Abstract
Background
Inhaled corticosteroid (ICS) non-adherence is common among patients with asthma; however, interventions to improve adherence have often been complex and not easily applied to large patient populations.
Objective
To assess the effect of supplying patient adherence information to primary care providers.
Methods
Patients and providers were members of a health system serving southeast Michigan. Providers (88 intervention; 105 control) and patients (1,335 intervention; 1,363 control) were randomized together by practice. Patients were age 5–56 years; had a diagnosis of asthma; and had existing prescriptions for ICS medication. Adherence was estimated using prescription and fill data. Unlike clinicians in the control arm, intervention arm providers could view updated ICS adherence information on their patients via electronic prescription software, and further details on patient ICS use could be viewed by selecting that option. The primary outcome was ICS adherence in last 3-months of the study period.
Results
At study end for the intention-to-treat analysis, ICS adherence was not different among patients in the intervention arm when compared with those in the control arm (21.3% vs. 23.3%, respectively; P=0.553). However, adherence was significantly higher among patients whose clinician elected to view their detailed adherence information (35.7%) when compared with both control arm patients (P=0.026) and intervention arm patients whose provider did not view adherence data (P=0.002).
Conclusions
Overall, providing adherence information to clinicians did not improve ICS use among patients with asthma. However, patient use may improve when clinicians are sufficiently interested in adherence to view the details of this medication use.
Keywords: Medication adherence, inhaled corticosteroids, asthma, randomized controlled trial
INTRODUCTION
Patient non-adherence to medications is common, especially in the treatment of chronic conditions where the tangible benefit or necessity of therapy may not be immediately evident.(1;2) A good example is the use of inhaled corticosteroid (ICS) medication, which is considered the cornerstone therapy for controlling persistent asthma.(3) Despite their described benefit, overall adherence to ICS medication is poor and non-adherence has been estimated to account for up to 60% of asthma hospitalizations.(4–6)
Equally discouraging has been the lack of efficacy of many interventions to improve adherence, despite often complex and labor-intensive designs.(7–9) However, data from a few small studies has suggested that routine monitoring of adherence with feedback of this information to patients may result in sustained high levels of adherence.(10;11) Implementing this on the scale of a health system, however, presents certain challenges. These include indentifying an inexpensive and reliable method of monitoring adherence on a large number of patients and providing these data to health care providers in a manner that is timely, feasible, and sustainable in clinical practice.
Here we describe a cluster-randomized trial (i.e., randomized by practice group) of providing ICS medication adherence information on patients with asthma to primary care providers within a large health system.
METHODS
Study participants
The primary purpose of this trial was to determine whether providing individual patient medication adherence information to physicians electronically for discussion with their patients with asthma could result in improved inhaled corticosteroid use. This study was approved by the Henry Ford Health System institutional review board and was in compliance with its Health Insurance Portability and Accountability Act policy. The study was implemented in a single, large integrated health system serving southeast Michigan and including metropolitan Detroit. Study findings are reported in a manner consistent with the CONSORT statement for cluster randomized trials.(12)
Health system primary care providers (i.e., in the areas of family practice, internal medicine, and pediatrics) were invited to participate. This group has had access to electronic prescription writing (i.e., ePrescribing) since January 19, 2005, and at the time of the intervention primary care clinicians were expected to use ePrescribing exclusively. Physicians who consented to participate were grouped according to practice, such that each practice comprised a group of physicians who worked together semi-autonomously (usually at single clinic site), cross-covered each others’ patients, and cared for a circumscribed patient population. Preliminary analysis showed that system patients received 96% of outpatient asthma care at a single clinic site. One hundred ninety-two (92%) of the 207 primary care staff clinicians, representing 34 pre-defined primary care practices within the health system, agreed to participate. One clinic was not included a priori since it functioned as the primary resident clinic for internal medicine trainees. Physicians were randomized with their practice as to whether or not the group would receive medication adherence information on their patients with asthma.
The decision to randomize by practice was based in part on preliminary small focus groups held with physicians in the design stage of the study. The purpose of these focus groups was to design the intervention to be acceptable, feasible, and sustainable in busy clinical settings. Focus group physicians expressed their desire to be able to share adherence information among the group cross-covering each others’ patients. Randomizing by practice also minimized overlap between physicians and patients in opposite arms of the study. Practices were randomized stratifying for whether the practice was a pediatric practice (i.e., pediatrics vs. family medicine and internal medicine) in order to achieve approximately equal partitioning of children and adults in both study arms. One researcher (ELP) generated the random allocation sequence within strata and the identities of the practices were concealed at the time of randomization.
Eligible patients had to fulfill the following criteria: a prior electronic prescription for an ICS between January 19, 2005 and April 30, 2007; age 5 to 56 years as of April 30, 2007; continuous enrollment in the affiliated health maintenance organization (HMO) for at least 1 year prior to April 30, 2007; prescription drug coverage as of April 30, 2007; at least one physician diagnosis of asthma and no diagnosis of chronic obstructive pulmonary or congestive heart failure following January 19, 2005; and at least one visit to a primary care provider in the year prior to April 30, 2007. Patients meeting these criteria were invited by letter to participate in the study, and those who refused were not included. Patients were excluded if their ICS medication was stopped and not restarted, or if they left the HMO prior to the start of the intervention (i.e., before August 1, 2007). Patients enrolled in the study were aligned to the practice in which they received their primary care. Therefore, all enrolled patients from the same practice were randomized to the same study arm.
Intervention
The intervention period ran from August 1, 2007 to July 31, 2008. The study staff was masked to the individual practice treatment assignment. Each participating physician received a packet which contained standardized information regarding their assigned study arm. Physicians assigned to both groups received an audio CD, video DVD, and booklet which contained information on the most recent national asthma guidelines (3) and methods for discussing medication non-adherence with their patients. The education material emphasized a non-confrontational approach to discussing adherence and included ways to identify barriers to taking medication, tips to help patients remember to take their medication, and methods to promote patient self-efficacy. Each educational packet contained redundant information in multiple formats (i.e., audio CD, video DVD, and booklet) so that physicians could choose according to their preference. Physicians in the intervention arm also received specific instructions on how to interpret the adherence data that they would be able to view via the ePrescribing application (DrFirst Inc., Rockville, MD).
We have previously described the calculation of ICS adherence from data which are routinely collected as part of electronic prescription writing and medication filling, and we have also shown that in our patient population we have near complete capture of ICS fills.(13;14) Briefly, we linked electronic prescription information with fill information from pharmacy claims data to estimate the number of days that a given fill of an ICS would last (i.e., days supplied). This was calculated by dividing the canister size (i.e., puffs per canister) as derived from National Drug Codes in pharmacy claims by the dosage information (i.e., puffs per day). The calculated days’ supply was then used to estimate adherence as a continuous measure of medication availability equal to the Cumulative days’ supplied/Number of days of observation.(15) This estimates the proportion of time that the patient took their medication, and has been shown to be associated with asthma outcomes.(4) Using a similar approach, we also estimated the frequency of the short-acting beta-agonist use as previously described.(16) Lastly, the strength of each patient’s current ICS prescription was estimated based on current national guidelines according to the patient’s age, type of ICS, and prescribed dose.(3) These metrics were calculated approximate every 2 weeks and uploaded to the ePrescribing system where they could be viewed by physicians in the intervention arm at the time of prescription writing, reviewing the medication list, or renewing medications (Figure E1 in the online supplemental material); this information is henceforth referred to as general adherence information. Physicians interested in the specific details of their patients’ ICS adherence over time could view this information by selecting a link following the general adherence message (Figure E2 in the online supplemental material); these data are henceforth referred to as detailed adherence information. We tracked the use of ePrescribing software by physicians in the intervention group to record the number of times that general and detailed adherence information was viewed. Control group physicians also used the ePrescribing system; however, use on their patients with asthma was not available.
Statistical analysis
Outcomes were ascertained by staff without knowledge of the treatment assignment. The primary outcome measure was patient adherence to inhaled corticosteroids in the last 3 months of intervention (i.e., an individual level outcome accounting for practice clusters). For individuals who disenrolled from the HMO or whose ICS medication was discontinued by a clinician, we carried forward their last 3 months adherence. Baseline ICS adherence was measured during the 3-months preceding the start of the intervention. Forty-five patients for whom adherence could not be calculated during the intervention period (e.g., their ICS prescription was without refills) were excluded from the analysis. However, we also performed a sensitivity analysis whereby we ascribed to these patients their baseline adherence, 0% adherence, and 100% adherence to determine whether this assumption substantially altered the results. No substantive differences were found (data not shown).
Given the cluster-randomized design, we used an intracluster correlation coefficient (ICC) of 0.02 for adherence when calculating study sample size for this primary outcome. The ICC was derived from baseline measures of the variance in adherence between and within practice clusters. We estimated that the study would require a total of 2,598 patients from 34 practices (i.e., 17 per arm) to have >80% power to detect a 9% absolute difference in adherence between study arms assuming a two-sided alpha value of 0.05. The effect size was based on another study by us which related this approximate change in adherence with disease outcomes.(17)
Secondary outcome measures included the time to and the number of the following events during the intervention period: an asthma-related emergency room visit, an asthma-related hospitalization, and oral steroid use. These outcomes were identified through electronic claims with algorithms validated against events in the pre-intervention period. Secondary outcomes associated with mailed patient surveys were not evaluated as part of this analysis. As a post-hoc analysis, we also assessed whether the change in adherence between baseline and study end differed between patients in the intervention and control arms.
Primary and secondary study outcomes were analyzed according to intention-to-treat. Because of the cluster randomized study design, all analyses also accounted for clustering at the practice level and stratification by practice type (i.e., pediatrics vs. family medicine and internal medicine).(18) Analysis of variance was used to assess difference in adherence levels between study arms (primary outcome), Cox proportional hazards regression models were used in the time-to-event analyses (secondary outcomes), and negative binomial regression models were used when analyzing the number of events during the intervention period (secondary outcomes). Regression models also adjusted for individual-level patient variables, including patient age, sex, and race-ethnicity. Among patients in the intervention group we assessed whether both adherence at study end and changes in adherence over the study period differed among patients who had a primary care visit, those whose provider viewed general adherence information, and those whose provider viewed detailed adherence information.
Lastly, we assessed whether individuals with stable or improved adherence (i.e., individuals whose adherence was the same or greater between baseline and study end) were less likely to experience an asthma-related emergency room visit, an asthma-related hospitalization, or oral steroid use when compared with individuals whose adherence decreased between these time-points. These outcomes were assessed using both Cox-proportional hazards regression models (i.e., time-to-event) and negative binomial regression models (i.e., total number of events).
All analyses were performed using SAS v9.1 (SAS Institute Inc., Cary, NC).(19) A P-value < 0.05 was considered statistically significant.
RESULTS
We enrolled 193 (93.2%) of 207 primary care providers from 34 practices groups throughout the health system (Figure 1). This group of providers included 49 family medicine practitioners, 88 internists, 5 internists/pediatricians, 41 pediatricians, 9 physician assistants, and 1 nurse practitioner. One pediatrician from the intervention group withdrew following enrollment. Seventeen practices (88 providers) were randomly assigned to the intervention arm, and 17 practices (105 providers) were assigned control. From these practices, we identified 3,003 patients who as of April 30, 2007 met the enrollment criteria. Of these 3,003 patients, 87 refused participation in the study; 126 had their ICS medication stopped (and not restarted) in the baseline period from May 1, 2007 to August 1, 2007, and 92 disenrolled from the heath plan prior to the start of the intervention. Of the remaining 2,698 patients, 1,335 were affiliated with intervention practices (and hence had their ICS adherence information available to the participating providers at these practices) and 1,363 were associated with the control practices.
Figure 1.
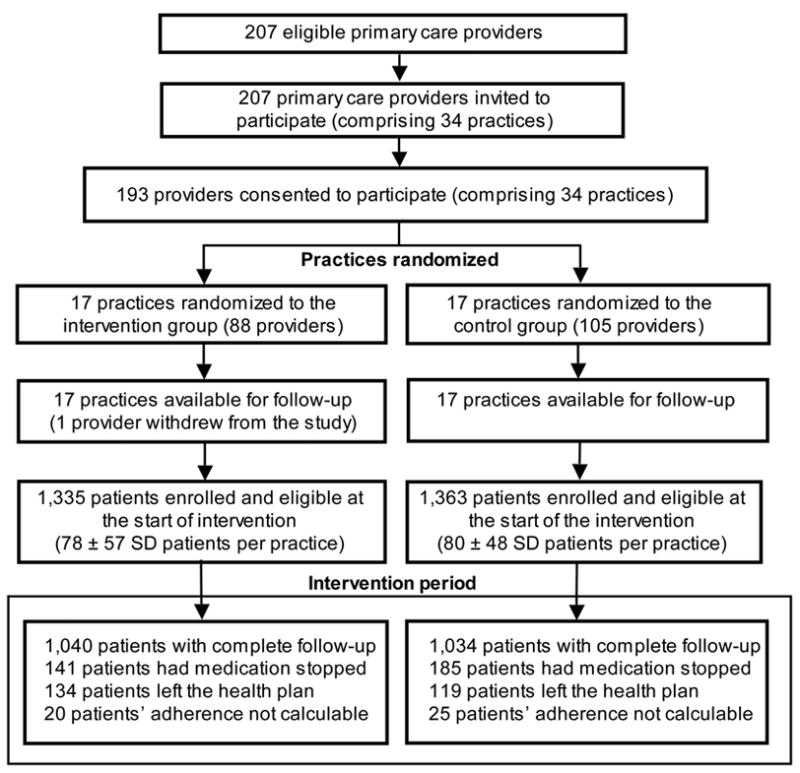
Flow chart of patients, providers, and practice clusters randomized in the intervention to provide clinicians with inhaled corticosteroid adherence information on their patients with asthma
The baseline characteristics of these patients are shown in Table 1. Only patient age differed significantly between patients in both study arms; average age was 26.8 years and 28.8 years for intervention arm and control arm patients, respectively (P=0.001). Baseline ICS adherence for the two comparison groups was similar at 25.6% and 27.7% for intervention arm and control arm patients, respectively (P=0.210).
Table 1.
Baseline characteristics of patients with asthma in the intervention and control study arms
| Characteristic | Intervention patients (n = 1,335) | Control patients (n = 1,363) | P-value |
|---|---|---|---|
| Age (years) – mean ± SD | 26.8 ± 17.4 | 28.8 ± 17.4 | 0.001 |
| Age categories – no (%) | 0.982 | ||
| 5–17 years | 591 (44.3) | 604 (44.3) | |
| 18–56 years | 744 (55.7) | 759 (55.7) | |
| Female – no (%) | 737 (55.2) | 753 (55.3) | 0.983 |
| Race-ethnicity | 0.569 | ||
| African-American | 511 (38.3) | 528 (38.7) | |
| White | 726 (54.4) | 749 (55.0) | |
| Other | 98 (7.3) | 86 (6.3) | |
| Baseline ICS adherence – mean ± SD* | 25.6 ± 37.3 | 27.7 ± 38.5 | 0.210 |
SE denotes standard deviation and ICS, inhaled corticosteroid.
Adherence for the 3-month period preceding the start of the intervention.
At study end, adherence was not significantly higher among patients in the intervention arm when compared with patients in the control arm (23.2% vs. 24.9%, respectively; P-value = 0.553) (Table 2). As required, this analysis accounted for both practice cluster and randomization stratum, but additionally adjusting for patient age, sex, and race-ethnicity did not impact these finding (data not shown). The ICC for the primary outcome was 0.063. Adherence was significantly higher among participants in both arms who had had a visit to a primary care provider in the first 9 months of the study period when compared with participants with no such visit (23.2% vs. 12.0%; P=0.001 among individuals in the intervention arm; 24.9% vs. 15.2%; P=0.001 among individuals in the control arm). However, within these strata (i.e., individuals with no primary care visit in the first 9 months of the study and individuals with ≥1 visit), adherence was not significantly different at study end between individuals in the intervention and control arms (P=0.379 and P=0.639, respectively). Even individuals who had their general adherence information viewed had similar adherence at study end when compared to patients in the control arm (25.1% vs. 23.3%; P=0.585). In contrast, individuals who had their detailed adherence information viewed by their primary care provider in the first 9 months had significantly higher adherence at study end when compared with patients in the intervention arm whose provider did not view adherence information (35.7% vs. 12.3%; P=0.002) and when compared with patients in the control group (35.7% vs. 23.3%; P=0.026).
Table 2.
Differences in inhaled corticosteroid adherence at study end between patients with asthma in the intervention and control study arms*
| Outcome | Adherence in intervention patients – mean ± SE (n = 1,335) | Adherence in control patients – mean ± SE (n = 1,363) | P-value† |
|---|---|---|---|
| ICS adherence at study end | 21.3 ± 2.5 | 23.3 ± 2.2 | 0.553 |
| ICS adherence at study end among patients with no primary care provider visit in first 9 months of study‡|| | 12.0 ± 2.3 | 15.2 ± 2.9 | 0.379 |
| ICS adherence at study end among patients with ≥1 primary care provider visit in first 9 months of study§|| | 23.2 ± 2.7 | 24.9 ± 2.2 | 0.639 |
| ICS adherence at study end among patients whose primary care provider did not view their adherence data in the first 9 months of study¶†† | 12.3 ± 3.0 | -- | -- |
| ICS adherence at study end among patients whose primary care provider viewed their general adherence data in the first 9 months of study**†† | 25.1 ± 2.4 | -- | -- |
| ICS adherence at study end among patients whose primary care provider viewed their detailed adherence data in the first 9 months of study‡‡|||| | 35.7 ± 5.1 | -- | -- |
SE denotes standard error and ICS, inhaled corticosteroid.
Adherence measured for the last 3 months of the intervention, May 1, 2008 through July 31, 2008.
P-value for the comparison of individuals in the intervention group with individuals in the control group. All analyses account for practice cluster and randomization stratum.
Number of individuals in each category was 228 and 221, respectively.
Number of individuals in each category was 1,087 and 1,117, respectively.
P=0.001 for the comparison of ICS adherence among individuals in the intervention group with and without a primary care visit, and P=0.001 for the comparison of adherence among individuals in the control group
Number of individuals was 396.
Number of individuals was 939.
P=0.001 for the comparison of ICS adherence among individuals in the intervention group whose primary care provider viewed their general adherence information with those whose primary provider did not view such information.
Number of individuals was 53
P=0.002 for the comparison of ICS adherence among individuals in the intervention group whose primary care provider viewed their detailed adherence information with those whose primary provider did not view such information.
Table 3 shows the difference in ICS adherence at study end depending on whether in the first 9 months of the study a participant in the intervention group had a primary care visit, had their general adherence information viewed, or had their detailed adherence information viewed. ICS adherence was highest for participants who had all three, and this group had significantly higher adherence when compared to individuals who had both a primary care visit and a provider who viewed their general adherence information alone (i.e., group 6 vs. group 4; P=0.017). Adjusting these comparisons for baseline adherence levels had little substantive effect on the P-values presented with the exception that the Group 6 vs. Group 3 comparison became statistically significant (P=0.049) and the Group 2 vs. Group 1 comparison was no longer significant (P=0.058) (data not shown).
Table 3.
Differences in inhaled corticosteroid adherence for patients in the intervention group by whether their primary care provider viewed adherence information and whether they had a primary care visit*
| Group no. | Primary care visit† | Primary care provider viewed general adherence information† | Primary care provider viewed detailed adherence information† | No.‡ | Adherence – mean ± SE§ | P-value for the comparison of ICS adherence to the following groups§ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | ||||||
| Group 1 | No | No | No | 170 | 7.6 ± 2.3 | -- | -- | -- | -- | -- |
| Group 2 | Yes | No | No | 219 | 16.0 ± 3.6 | 0.001 | -- | -- | -- | -- |
| Group 3 | No | Yes | No | 53 | 24.6 ± 3.2 | 0.001 | 0.123 | -- | -- | -- |
| Group 4 | Yes | Yes | No | 821 | 24.4 ± 2.5 | 0.001 | 0.001 | 0.958 | -- | -- |
| Group 5 | No | Yes | Yes | 5 | 27.6 ± 13.0 | 0.154 | 0.428 | 0.820 | 0.815 | -- |
| Group 6 | Yes | Yes | Yes | 47 | 36.6 ± 5.6 | 0.001 | 0.001 | 0.070 | 0.017 | 0.523 |
SE denotes standard error
Adherence measured for the last 3 months of the intervention, May 1, 2008 through July 31, 2008.
Occurrence in the first 9 months of the intervention.
Total of 1,315 rather than 1,335 due to some individuals with missing data.
Accounts for practice cluster and randomization stratum.
Among the patients in the intervention arm, 127 subjects had 177 asthma-related emergency rooms visits, 10 subjects had 10 asthma-related hospitalizations, and 307 subjects had 550 separate occasions in which they were treated with an oral steroid during the study period. Among the patients in the control arm, 111 subjects had 159 asthma-related emergency rooms visits, 11 subjects had 11 asthma-related hospitalizations, and 300 subjects had 522 separate occasions in which they were treated with an oral steroid. Therefore, when compared to patients in the control arm, intervention patients did not differ in the time to first asthma-related emergency room visit (adjusted hazard ratio [aHR] 1.22; 95% CI 0.83–1.78), asthma-related hospitalization (aHR 0.86; 95% CI 0.32–2.29), or oral steroid use (aHR 1.07; 95% CI 0.89–1.29) (Table 4). Likewise, intervention patients had a similar rate of asthma-related emergency room visits (adjusted relative rate [aRR] 1.12; 95% CI 0.74–1.69), asthma-related hospitalizations (aRR 0.87; 95% CI 0.33–2.29), and oral steroid use (aRR 1.11; 95% CI 0.92–1.34) when compared to patients in the control arm during the study period.
Table 4.
Differences in secondary outcomes between patients with asthma in the intervention and control study arms*
| Outcome | Time-to-event analysis† | Relative rate of total events‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | aHR (95% CI)§ | P-value | RR (95% CI) | P-value | aRR (95% CI)§ | P-value | |
| Asthma-related emergency room visits | 1.20 (0.80–1.79) | 0.374 | 1.22 (0.83–1.78) | 0.315 | 1.13 (0.71–1.80) | 0.607 | 1.12 (0.74–1.69) | 0.596 |
| Asthma-related hospitalization | 0.93 (0.34–2.57) | 0.885 | 0.86 (0.32–2.29) | 0.765 | 0.93 (0.34–2.56) | 0.886 | 0.87 (0.33–2.29) | 0.774 |
| Oral steroid use|| | 1.05 (0.88–1.25) | 0.621 | 1.07 (0.89–1.29) | 0.459 | 1.07 (0.89–1.29) | 0.450 | 1.11 (0.92–1.34) | 0.277 |
HR denotes hazard ratio; aHR, adjusted hazard ratio; RR, relative rate; and aRR, adjusted relative rate.
All analyses account for practice cluster and randomization stratum.
Proportional hazards regression models for the time to first event.
Negative binomial regression for the overall rate of events during the study period, August 1, 2007 through July 31, 2008.
Analyses account for practice cluster and randomization stratum and adjust for patient’s age, sex, and self-reported race.
Based on claims for oral corticosteroid fills during the study period, August 1, 2007 through July 31, 2008.
In both intervention and control groups patients combined, those who had stable (i.e., unchanged) or improved adherence during the course of study had significantly lower relative rates of asthma-related emergency room visits (aRR 0.73; 95% CI 0.55–0.98) and oral steroid use (aRR 0.77; 95% CI 0.63–0.93) when compared with those whose adherence fell during this time (Table 5).
Table 5.
Differences in outcomes between patients who had stable or improved inhaled corticosteroid adherence during the study period compared with those who did not, stratified by study arm assignment*
| Outcome | Time-to-event analysis† | Relative rate of total events‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | Patients in the intervention arm | Patients in the control arm | All patients | Patients in the intervention arm | Patients in the control arm | |||||||
| RR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Asthma-related emergency room visits | 0.70 (0.54–0.91) | 0.008 | 0.71 (0.53–0.96) | 0.025 | 0.69 (0.45–1.07) | 0.100 | 0.73 (0.55–0.98) | 0.039 | 0.90 (0.65–1.24) | 0.522 | 0.58 (0.37–0.90) | 0.016 |
| Asthma-related hospitalization | 0.79 (0.34–1.80) | 0.566 | 0.72 (0.33–1.58) | 0.412 | 0.85 (0.20–3.55) | 0.822 | 0.77 (0.34–1.76) | 0.540 | 0.70 (0.33–1.52) | 0.374 | 0.84 (0.20–3.52) | 0.814 |
| Oral steroid use§ | 0.90 (0.76–1.08) | 0.262 | 0.91 (0.75–1.11) | 0.364 | 0.89 (0.67–1.20) | 0.453 | 0.77 (0.63–0.93) | 0.007 | 0.81 (0.63–1.04) | 0.096 | 0.72 (0.55–0.96) | 0.024 |
HR denotes hazard ratio; aHR, adjusted hazard ratio; RR, relative rate; and aRR, adjusted relative rate.
All analyses account for practice cluster and randomization stratum. Individuals with stable or improved adherence (i.e., individuals whose adherence was the same or greater between baseline and study end) were compared with individuals whose adherence decreased between these time-points.
Proportional hazards regression models for the time to first event.
Negative binomial regression for the overall rate of events during the study period, August 1, 2007 through July 31, 2008.
Based on claims for oral corticosteroid fills during the study period, August 1, 2007 through July 31, 2008.
As a post-hoc analysis, we also examined whether there were differences in the change in adherence (i.e., the change in adherence between the baseline period and study end) between study arms. As previously noted in Table 1, individuals in both arms had similar baseline levels of ICS adherence. We found that in general, the change in adherence over the study period did not differ between patients in the intervention group and those in the control group (Table E1 in the online supplemental material). Adherence fell over the study period for patients in both arms, and it fell to a lesser extent in those who had a primary care visit in the first 9 months of the study when compared to those who had no such visit. In contrast, among individuals in the intervention group, participants whose primary care provider viewed their detailed adherence information in the first 9 months of the study had an overall increase in their adherence over the study period, and this change was significantly different when compared to those whose primary care provider did not view their adherence data (3.8% vs. −6.7%, respectively; P=0.039).
Among participants in the intervention arm, the only patients that experienced an overall increase in ICS adherence over the study period were those whose primary care provider had viewed their detailed adherence information (Table E2 in the online supplemental material). Individuals in Group 6 (i.e., those with a clinic visit and who had their detailed adherence information viewed) showed significantly greater improvement when compared with individuals who did not have adherence information viewed, regardless of the presence of a clinic visit (i.e., Group 6 vs. Group 1, P=0.037; Group 6 vs. Group 2, P=0.029). These comparisons were similar for individuals in Group 5 (i.e., those with no clinic visit but who had their detailed adherence information viewed) but were of borderline statistical significance (i.e., Group 5 vs. Group 1, P=0.075; Group 5 vs. Group 2, P=0.074). The consistency of these findings when compared to the analyses presented in Table 3 again suggests that individuals experienced an overall improvement in their adherence when providers viewed their detailed adherence information.
DISCUSSION
To our knowledge, this is the first large-scale, controlled study to test the effectiveness of routinely providing patient medication adherence information to clinicians. Although smaller studies have suggested that medication use improves when clinicians are provided measures of adherence to discuss with their patients,(10;20–23) the processing and delivery of these data to health care providers in these earlier interventions was more labor intensive, thus limiting their applicability to large patient populations. Although our cluster randomized trial did not demonstrate a significant difference in the primary (i.e., ICS adherence) and secondary outcomes between patients in both study arms, it did suggest that patients’ adherence improved when their primary care clinician used the application as intended.
For example, we could infer clinician intent by the type of adherence information viewed. Because general adherence information was available at any time electronic prescribing application was accessed, “viewing” may not have necessarily signified an interest to see or act on that information. In contrast, detailed adherence information had to be accessed by selecting an additional link after the general adherence message, suggesting the clinician specifically sought further clarification on the pattern of patient medication use. While we cannot verify that this information was then discussed with patients, we did observe that patients in the intervention group whose clinicians opted to view this detailed information had an improvement in their adherence over the study period, whereas adherence tended to fall among patients whose clinicians did not view this information. Unfortunately, few clinicians in the intervention group accessed the detailed adherence information during the study period.
Slow adoption of information technology by physicians has been described.(24;25) To avoid this reticence, we sought physician input in both the design of the adherence application and education in its use. This early planning and physician involvement likely accounts for the high levels of clinician enrollment into the study since the content and delivery of adherence information was designed to be relevant yet of minimal burden to clinical practice. Nevertheless, we still saw lower than expected use of the application by primary care providers. Perhaps a longer intervention period or more intensive clinician education could have overcome this apparent inertia. While the finding of improved adherence in those patients whose physicians viewed the detailed adherence information is encouraging, this study underscores the importance of changing both patient and clinician behavior in order to improve asthma controller use.
In this study we estimated medication adherence using both electronic prescriptions and pharmacy claims for medication fills and refills. We and others have previously shown claims-based measures of adherence to be associated with disease outcomes,(4;17;26;27) and therefore these adherence metrics were felt to be reasonable targets for improvement. Here we show that over the course of a year patients with stable to improved claims-based adherence levels were less likely to have an asthma-related emergency room visit and required fewer oral corticosteroid bursts. These findings further support the use of these metrics in clinical practice.
Moreover, as the source data for these claims-based adherence measures were readily available within our health system, ICS adherence estimates could be generated, easily, inexpensively, and repeatedly on our large patient population with asthma. While many health care systems may not currently have electronic prescription data from which to generate these estimates, these data will likely become more common with the recent passage of the Medicare Improvements for Patients and Providers Act of 2008 which incentivizes electronic prescription writing.(28) Since clinician views of adherence information can be tracked within an electronic prescription application, direct compensation based on use may also be way to promote integration of this new information into practice.(29)
This study must also be interpreted in light of additional limitations. First, provider input resulted in a cluster-randomized study design, as focus group physicians felt that adherence information should be shared between colleagues who cross-cover each other’s patients. However, cluster-randomization does not guarantee equal distribution of patient-level characteristics,(30) and these may have differed between study arms in ways not assessed or fully accounted for in our analyses.
We have also recently shown various patient internal factors (i.e., those pertaining to patient beliefs, knowledge, motivation regarding asthma and asthma treatments) to be related to ICS adherence.(14) This suggests that an added behavioral intervention may have resulted in improved medication adherence. However, this intervention was explicitly designed to assess the real world impact (i.e., effectiveness) of introducing adherence metrics into clinical practice without an intensive behavioral component (i.e., with the exception of the standardized adherence education provided to all participating clinicians). Given the ineffectiveness of many intensive behavioral interventions,(8) we felt that this was a reasonable first approach. Moreover, our study addressed some of the other limitations of earlier study designs, namely the use of objective measures of adherence and minimal population selection bias.(9)
As mentioned previously, we did not directly observe the manner in which clinicians used the adherence information provided to them in the clinic, and therefore we could not assess the quality or quantity of physician-patient discussions. Nevertheless, we specifically avoided interfering with normal clinic processes as our presence could have had unintended consequences on patient and physician behavior. As a result, we likely have a more reliable estimate of the intervention’s effectiveness.
Adherence levels at baseline were lower than we have previously described for ICS use.(13;14;31) In the long term follow-up of patients with asthma previously prescribed an ICS, some may have experienced an improvement in their asthma without documented discontinuation of the medication by their physician. In this situation, some of our adherence measures may have been inappropriately low. Despite the low estimated levels of adherence, further decreases over the study period were associated with poor outcomes, suggesting that there was underuse of controller medication. In addition, seasonal variation in ICS use (i.e., lower summertime use) could have accounted for the low baseline and follow-up adherence measures;(32) however, we do not anticipate that this would have affected the differences observed by level of physician viewing.
Outcomes were analyzed as intention-to-treat and included individuals who neither had a primary care visit nor had their adherence information viewed during the study period. Overall, individuals with a clinic visit had higher adherence when compared with those who did not. Yet, individuals who had their detailed adherence information viewed had the largest improvement in adherence, and this improvement was similar in magnitude for individuals who did and did not have a clinic visit. This suggests that important patient-clinician interactions regarding this information may have also occurred over the phone, via the mail, or by another method.
In summary, our data suggest that providing clinicians with adherence information may improve patients’ ICS adherence when clinicians are sufficiently motivated to review the details of their patients’ medication use. Although this intervention was explicitly designed to be feasible and minimally obtrusive in the clinical setting, our study suggests that further inducements are needed to get clinicians to use this information as intended. In other words, while providing adherence information may be a necessary first step, physicians may require further training or financial incentives structured around the use of this information. Yet, it is also important to note that once the electronic process is established, these measures can be generated on a recurring basis with little marginal effort. Therefore, across a large patient population and over time, the actual number of physicians using the information and number of patients benefiting may eventually outweigh the continued investment even if these numbers are relatively small; however, this will require further study.
Clinical Implications: Adherence information can be generated for large numbers of patients, but clinician use is a barrier. Improvements in patient ICS adherence may occur when clinicians use the application as designed.
Acknowledgments
This work was supported by grants the National Heart, Lung, and Blood Institute (HL79055) and the National Institute of Allergy and Infectious Diseases (AI61774), National Institutes of Health; the Fund for Henry Ford Hospital, and the Strategic Program for Asthma Research of the American Asthma Foundation.
We would like to thank Drs. Andrea Apter, Cynthia Rand, and Allan Donner for their contributions to the design of this study. We similarly would like to thank all the patients and physicians whose input in focus groups helped shaped this study.
Abbreviations
- ICS
Inhaled Corticosteroid
- HR
Hazard Ratio
- RR
Relative Rate
Footnotes
Trial Registration clinicaltrials.gov identifier: NCT00459368
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. [Google Scholar]
- 3.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. The Journal of Allergy and Clinical Immunology. 2004;114(6):1288–93. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Apter AJ, Boston RC, George M, Norfleet AL, Tenhave T, Coyne JC, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol. 2003;111(6):1219–26. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 6.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98(6 Pt 1):1051–7. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 7.Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002;(2):CD000011. doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 8.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Bender B, Milgrom H, Apter A. Adherence intervention research: what have we learned and what do we do next? J Allergy Clin Immunol. 2003;112(3):489–94. doi: 10.1016/s0091-6749(03)01718-4. [DOI] [PubMed] [Google Scholar]
- 10.Onyirimba F, Apter A, Reisine S, Litt M, McCusker C, Connors M, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–5. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 11.Reddel HK, Toelle BG, Marks GB, Ware SI, Jenkins CR, Woolcock AJ. Analysis of adherence to peak flow monitoring when recording of data is electronic. BMJ. 2002;324(7330):146–7. doi: 10.1136/bmj.324.7330.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328(7441):702–8. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120(5):1153–9. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Wells K, Pladevall M, Peterson EL, Campbell J, Wang M, Lanfear DE, et al. Race-ethnic differences in factors associated with inhaled steroid adherence among adults with asthma. Am J Respir Crit Care Med. 2008;178(12):1194–201. doi: 10.1164/rccm.200808-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Paris J, Peterson EL, Wells K, Pladevall M, Burchard EG, Choudhry S, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol. 2008;101(5):482–7. doi: 10.1016/S1081-1206(10)60286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–5. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donner A, Klar N. Design and Analysis of Cluster Randomization - Trials in Health Research. London, England: Arnold Publishers; 2000. [Google Scholar]
- 19.SAS Institute Inc. SAS/STAT Users Guide. Cary, NC: SAS Institute Inc; 2004. Version 9.1 ed. [Google Scholar]
- 20.Cramer JA, Rosenheck R. Enhancing medication compliance for people with serious mental illness. J Nerv Ment Dis. 1999;187(1):53–5. doi: 10.1097/00005053-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O’Malley SS, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–7. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schectman JM, Schorling JB, Nadkarni MM, Voss JD. Can prescription refill feedback to physicians improve patient adherence? Am J Med Sci. 2004;327(1):19–24. doi: 10.1097/00000441-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42(4):409–22. doi: 10.1016/S0005-7967(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 24.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348(25):2526–34. doi: 10.1056/NEJMsa020847. [DOI] [PubMed] [Google Scholar]
- 25.Eger MS, Godkin RL, Valentine SR. Physicians’ adoption of information technology: a consumer behavior approach. Health Mark Q. 2001;19(2):3–21. doi: 10.1300/J026v19n02_02. [DOI] [PubMed] [Google Scholar]
- 26.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25(6):1015–21. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 27.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–9. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Medicare Improvements for Patients and Providers Act of 2008, Public Law 110–275. H.R. 6331, 132. 7-15-2008. Ref Type: Bill/Resolution
- 29.Menachemi N, Struchen-Shellhorn W, Brooks RG, Simpson L. Influence of pay-for-performance programs on information technology use among child health providers: the devil is in the details. Pediatrics. 2009;123 (Suppl 2):S92–S96. doi: 10.1542/peds.2008-1755H. [DOI] [PubMed] [Google Scholar]
- 30.Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health. 2004;94(3):416–22. doi: 10.2105/ajph.94.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams LK, Joseph CL, Peterson EL, Moon C, Xi H, Krajenta R, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. J Allergy Clin Immunol. 2007;119(1):168–75. doi: 10.1016/j.jaci.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Spahn J, Sheth K, Yeh WS, Stempel DA, Stanford RH. Dispensing of fluticasone propionate/salmeterol combination in the summer and asthma-related outcomes in the fall. J Allergy Clin Immunol. 2009;124(6):1197–203. doi: 10.1016/j.jaci.2009.08.042. [DOI] [PubMed] [Google Scholar]


