Abstract
Several curcuminoids and sesquiterpenoids isolated from Curcuma longa (CL) have been shown to have many pharmacological activities. In the present study, the immunomodulatory activities of the polar fractions of CL hot water extracts were investigated using human peripheral blood mononuclear cells (PBMC). Our results showed that the high polarity fraction of the hot water extract exhibited stimulatory effects on PBMC proliferation as shown in [methyl-3H]-thymidine incorporation assay. In an attempt to isolate the active components responsible for the activities, further partition with ethyl-acetate, n-butanol and ethanol, progressively were performed. The cytokine productions (TGF-β, TNF-α, GM-CSF, IL-1α, IL-5, IL-6, IL-8, IL-10 and IL-13, etc.) have been modulated by a polysaccharide-enriched fraction as shown in ELISA and cytokine protein array. The proportion of CD14 positive stained PBMC was increased by such fraction. The composition of monosaccharide of the active fraction has been determined by GC-MS and gel permeation chromatography. The immunostimulatory effects of Curcuma longa polysaccharides on PBMC were shown for the first time. The findings revealed the potential use of Curcuma longa crude extract (containing curcuminoids and polysaccharides) as an adjuvant supplement for cancer patients, whose immune activities were suppressed during chemotherapies.
Keywords: Curcuma longa, immunostimulatory, peripheral blood mononuclear cells, polysaccharide
1. Introduction
Curcuma longa (CL, common name Turmeric), a plant species from the Curcuma genus (family Zingiberaceae), is common ingredient in many health supplements in Asia. According to the Ayurvedic Pharmacopoeia of India, essential oil from rhizome of CL was used as a carminative, stomachic and tonic [1]. According to the Chinese Pharmacopoeia, CL was suggested to have the functions of eliminating blood stasis, promoting the flow of “qi”, stimulating menstrual discharge and relieving pain [2].
In modern pharmacological studies, the active components of CL such as curcuminoids and sesquiterpenoids had previously been shown to possess anti-inflammatory, antioxidant, and chemopreventive properties. Curcumin was known to suppress NF-κB activation [3]. Recent reports suggested that curcumin down-regulated expression of cell proliferation and anti-apoptotic and metastatic gene products [4] and potentiated antitumor activity of gemcitabine in pancreatic cancer model [5]. Curcumin has also been shown to inhibit proliferation and induce apoptosis in human leukemic cell lines [6]. On the other hand, curcumin has been suggested to modulate the proliferation and cellular response of various immune cell types, such as T cells, B cells, macrophages, neutrophils, NK cells and dendritic cells [7, 8]. Previous studies have shown that aromatic turmerone isolated from CL possessed antioxidant [9] and antiplatelet activities [10]. The zedoarondiol isolated from another Curcuma species showed anti-inflammatory properties in murine macrophages [11]. Our recent findings also suggested that aromatic turmerone possess immunostimulating activities in human peripheral blood mononuclear cells [12]. However, very few pharmacological studies are found regarding the polysaccharides of Curcuma species. A group of polysaccharides isolated from CL, named ukonan A, B, C and D, showed reticuloendothelial system-potentiating activity, anti-complementary and alkaline phosphatase-inducing activities [13–15]. Polysaccharides isolated from related species C. zedoaria and C. xanthorrhiza have been shown to have macrophage-stimulating activity via specific activation of NFκ-B [16, 17]. Nevertheless, the immunostimulatory activities of polysaccharides isolated from CL in human peripheral blood mononuclear cells have never been reported.
The present study aimed to isolate and characterize bioactive components from the polar fractions of CL extracts using bioassay-guided fractionation and to evaluate the immunomodulatory activities of such components using human peripheral blood mononuclear cells (PBMC). The chemical profile of the bioactive component would be characterized by GC-MS and gel permeation chromatography. The cell proliferation responses, cytokine production as well as the distribution of T-cell subsets of PBMC would be evaluated.
2. Materials and Methods
2.1 Materials
Cell culture medium RPMI-1640, fetal bovine serum (FBS), penicillin, streptomycin, and phosphate-buffered saline (PBS) were purchased from Invitrogen (NY, USA). Ficoll-Paque™ Plus and [methyl-3H]-thymidine were obtained from GE healthcare (UK). All other chemicals including phytohaemagglutinin (PHA), polymyxin B, 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), trypan blue were purchased from Sigma (MO, USA). The ELISA kits for TNF-α, IFN-γ, IL-6, TGF-β and the antibodies against CD4, CD8 and CD14 were purchased from BD Pharmingen (CA, USA). The antibody-based human cytokine array III was obtained from RayBiotech Inc (GA, USA). The organic solvents, dichloromethane, 95% ethanol, ethyl acetate, n-hexane and methanol were purchased from Lab-Scan (Thailand) and were either HPLC-grade or AR grade.
2.2 Plant Material
Dried rhizome of Curcuma longa L. (CL) was purchased from a herbal supplier in Hong Kong with the source of origin in India. Microscopic and chemical authentication was accomplished in accordance with the Chinese Pharmacopoeia [2]. Authenticated voucher specimen (no. HK 40400) was deposited in the Hong Kong Herbarium of the Agriculture, Fisheries and Conservation Department of Hong Kong Special Administration Region, China.
2.3 Preparation of crude extract and sub-fractions
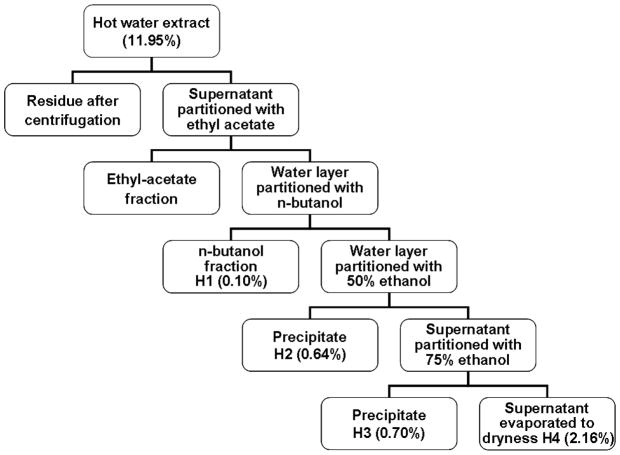
The dried rhizomes Curcuma longa (500 g) was cut into small pieces and soaked in 1.0 L of water for 1 hour. It was then extracted twice with water under reflux for 2 hours. Following centrifugation, the hot water extract supernatant was subjected to sequential partition with ethyl acetate, n-butanol and ethanol (Fig. 1). Four fractions (H1–H4) were obtained from the hot water extracts. The n-butanol fraction was evaporated under reduced pressure at 60°C to dryness to give H1 fraction. The water layer was mixed with 50% ethanol. The precipitate obtained as H2 fraction. The supernatant was further mixed with 75% ethanol. The precipitate obtained as H3 fraction. The supernatant was evaporated to dryness to give H4 fraction. The yield of each fraction with regards to the weight of raw herb was shown in Fig. 1. Prior to bioassays, H2 and H3 fractions were dissolved in culture medium and diluted to working concentrations, while H1 and H4 were dissolved in DMSO and the stock was diluted in culture medium. The vehicle control cultures received the vehicle solvent (0.2% v/v DMSO).
Fig. 1.
Schematic chart showing the fractionation of CL hot water extract. Numbers in brackets are the percentage yield (w/w).
2.4 Chemical analysis of the bioactive fraction
2.4.1 Determination of the molecular weight of polysaccharides
The relative molecular weights and the monosaccharide compositions in fraction H2 were analyzed as described by Huang et al. and in our previous paper [18, 19]. The fraction H2 was dissolved or suspended in 0.05 M NaH2PO4-Na2HPO4 buffer (pH = 6.7, 0.05% NaN3) and were filtered through a 0.45 μm filter. The sample was run on a TSK-Gel G3000SWXL column (5 μm, 7.8 × 300 mm, Tosoh Bioscience LLC, PA, USA) eluted with 0.05 M NaH2PO4-Na2HPO4 buffer to determine the molecular weight by gel filtration. The flow rate was 0.5 ml/min. Phenol-sulfuric acid method was utilized to monitor the collected fractions. A calibration curve was constructed using standard dextran with molecular weights of 0.738×103, 5.8×103, 1.22×104, 2.37×104, 4.8×104, 1.0×105, 1.86×105, 3.8×105, 8.35×105 Daltons (Da).
2.4.2 Determination of the monosaccharide composition of polysaccharides
The monosaccharide composition in the polysaccharide from fraction H2 was determined by GC-MS. The tested sample were hydrolyzed (2M H2SO4, 100°C, 6 hours) and evaporated to dryness. The hydrolyzed samples were then acetylated as described by Huang et al. [18] and analyzed by GC-MS on DB-1 capillary column (15 m× 0.2 mm) with flame ionization detector (Agilent 6890GC/5973MS instrument) in a temperature gradient of 100–280°C at 10°C/min. Identification and quantification of monosaccharides were made in comparison with standards.
2.5 Human peripheral blood mononuclear cells (PBMC) preparation
Fresh human buffy coat obtained from the Hong Kong Red Cross Blood Transfusion Service was diluted with PBS at a ratio of 1:1. The diluted buffy coat sample was layered on equal volume of Ficoll-Paque™ Plus solution and then centrifuged at 800×g for 20 minutes at 18°C. The thin white middle PBMC layer was collected and the PBMC were washed with PBS twice and centrifuged at 100×g for 10 minutes at 18°C. The supernatant was discarded and the PBMC were resuspended in RPMI-1640 medium plus 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cell number was counted with a hematocytometer and the viability of the cells was checked by trypan blue exclusion assay. Only the isolated cells with 95% or above viability were resuspended to the target density, which was 2 × 106 cells/ml, for experiments. The PBMC were incubated at 37°C in a humidified atmosphere of 5% CO2. The use of human buffy coat for experiment was approved by Joint The Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee.
2.6 Cell viability assay
The isolated PBMC (3 × 106/ml) were seeded in 96-well flat-bottom culture plates (Iwaki, Japan) and incubated with various concentrations (100 - 800 μg/ml) of H1-H4 fractions from CL for 72 hours. Plain medium were added to the control wells. The fractions were dissolved in medium and filtered with 0.22 μm syringe filters (Iwaki, Japan). Following the incubation of cells with the extracts, the medium was discarded and 30 μl of 5 mg/ml 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl-tetrazolium bromide (MTT; Sigma, USA) in PBS were added to each well and the plates were further incubated for 3 hours at 37°C. The plates were then centrifuged at 300×g for 10 minutes. The supernatant was then removed and 100 μl of DMSO was added to each well to dissolve the purple formazan crystals. The absorbance at a wavelength of 540 nm was measured spectrophotometrically with a BMG FLUOstarOptima microplate reader (BMG LABTECH GmbH, Germany). Results were expressed as the percentage of MTT absorbance with respect to control cells.
2.7 Cell proliferation assay of PBMC and collection of culture supernatant
The isolated PBMC were seeded in a 96-well flat bottom microplate and incubated with various concentrations (100–800 μg/ml) of H1-H4 fractions for 72 hours. To ensure the effects of the fractions on the PBMC were not due to the presence of lipopolysaccharide (LPS), polymyxin B (10 μg/ml), an antibiotic that can bind LPS [20], was added to the extracts. The mitogen, phytohaemagglutinin (PHA), was added to target wells when appropriate at a final concentration of 10 μg/ml. Proliferation rates were estimated by [methyl-3H]-thymidine incorporation. After the incubation period of 72 hours, the microplates were centrifuged at 300×g for 10 minutes to obtain cell-free supernatant. The supernatant was collected and stored at −80°C until cytokine ELISA experiments.
The effects of fractions on the proliferation of resting and mitogen (PHA)-activated PBMC were determined by [methyl-3H]-thymidine incorporation. Following incubation of cells with the extracts for 72 hours, tritiated thymidine (0.5 μCi/well) was added into each well and further incubated for 6 hours. Then the cells were harvested on glass fiber filters by cell harvester. Radioactivity in the filters was measured by Packard TopCount NXT™ Microplate Scintillation and Luminescence Counter (PerkinElmer Inc., USA).
2.8 Determination of human cytokine production of PBMC using ELISA and protein array
The PBMC culture supernatants were subjected to test for the concentrations of cytokines such as IL-6, IFN-γ, TGF-β and TNF-α by enzyme-linked immunosorbent assay (ELISA). The assay was carried out according to the procedures recommended in the ELISA kit manual. The expression profile of 42 different cytokines in PBMC culture supernatant was further assessed semi-quantitatively using antibody-based RayBio human cytokine array III. The assay was carried out according to the procedures recommended in the assay kit manual.
2.9 CD marker staining assay
The isolated PBMC (3 × 106/ml) were seeded in a 24-well flat bottom microplate and incubated with various concentrations (200–800 μg/ml) of selected fractions for 72 hours. The cells were resuspended with cold PBS and were blocked with 1% bovine serum albumin in PBS for 5 min at room temperature. Then the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD4 and CD14, phycoerythrin-conjugated CD8, mouse IgG1 isotype for 30 min at 4°C in the dark. After washing with PBS supplemented with 1% bovine serum albumin and 0.05% sodium azide, the cells were subjected to flow analysis. Expressions of CD4, CD8 and CD14 on 20,000 viable cells were then gated and analyzed by flow cytometry (Becton Dickinson FACSCanto II, USA).
2.10 Statistical analysis
Data were expressed as the mean ± standard error of mean (S.E.M.). Statistical analyses and significance, as measured by the Mann Whitney test unpaired samples, were performed using GraphPad PRISM software version 5.0 (GraphPad Software, USA). In all comparisons, p < 0.05 was considered statistically significant.
3. Results
3.1 Effects of H1-H4 fractions on cell proliferation of human PBMC
To evaluate the effects of fractions from CL extracts on cell viability of PBMC, cells were treated with 100–800 μg/ml of fractions H1–H4 for 72 hours and subjected to MTT assay. Fraction H1 showed cytotoxic effect on PBMC at concentration higher than 200 μg/ml, while fractions H2, H3 and H4 did not show significant cytotoxic activities (data not shown). Assays for proliferative response in human PBMC were performed to evaluate the stimulatory activities of the four fractions obtained from CL extracts. The cells were treated with 200 μg/ml of fractions H1–H4 for 72 hours. The fractions were added to PBMC with polymyxin B in order to rule out the possible contamination by the endotoxin. As shown in Fig. 2A, fraction H2 (200 μg/ml) were found to significantly stimulate the proliferation of PBMC after 72 hours treatment. Fractions H3 and H4 could also stimulate the proliferation of PBMC but the increase was not statistically significant. Therefore, the immune responses towards H2 in PBMC were further investigated.
Fig. 2.
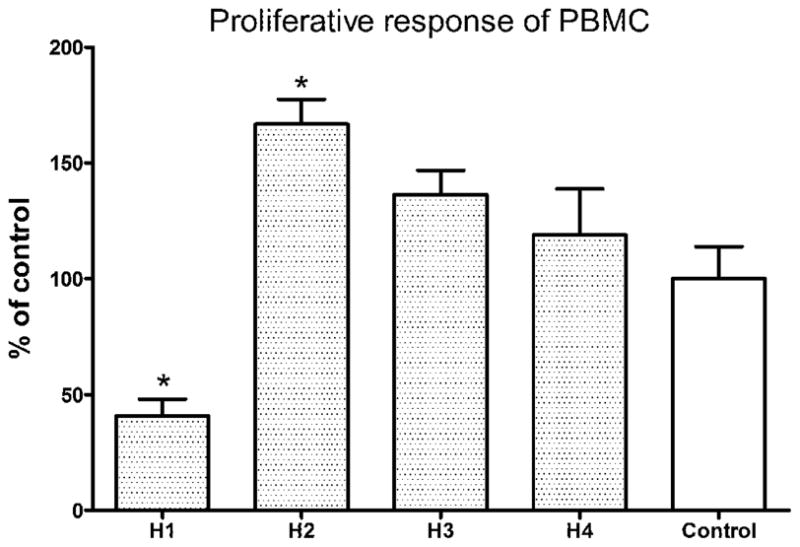
Proliferative response of H1–H4 fraction-treated PBMC. Cells were seeded in 96-well plates and incubated with 200 μg/ml of H1–H4 fractions in culture medium for 72h, and expressed as the mean % ratio of count per minute in treated and untreated control cells of 8 individual blood donors. Results were expressed as mean concentration + S.E.M. of 8 blood samples with four wells each. Differences between the treated and untreated control group were compared using Mann Whitney test. * p < 0.05 as compared to the control group.
3.2 Immunostimulating activities of fraction H2 in PBMC
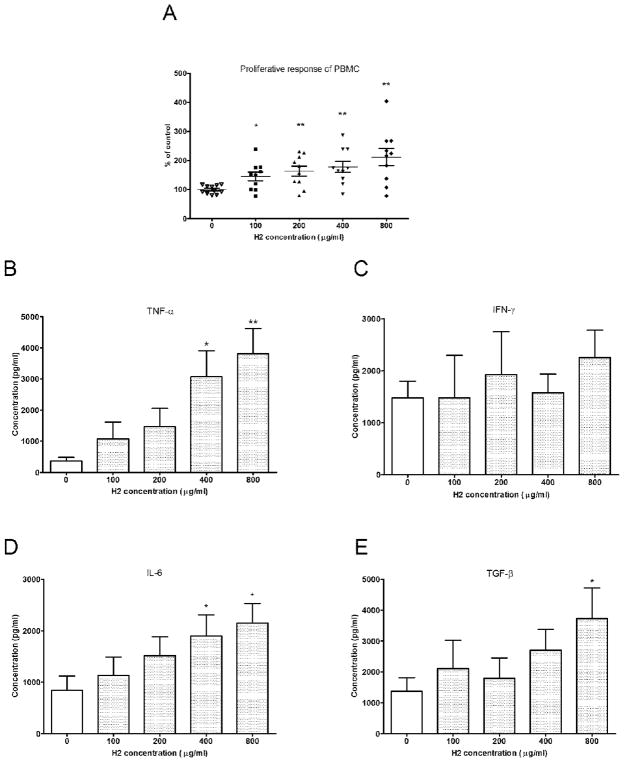
Different concentrations of fraction H2 were tested in cultured PBMC to examine its effects on the proliferation of PBMC. As shown in Fig. 3A, fraction H2 (100–800 μg/ml) were found to significantly stimulate the proliferation of PBMC after 72 hours treatment (n = 10, p<0.05). After incubating the PBMC with fraction H2 (100–800 μg/ml) for 24 hours, the culture supernatants were collected. The concentrations of TNF-α, IFN-γ, IL-6 and TGF-β were determined by ELISA. The TNF-α and IL-6 production was significantly increased by H2 at 400 and 800 μg/ml (Fig. 3B and 3D). The TGF-β production was increased by H2 at 800 μg/ml while the IFN-γ production was not affected by H2-treatment (Fig. 3C and 3E).
Fig. 3.
Proliferative response and TNF-α productions of H2 fraction-treated PBMC. (A) PBMC were seeded in 96-well plates and incubated with 100–800 μg/ml of fraction H2 in culture medium for 72h, and expressed as the mean % ratio of count per minute in treated and untreated control cells of 9–10 individual blood donors. Culture supernatants were collected 24h after incubation with H2 fraction and the cytokine TNF-α (B), IFN-γ (C), IL-6 (D) and TGF-β (E) concentrations were specifically determined by ELISA. Results were expressed as mean concentration + S.E.M. of 10 blood samples with four wells each. Differences between the treated and untreated control group were compared using Mann Whitney test. * p < 0.05, ** p < 0.005 as compared to the control group.
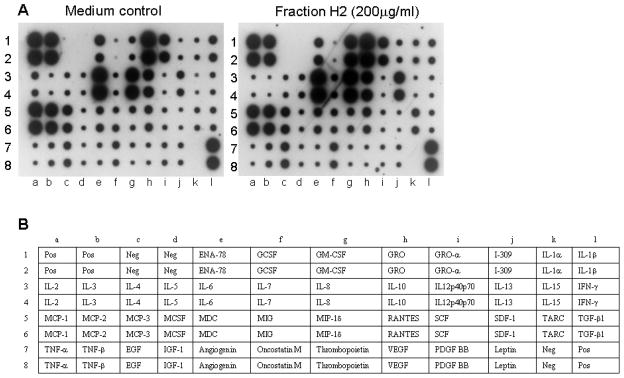
The cytokine expression profile of PBMC stimulated by fraction H2 was further assessed using antibody-based RayBio human cytokine array III (RayBiotech) [21]. As the fraction H2 at 200 μg/ml did not significantly increase the above mentioned cytokine production, cytokine array was used to screen whether the fraction H2 at 200 μg/ml could stimulate other cytokines or chemokines. Results showed that fraction H2 could induce protein expression of cytokines and chemokines GM-CSF, IL-1α, IL-5, IL-8, IL-10, IL-13 and TARC (thymus and activation-regulated chemokine) among 42 different cytokines being screened (Fig. 4).
Fig. 4.
A: Representative profile of the production of cytokines from PBMC stimulated by fraction H2. PBMC (2 × 106 cells) were cultured with or without H2 (200 μg/ml) for 24 hours in a 96-well plate. Cell free supernatant was collected and cytokines in culture supernatant were semi-quantitated using antibody-based RayBio human cytokine array III. Positive and negative controls were designed at (1a, 1b, 2a, 2b, 7l, 8l) and (1c, 1d, 2c, 2d, 7k, 8k), respectively. B: Table lists the format of antibodies on the cytokine array.
3.3 Effects of fraction H2 on cell surface expression of CD4, CD8 and CD14 in PBMC
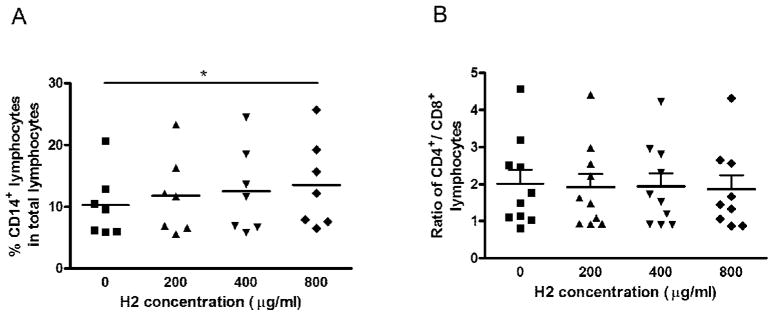
To evaluate the effects of fraction H2 on cell surface expression of CD4, 8 and 14 in PBMC, cells were treated with a series of concentrations of fraction H2 from 200 – 800 μg/ml for 72 hours. In Fig. 5A, fraction H2 at concentration of 800 μg/ml induced significant increase in CD14+ lymphocyte population in total lymphocytes. However, the CD4+/CD8+ ratio was not changed by fraction H2 treatment (Fig. 5B).
Fig. 5.
Effects of fraction H2 on the CD14, CD4 and CD8 expression in PBMC. Cells were incubated with various concentrations (200 – 800 μg/ml) of H2 fractions for 72 hours. A: The number of PBMC from 7–8 blood donors that stained single-positive for CD14 were quantified by fluorescence activated cell sorting and are depicted here as the percentage of positively stained cells per 20,000 viable PBMC. B: The ratio of CD4+ and CD8+ co-stained cells per 20,000 viable PBMC (n=10). The line represents the median. Differences between the treated and untreated control group were compared using Mann Whitney test, * p < 0.05 as compared to the control group.
3.4 Characterization of H2 fraction
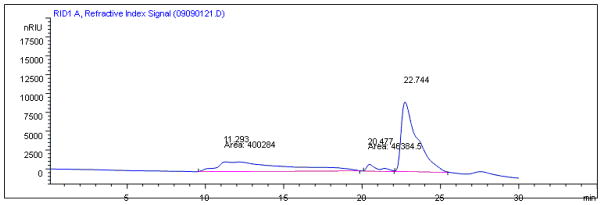
Since fraction H2 was prepared from sequential partition with ethyl acetate, n-butanol and ethanol, the contents of fraction H2 were likely to be polysaccharides. Therefore, the composition of monosaccharides and molecular weight of fraction H2 were determined by GC-MS and gel filtration chromatography (Table 1). The molecular weight parameters shown are relative to the dextran reference. The gel permeation chromatograph (Fig. 6) showing 3 peaks detected in fraction H2.
Table 1a.
Monosaccharide composition of the H2 fraction
| Monosaccharide composition | Relative contents (%) |
|---|---|
| Ribose | 2.58 |
| Arabinose | 3.31 |
| Xylose | 0.59 |
| Mannose | 14.77 |
| Glucose | 70.69 |
| Galactose | 5.73 |
Fig. 6.
Gel permeation chromatograph showing the contents of fraction H2. Three peaks were detected at 11.29, 20.48 and 22.74 min of retention time.
4. Discussion
The use of health supplement containing Chinese traditional medicine has expanded. Curcuma longa (CL) and its extracts, curcumin, widely consumed as food additive and medicine, are believed to possess anti-cancer, anti-inflammatory, anti-oxidant and immunomodulatory properties. However, the immunomodulatory activity of CL polar fraction is seldom reported. In the present study, the immunostimulating activities in human PBMC of the CL polar fractions were evaluated in vitro. All the extract fractions were prepared by bioassay-guided fractionation process in our laboratory.
The present study demonstrated that the fraction H2 of CL polar fractions significantly stimulated PBMC proliferation and cytokine production (Fig. 3 & 4). Since the activities of fraction H2 were not affected by the addition of polymyxin B (10 μg/ml) (data not shown), the observed mitogenic effect was unlikely to be mediated by the endotoxin (lipopolysaccharide), which is a potent activator of B cells. The fraction H2 at 200 μg/ml could marginally increase the TNF-α and IL-6 production in PBMC. In order to evaluate the immunomodulatory properties of H2 in cytokine induction, H2-treated PBMC culture supernatants were subjected to cytokine protein array analysis. There were several cytokines and chemokines increased in expression in H2-treated (200 μg/ml) PBMC (Fig. 4). Along with these results, some of the increased cytokines are involved in B cells proliferation and activation as well as T cell growth. The elevated CD14+ lymphocyte population which consists of monocytes and B cells was observed in fraction H2-treated PBMC. Fraction H2 may be beneficial for immunomodulatory function by enhancing humoral immunity. However, the CD4+/CD8+ ratio did not significantly change by 72-hour treatment of fraction H2. It may due to insufficient incubation time for the functional T cell subsets to be generated [22].
Previous studies on Curcuma polysaccharides have focused on the anti-inflammatory effects using macrophages. Polysaccharides purified from C. zedoaria increased phagocytic activities of RAW 264.7 cells towards gram-negative and gram-positive bacteria as well as nitric oxide and TNF-α production [4]. Crude polysaccharide extract isolated from C. xanthorrhiza also stimulated the phagocytosis and the release of nitric oxide, TNF-α and PGE2 of RAW 264.7 cells [5]. Nevertheless, little information is available about the immunomodulatory activities in other cell types of the immune system. Fraction H2, whose molecular weight and composition of monosaccharides were determined, was shown for the first time to exert stimulating activities in human PBMC. The extracts of CL contain curcuminoids, turmerones and polysaccharides. In our previous study, the former components have been shown for their antitumor and immunostimulating activities [6]. The polysaccharide in CL polar fraction was demonstrated for its immunostimulating effect in human PBMC in the present study. The multiple targets abilities of CL extracts could be manifested in the present study. For future study, the multi-functionality of CL extracts can be evaluated using a tumor-bearing Balb/c mouse model so that both the direct antitumor effects exerted by curcumin as well as the immunostimulatory activities exerted by turmerones and polysaccharides can be anticipated.
In conclusion, bioassay-guided fractionation of the rhizome of Curcuma longa led to the isolation of the bioactive polysaccharide. Fraction H2 significantly stimulated PBMC proliferation and cytokine production in vitro. To the best of our knowledge, this is possibly the first report on the immumodulatory activity exerted by Curcuma longa polysaccharide in PBMC. The finding revealed the potential use of whole Curcuma longa extracts (including curcuminoids, volatile oil and water soluble polysaccharides) as chemopreventive health supplement.
Table 1b.
Relative molecular weight distribution of the H2 fraction
| Mw | Mw/Mna | |
|---|---|---|
| Peak 1 | 887,000 | 2.44 |
| Peak 2 | 5,080 | 1.04 |
| Peak 3 | 500 | 7.17 |
Mw: weight average molecular weight; Mn: number average molecular weight
Acknowledgments
This project was supported by Grant Number 5 P50 AT002779-02 from the National Institutes of Health of USA and the Botanical Research Center at Memorial Sloan-Kettering Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Government of India, Ministry of Health & Family Welfare. The Ayurvedic Pharmacopoeia of India. The Controller of Publications Civil Lines; New Delhi: 2001. [Google Scholar]
- 2.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia. Shang wu yin shu guan; Shanghai: 2010. [Google Scholar]
- 3.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 5.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 6.Anuchapreeda S, Tima S, Duangrat C, Limtrakul P. Effect of pure curcumin, demethoxycurcumin, and bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines. Cancer Chemother Pharmacol. 2008;62:585–594. doi: 10.1007/s00280-007-0642-1. [DOI] [PubMed] [Google Scholar]
- 7.Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- 8.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 9.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch. 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 10.Lee HS. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour Technol. 2006;97:1372–1376. doi: 10.1016/j.biortech.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Cho W, Nam JW, Kang HJ, Windono T, Seo EK, Lee KT. Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-kappaB pathway in LPS-stimulated murine macrophages. Int Immunopharmacol. 2009;9:1049–1057. doi: 10.1016/j.intimp.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Yue GGL, Chan BC, Hon PM, Lee MY, Fung KP, Leung PC, Lau CBS. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food and Chemical Toxicology. 2010 doi: 10.1016/j.fct.2010.04.039. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonda R, Tomoda M, Shimizu N, Kanari M. Characterization of polysaccharides having activity on the reticuloendothelial system from the rhizome of Curcuma longa. Chem Pharm Bull (Tokyo) 1990;38:482–486. doi: 10.1248/cpb.38.482. [DOI] [PubMed] [Google Scholar]
- 14.Gonda R, Takeda K, Shimizu N, Tomoda M. Characterization of a neutral polysaccharide having activity on the reticuloendothelial system from the rhizome of Curcuma longa. Chem Pharm Bull (Tokyo) 1992;40:185–188. doi: 10.1248/cpb.40.185. [DOI] [PubMed] [Google Scholar]
- 15.Gonda R, Tomoda M, Ohara N, Takada K. Arabinogalactan core structure and immunological activities of ukonan C, an acidic polysaccharide from the rhizome of Curcuma longa. Biol Pharm Bull. 1993;16:235–238. doi: 10.1248/bpb.16.235. [DOI] [PubMed] [Google Scholar]
- 16.Kim AJ, Kim YO, Shim JS, Hwang JK. Immunostimulating activity of crude polysaccharide extract isolated from Curcuma xanthorrhiza Roxb. Biosci Biotechnol Biochem. 2007;71:1428–1438. doi: 10.1271/bbb.60241. [DOI] [PubMed] [Google Scholar]
- 17.Kim KI, Shin KS, Jun WJ, Hong BS, Shin DH, Cho HY, Chang HI, Yoo SM, Yang HC. Effects of polysaccharides from rhizomes of Curcuma zedoaria on macrophage functions. Biosci Biotechnol Biochem. 2001;65:2369–2377. doi: 10.1271/bbb.65.2369. [DOI] [PubMed] [Google Scholar]
- 18.Huang XL, Wu HQ, Huang F, Lin XS. Analysis of polysaccharide from broken cellular wall and unbroken spore of Ganoderma lucidum. Chinese Traditional and Herbal Drugs. 2006;37:813–816. [Google Scholar]
- 19.Yue GG, Fung KP, Leung PC, Lau CB. Comparative studies on the immunomodulatory and antitumor activities of the different parts of fruiting body of Ganoderma lucidum and Ganoderma spores. Phytother Res. 2008;22:1282–1291. doi: 10.1002/ptr.2478. [DOI] [PubMed] [Google Scholar]
- 20.Duff GW, Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982;52:333–340. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- 21.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 22.Janeway CA., Jr How the immune system protects the host from infection. Microbes Infect. 2001;3:1167–1171. doi: 10.1016/s1286-4579(01)01477-0. [DOI] [PubMed] [Google Scholar]