| 28 | 1 | 28 | 2 |
| Ouabain | 0.05 | Ouabain | 0.05 |
Maximal contractions after treatment with cardiac glycosides are expressed as a percentage of those before glycoside treatment. For ouabain, 23, 16 and 8, these values were 775, 660, 165 and 144%, respectively, in left atria. In right ventricular muscle, the maximal contractions were 249, 446, 240 and 260% compared to basal values for ouabain, 23, 16 and 8, respectively (Table 4).
Table 4.
Maximal Positive Inotropic Effect (PIEmax) of Cardiac Glycosides in Atria and Ventricular Strips.
| Left atria strips | Right ventricular strips | ||
|---|---|---|---|
| Cmpd | PIEmax (% of basal) | Cmpd | PIEmax (% of basal) |
| 1 | 158±28 | 1 | 165±33 |
| 2 | 373±187 | 2 | 382±99 |
| 3 | 208±34 | 3 | 229±35 |
| 4 | 201±1 | 4 | 369±47 |
| 5 | 264±38 | 5 | 230±19 |
| 6 | 140±28 | 6 | 618±321 |
| 7 | 419±31 | 7 | 221±69 |
| 8 | 144±27 | 8 | 260±33 |
| 9 | 562±236 | 9 | 1246±49 |
| 10 | 367 | 10 | - |
| 11 | 381±104 | 11 | 355±33 |
| 12 | 278 | 12 | 150 |
| 13 | 205±5 | 13 | 408±128 |
| 14 | 138 | 14 | 625 |
| 16 | 165±15 | 16 | 240±74 |
| 17 | 467 | 17 | 300 |
| 18 | 567 | 18 | 200 |
| 19 | 300 | 19 | 300 |
| 20 | 225 | 20 | 350 |
| 21 | 443±105 | 21 | 292±17 |
| 22 | 489 | 22 | 350 |
| 23 | 660±88 | 23 | 464±89 |
| 24 | 121 | 24 | - |
| 25 | 220 | 25 | 200 |
| 26 | 350 | 26 | - |
| 27 | 163 | 27 | - |
| 28 | 243 | 28 | 250 |
| Ouabain | 775±128 | Ouabain | 249±26 |
The safety index (therapeutic index) was calculated from the ratio of the arrhythmogenic concentration to the minimal effective positively inotropic concentration. A narrow margin of safety index restricts the therapeutic use of this class of positive inotropic drugs. For example, the safety index of digitalis is narrow, and arrhythmias are common problems in clinical practice.19 Safety indexes were 20, 20, 9 and 7.5 for ouabain, 23, 16 and 8, respectively, in left atria. Safety indexes of ouabain, 23, 16 and 8 were 20, 24, 8.7 and 9.7, respectively, in right ventricular muscle (Table 5).
Table 5.
Safety Index of Cardiac Glycosides in Atria and Ventricular Strips.
| Left atria strips | Right ventricular strips | ||
|---|---|---|---|
| Cmpd | Safety index | Cmpd | Safety index |
| 1 | 4.3±0.3 | 1 | 18±11.1 |
| 2 | 5.0±1.0 | 2 | 100±60 |
| 3 | 15 | 3 | 9.7±3.7 |
| 4 | 7.3±0.7 | 4 | 6.3±0.3 |
| 5 | 10 | 5 | 7.7±3.5 |
| 6 | 6.0±2.3 | 6 | 5.8±1.0 |
| 7 | 13±1 | 7 | 20.7±10 |
| 8 | 7.5±2.5 | 8 | 9.7±3.3 |
| 9 | 6.7±1.7 | 9 | 10.7±1.8 |
| 10 | 1000 | 10 | - |
| 11 | 11±3.7 | 11 | 8.8±3.3 |
| 12 | 10 | 12 | 2 |
| 13 | 6.3±3.8 | 13 | 65±25 |
| 14 | 5 | 14 | 20 |
| 16 | 9 | 16 | 8.7±3.0 |
| 17 | 10 | 17 | 8 |
| 18 | 10 | 18 | 5 |
| 19 | 50 | 19 | 10 |
| 20 | 2.5 | 20 | 4 |
| 21 | 20 | 21 | 9 |
| 22 | 10 | 22 | 10 |
| 23 | 20 | 23 | 24 |
| 24 | 20 | 24 | - |
| 25 | 10 | 25 | 15 |
| 26 | 80 | 26 | - |
| 27 | 10 | 27 | - |
| 28 | 10 | 28 | 15 |
| Ouabain | 20 | Ouabain | 20 |
Other compounds, such as 2 and 13, had larger safety indexes than ouabain and 23 (100 and 65 for 2 and 13 versus 20 and 24 for ouabain and 23). Maximal contractions after treatment with 2 and 13 were 382 and 408%, respectively. In our previous study, we found that 23 had a larger safety index than ouabain in vivo.20 Whether 2 and 13 have better safety indexes than ouabain or 23 in animals remains to be determined.
The following structure-activity relationships were identified in these studies. Changing the β-O-α-rhamnose in 7 to β-O-β-antiarose in 8 increased PIECmin in atria from 3.75 μM to 7.5 μM and in right ventricular muscle from 3.67 μM to 8.33 μM (Table 3). Comparison of 3 with one sugar (α-O-α-rhamnose) and 4 with two sugars [α-O-α-rhamnosyl(4→1)β-glucose] showed a decrease in PIECmin from 4 to 2.75 μM, in right ventricular muscles. Substitution of the C-18 CH3 of 11 with CH2OH in 14 increased PIECmin in atria from 0.05 to 0.1 μM, but PIECmin in right ventricular muscle remained at 0.25 μM (Table 3). Similarly, changing the C-18 CHO of 23 to COOH in 5 increased PIECmin from 0.25 μM to 0.42 μM in atria and from 0.25 μM to 1.33 μM in right ventricular muscle. Finally, glycosylation of the carboxylic acid increased PIECmin in atria from 0.42 μM (5, COOH) to 1.42 μM (6, COOglc).
To confirm that, like digitalis, 23 exerts an inotropic effect through inhibition of Na+/K+ ATPase, sodium pump current (Ipump) was measured by the whole-cell patch clamp technique. Ipump currents before and after 23 treatment were recorded. Figure 1 shows basal Ipump (filled square), Ipump with 23 at 1 μM (filled triangle) and Ipump with 23 at 3 μM (filled circle). Compound 23 inhibited sodium pump current in a concentration-dependent manner. A detailed mechanistic study of the inotropic effect of 23 in guinea pig has been reported.20
Figure 1.
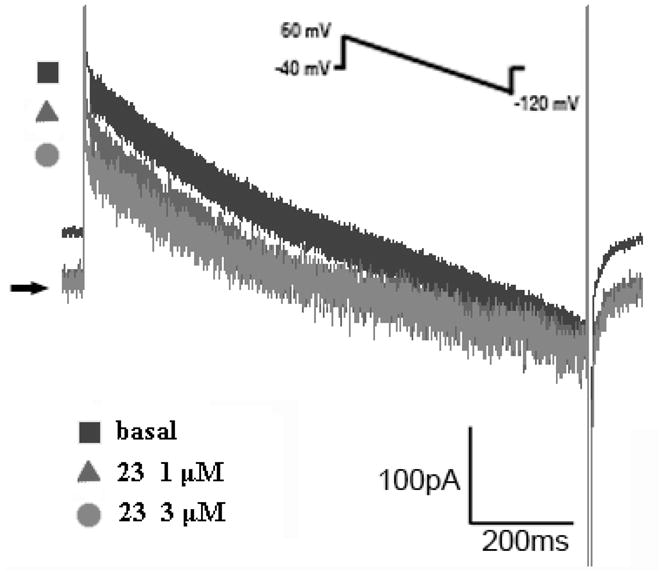
Drug effects on Ipump of basal (filled square), 23 at 1 μM (filled triangle) and 3 μM (filled circle) treated cells.
Several noteworthy conclusions were obtained from this study. Nine new cardiac glycosides (1–9) and one aglycone (10), together with eighteen known cardiac glycosides/aglycones, were isolated from A. toxicaria. The first compounds with COOH (5, 7, 8) and COOglc (6) from this plant were reported. These groups are representative units for the metabolic pathway of cardiac glycosides. A side-by-side evaluation of biological activity properties of the isolated cardiac glycosides provided additional insights into the pharmacological profile of this compound class. Our data showed that α-O-α-rhamnosyl(4→1)-β-glucose linked at the C-3 OH resulted in better PIECmin in right ventricular muscle. The presence of -CH3 at C-10 was better than -CH2OH, -CHO, and -COOH, as measured by PIECmin, in atria and right ventricular muscle. Increasing the polarity of this substituent may be beneficial in cardiac glycosides. Most significantly, 23 increased contractility and inhibited sodium pump current in guinea pig heart preparations in a concentration-dependent manner, and the safety indexes of 2, 13, and 23 were better than those of ouabain in vitro.
Experimental Section
General Experimental Procedures
Proton NMR spectra were recorded on Bruker Avance 300 (300 MHz) and AMX 400 (400 MHz) spectrometers. The chemical shifts (ppm) were measured with tetramethylsilane (TMS) as internal standard and deuterated pyridine as solvent. Mass spectra were performed in the EI mode on a VG 70-250S spectrometer. The optical rotation was recorded on JASCO DIP-370 polarimeter. Merck silica gel 60 (Merck 70–230, 230–400 mesh) was used for column chromatography. Glass sheets of silica gel 60 F254 (Merck 0.2 mm thick) were used for TLC. Melting points were measured on a Yanagimoto MP-S3 micromelting point apparatus and are uncorrected. The UV spectra were recorded on a Hitachi UV-3210 spectrophotometer and IR spectra were determined as KBr discs on a Shimazu FTIR-8501 spectrophotometer.
Plant Material
Trunk bark of A. toxicaria was collected from Yunnan, China, and authenticated by C. S. Kuoh (Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan). A voucher specimen (NCKUWu 92012) has been deposited in the Herbarium of National Cheng Kung University, Tainan, Taiwan, R.O.C.
Extraction and Isolation
The trunk bark of A. toxicaria (6.0 kg) was cut into small pieces and extracted with 95% EtOH (20 L × 3). Evaporation of the solvent under reduced pressure provided 239.0 g of crude extract, which was partitioned between CHCl3-H2O, and n-BuOH-H2O, successively to yield CHCl3 (65.1 g), n-BuOH (100.2 g), and H2O (73.7 g) fractions. The CHCl3 fraction was subjected to silica gel CC using increasing polarity mixtures of n-hexane-acetone as eluant to give 14 fractions. Fraction 6 was chromatographed on silica gel using diisopropyl ether-MeOH (40:1) to obtain 10 (8.7 mg), and 16 (103.2 mg). Fraction 7 was chromatographed on silica gel and eluted with CHCl3-MeOH (15:1) to give 13 (6.6 mg), and 18 (7.0 mg). Fraction 8 was chromatographed on silica gel using EtOAc-MeOH (20:1) to obtain 5 (37.6 mg), 15 (3.2 mg), and 27 (8.8 mg), successively. Fraction 9 was chromatographed on silica gel using EtOAc-MeOH (20:1) to afford 23 (21.4 mg). Fraction 10 was chromatographed on silica gel using EtOAc-MeOH (20:1) to yield 17 (117.2 mg).
The n-BuOH fraction was subjected to Diaion HP-20 CC eluting with a H2O-MeOH gradient system to give 12 fractions. Fraction 5 was chromatographed on silica gel using CHCl3-MeOH (9:1) to obtain 7 (34.7 mg), 8 (147.4 mg), 9 (1.5 mg), 24 (58.9 mg), 25 (11.0 mg), 26 (4.3 mg), and 28 (7.3 mg), successively. Fraction 6 was chromatographed on silica gel using CHCl3-MeOH (9:1) to obtain 25 (118.4 mg). Fraction 7 was chromatographed on silica gel using CHCl3-MeOH-H2O (9:1:0.05) to obtain 21 (11.4 mg). Fraction 8 was chromatographed on silica gel using CHCl3-MeOH-H2O (9:1:0.05) to afford 2 (12.3 mg), 3 (112.3 mg), 4 (12.2 mg), 6 (5.9 mg), 12 (9.1 mg), 14 (6.1 mg), 17 (431.2 mg), 18 (4.3 mg), 20 (5.9 mg), 21 (79.8 mg), and 22 (18.3 mg). Fraction 9 was chromatographed on silica gel using CHCl3-MeOH-H2O (9:1:0.05) to obtain 11 (7.6 mg), 17 (12.5 mg), 19 (19.7 mg), and 20 (14.3 mg). Fraction 10 was chromatographed on silica gel using CHCl3-MeOH-H2O (9:1:0.05) to obtain 1 (9.8 mg), 11 (3.3 mg), 19 (10.2 mg), 20 (3.5 mg), and 27 (13.5 mg).
The water fraction was directly subjected to Diaion HP-20 CC eluting with water containing increasing proportions of MeOH to give twelve fractions. Fraction 8 was chromatographed on a Sephadex LH-20 column using mixtures of MeOH-H2O of increasing polarity to obtain 25 (20.2 mg), and 26 (1.3 mg). Fraction 9 was chromatographed on Sephadex LH-20 eluting with water containing increasing proportions of MeOH to give 4 (26.4 mg), 17 (43.2 mg), and 21 (26.8 mg).
Antiaroside A (1): colorless needles (CHCl3-MeOH); mp 184–186 °C; [α]25 D −24.4 (c 0.09, MeOH); UV (MeOH) λmax (log ε) 213 (4.67) nm; IR (KBr) υmax 3450, 2939, 1738, 1622, 1450, 1383, 1078, 1038 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 559 [M+23]+ (16), 537 (47), 391 (29), 373 (43), 355 (100), 337 (54), 277 (47), 185 (98); HRFABMS m/z 537.3063 [M + 1]+ (calcd for C29H45O9, 537.3064).
Antiaroside B (2): colorless syrup; [α]25 D −20.09 (c 0.12, MeOH); UV (MeOH) λmax (log ε) 218 (4.32) nm; IR (KBr) υmax 3400, 2936, 1738, 1730, 1655, 1067, 1030 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 737 [M + 39]+ (3), 721 [M + 23]+ (3), 699 (2), 519 (3), 356 (5), 185 (100), 147 (25); HRFABMS m/z 737.8260 [M + 39]+ (calcd for C35H54KO14 737.8262).
Antiaroside C (3): colorless powder (CHCl3-MeOH); mp 231–232 °C; [α]25 D −11.31 (c 1.03, MeOH); UV (MeOH) λmax (log ε) 213 (4.17) nm; IR (KBr) υmax 3440, 2934, 1734, 1715, 1618, 1454, 1344, 1198, 1057 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 551 [M + 1]+ (25), 405 (13), 387 (35), 369 (36), 351 (18), 341 (22), 323 (26), 185 (100), 179 (12), 147 (54); HRFABMS m/z 551.2855 [M + 1]+ (calcd for C29H43O10, 551.2856).
Antiaroside D (4): colorless syrup; [α]25 D −21.73 (c 0.39, MeOH); UV (MeOH) λmax (log ε) 213 (4.37) nm; IR (KBr) υmax 3400, 2932, 1734, 1647, 1456, 1067, 1030 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 751 [M + 39]+ (7), 549 (2), 403 (6), 387 (6), 369 (6), 359 (4), 342 (6), 341 (8), 323 (12), 207 (16), 185 (100), 179 (3), 163 (7), 147 (19); HRFABMS m/z 751.2943 [M + 39]+ (calcd for C35H52KO15, 751.2943).
Antiaroside E (5): colorless syrup; [α]25 D −29.07 (c 0.25, MeOH); UV (MeOH) λmax (log ε) 216 (4.40) nm; IR (KBr) υmax 3460, 2936, 1738, 1670, 1453, 1076, 1036 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 551 [M + 1]+ (30), 507 (10), 462 (17), 417 (38), 387 (23), 359 (15), 341 (26), 323 (35), 315 (57), 277 (25), 185 (100), 147 (63); HRFABMS m/z551.2856 [M + 1]+ (calcd for C29H43O10, 551.2856).
Antiaroside F (6): colorless syrup; [α]25 D −19.61 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 216 (4.42) nm; IR (KBr) υmax 3400, 1726, 1655, 1647, 1642, 1545, 1533, 1460 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 751 [M + 39]+ (7), 713 (3), 550 (3), 534 (2), 490 (3), 241 (6), 185 (100), 147 (6); HRFABMS m/z 751.2943 [M + 39]+ (calcd for C35H53O15, 751.2945).
Antiaroside G (7): colorless syrup; [α]25 D +3.07 (c 0.3472, MeOH); UV (MeOH) λmax (log ε) 218 (4.67) nm; IR (KBr) υmax 3440, 2941, 1738, 1726, 1514, 1036 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 583 [M + 1]+ (11), 437 (6), 383 (3), 277 (9), 241 (7), 207 (9), 185 (100), 149 (18), 147 (6), 115 (116); HRFABMS m/z 583.2752 [M + 1]+ (calcd for C29H43O12, 583.2754).
Antiaroside H (8): colorless syrup; [α]25 D +0.77 (c 1.47, MeOH); UV (MeOH) λmax (log ε) 219 (4.20) nm; IR (KBr) υmax 3420, 2970, 2941, 2878, 1739, 1710, 1618, 1450, 1416, 1377, 1313, 1030, 993 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 583 [M + 1]+ (21), 437 (25), 401 (8), 383 (10), 337 (11), 185 (100), 147 (28), 129 (33); HRFABMS m/z 583.2753 [M + 1]+ (calcd for C29H43O12, 583.2754).
Antiaroside I (9): colorless syrup; [α]25 D −28.22 (c 0.08, MeOH); UV (MeOH) λmax (log ε) 217 (4.17) nm; IR (KBr) υmax 3430, 2934, 1742, 1647, 1454, 1364, 1225, 1049, 987 cm−1; 1H and 13C NMR data, see Tables 1 and 2; FABMS m/z 523 [M + 1]+ (28), 360 (11), 359 (25), 341 (30), 323 (14), 225 (39), 185 (100), 147 (27), 131 (56), 129 (36); HRFABMS m/z 523.2908 [M + 1]+ (calcd for C28H43O9, 523.2907).
Antiarotoxinin A (10): colorless powder; [α]25 D +30.96 (c 0.087, MeOH); UV (MeOH) λmax (log ε) 212 (3.95) nm; IR (KBr) υmax 3304, 2943, 1761, 1643, 1275, 1036 cm−1; 1H and 13C NMR data, see Tables 1 and 2; EIMS m/z 408 (M+, 2), 390 (9), 372 (25), 354 (26), 318 (100), 219 (29), 201 (43), 145 (21), 124 (39), 121 (28), 111 (37), 109 (26), 107 (36), 93 (40), 91 (46), 81 (55), 55 (60); HREIMS m/z 408.2513 [M]+ (calcd for C23H36O6, 408.2511).
Assay Methods of Positive Inotropic Action and Arrhythmogenic Action
Adult guinea pigs (300–500 g) were anesthetized with pentobarbital (25 mg/kg, i.p.). The heart was excised, and retrograde coronary perfusion was performed with normal Tyrode’s solution containing (in mM) NaCl 137, KCl 5.4, CaCl2 2, MgCl2 1.1, NaH2PO4 0.33, NaHCO3 11.9 and glucose 11 through coronary artery. Tyrode’s solution was maintained at 37 °C and continuously aerated with 95% O2 + 5% CO2 (pH 7.2–7.4 under these conditions). Left atria and right ventricular muscles were separated from the heart. One end of the muscle was attached to a rigid support and the other end was attached to a transducer in the 10 mL bath. Each tissue was placed under 1 g tension and stimulated at 2 Hz, with pulses of 2 ms duration and amplitude twice the threshold. Following stabilization for about 60 min, drugs were cumulatively added. The positive inotropic effects (PIEmax) and safety indexes were studied according to the methods described previously.20,21 Briefly, the minimal positive inotropic effective concentration (PIECmin) to increase myocardial contraction and the arrhthymogenic concentration to induce arrhythmia in these isolated cardiac preparations were measured. The maximal positive inotropic effect was determined at a concentration level immediately before the occurrence of cardiac arrhythmia and the safety index was then measured from the ratio of arrhthymogenic concentration to minimal effective positively inotropic concentration.
Electrophysiological Recording of Malayoside (23)
Cardiomyocytes were isolated by using the enzymatic method previously described.20 Adult male guinea pigs (200–250 g) were intraperitoneally injected with sodium pentobarbital (25 mg/kg) plus heparin (16 mg/kg). After the guinea pig was deeply anesthetized, heart was excised and the coronary artery was antegradely perfused with oxygenated Ca2+-free HEPES solution containing (in mM) 137 NaCl, 22 glucose, 6 HEPES, 1.2 MgSO4, 1.2 KH2PO4, and 5.4 KCl; pH was adjusted to 7.4 using NaOH. The heart was then perfused with the same solution containing 0.4 mg/mL collagenase (type II, Sigma Chemical Co., St. Louis, Mo, USA), 0.06 mg/mL protease (type XIV, Sigma) and bovine serum albumin 1 mg/mL.
After 4–5 min of digestion, enzymes were washed out in Kruftbruhe solution containing (in mM) 10 taurine, 10 oxylate, 70 glutamate, 25 KCl, 10 KH2PO4, 11 glucose, 0.5 EGTA; pH was adjusted to 7.4 using KOH. The ventricles were then chopped and resuspended under gentle mechanical agitation and stored in Kruftbruhe solution at room temperature.
The whole-cell patch clamp technique was used to record ionic currents in voltage clamp mode with a Dagan 8900 voltage clamp amplifier (Dagan Co., Minneapolis, Minn., U.S.A.). A droplet of the cell suspension was placed in a chamber mounted on the stage of an inverted microscope (Nikon, Diaphot, Japan). After settling down, cells were finally exposed to the bath solution containing (in mM) 137 NaCl, 5.4 KCl, 2.9 MgCl2, 6 HEPES, 22 glucose, 0.33 NaH2PO4, 2 BaCl2, and 0.2 CdCl; pH was adjusted to 7.4 using NaOH. For the measurement of Ipump, a pipette was filled with the internal solution containing (mM) 80 CsOH, 50 NaOH, 3 MgCl2, 20 TEA-Cl, 100 aspartic acid, 10 HEPES, 10 ATP-Mg, 0.2 GTP-Na3, 5.5 glucose, 5 Na-creatine phosphate, and 5 pyruvic acid; pH was adjusted to 7.2 using CsOH. Heat-polished glass electrodes (tip resistances about 1 MΩ when filled with pipette internal solution) were used. After rupture of the patch, the holding potential was set at −40 mV to inactivate Na+ channels and the cell interior was allowed to equilibrate 5 min with the pipette solution. Then membrane currents were elicited by voltage ramps from +60 mV to −120 mV. Junction potentials were zeroed before the formation of the membrane–pipette seal in bath solution. The series resistance was electronically compensated by about 80% to minimize the duration of the capacitive surge on the current recorded and the voltage drop across the pipette. Currents were elicited and acquired using Digidata 1200 data acquisition system controlled using pClamp software (Axon Instruments). Recordings were lowpass filtered at 10 kHz and stored on the hard disk of a computer.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the National Science Council, Taiwan, Republic of China awarded to T.S. Wu. Partial support was received from NIH Grant No. CA-17625 awarded to K.H. Lee.
Footnotes
Supporting Information Available. NMR spectra of new compounds 1–10 and structures of known compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Philippe G, Angenot L. J Ethnopharmacol. 2005;100:85–91. doi: 10.1016/j.jep.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Bisset NG. J Ethnopharmacol. 1979;1:325–384. doi: 10.1016/s0378-8741(79)80002-1. [DOI] [PubMed] [Google Scholar]
- 3.Carter CA, Forney RW, Gray EA, Gehring AM, Schneider TL, Young DB, Lovett CM, Jr, Scott L. Tetrahedron. 1997;53:13557–13566. and references therein. [Google Scholar]
- 4.Fu LK, Jin JM. China Plant Red Data Book, Rare and Endangered Plants. Vol. 1. Science Press; Beijing: 1992. p. xviii.p. 741. [Google Scholar]
- 5.Wehrli W, Schindler O, Reichstein T. Helv Chem Acta. 1962;45:1183–1205. and references therein. [Google Scholar]
- 6.Kopp B, Bauer WP, Bernkop-Schnurch A. J Ethnopharmacol. 1992;36:57–62. doi: 10.1016/0378-8741(92)90061-u. [DOI] [PubMed] [Google Scholar]
- 7.Carter CA, Gray EA, Schneider TL, Lovett CM, Jr, Scott L, Messer AC, Richardson DP. Tetrahedron. 1997;53:16957–16968. [Google Scholar]
- 8.Robinson JA, Ling HW. Br J Pharmacol Chemother. 1953;8:79–82. doi: 10.1111/j.1476-5381.1953.tb00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto Y, Suzuki Y, Kanaiwa T, Amiya T, Hoshi K, Fujino S. J Pharm Dyn. 1983;6:128–135. doi: 10.1248/bpb1978.6.128. [DOI] [PubMed] [Google Scholar]
- 10.Scott AI. Interpretation of the Ultraviolet Spectra of Natural Products. Pergamon Press; New York: 1964. p. 235. [Google Scholar]
- 11.Lei ZH, Yahara S, Nohara T, Tai BS, Xiong JZ, Ma YL. Chem Pharm Bull. 2000;48:290–292. doi: 10.1248/cpb.48.290. [DOI] [PubMed] [Google Scholar]
- 12.Cai MS, Li ZJ. Carbohydrate Chemistry. Chemical Industry Press; Bei-Jing: 2006. p. 373. [Google Scholar]
- 13.Muehlradt P, Weiss Ek, Reichstein T. Helv Chem Acta. 1964;47:2164–2186. [Google Scholar]
- 14.Juslen C. Soc Sci Fennica, Commentationes Phys. 1962;(2):61. Math 27. [Google Scholar]
- 15.Wehrli W. Helv Chem Acta. 1962;45:1206–1211. [Google Scholar]
- 16.Muehlradt P, Weiss Ek, Reichstein T. Ann Chem. 1965;685:253–261. [Google Scholar]
- 17.Brandt R, Kaufmann H, Reichstein T. Helv Chem Acta. 1966;49:2469–2481. [Google Scholar]
- 18.Makarevich IF, Kolesnikov DG, Belokon VF. Chem Nat Compd. 1974;10:616–618. [Google Scholar]
- 19.Khatter JC, Agbanyo M, Hoeschen RJ, Navaratnam S, Bains R. J Pharmacol Exp Ther. 1986;239:206–210. [PubMed] [Google Scholar]
- 20.Lee AS, Wu TS, Su MJ. Eur J Pharmacol. 2008;580:224–230. doi: 10.1016/j.ejphar.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 21.Kass RS, Lederer WJ, Tsien RW, Weingart R. J Physiol (Lond) 1978;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


