Abstract
Histone/protein deacetylases (HDACs) decrease histone and protein acetylation, typically leading to suppression of gene transcription and modulation of various protein functions. We found significant differences in expression of HDAC before and after stimulation of human T regulatory (Treg) and T effector cells, suggesting the potential for future selective targeting of Tregs with HDAC inhibitors (HDACi). Use of various HDACi small molecules enhanced, by up to 4.5-fold (average 2-fold), the suppressive functions of both freshly isolated and expanded human Tregs, consistent with our previous murine data. HDACi use increased Treg expression of CTLA-4, a key negative regulator of immune response, and we found a direct and significant correlation between CTLA-4 expression and Treg suppression. Hence, HDACi compounds are promising pharmacologic tools to increase Treg suppressive functions, and this action may potentially be of use in patients with autoimmunity or post-transplantation.
INTRODUCTION
FOXP3+ T regulatory cells (Tregs) are important to normal homeostasis of the immune system and play key roles in immunological processes ranging from transplant rejection and autoimmunity to allergy and cancer [1-4]. Therapeutic strategies proposed for Treg use mainly involve increasing the conversion of naïve T cells into induced Tregs, or expanding autologous or even allogeneic naturally occurring Tregs, prior to their adoptive transfer into patients [5]. However, the clinical applicability of these approaches may be limited by the stability of Treg suppressive functions after ex vivo expansion [5, 6], and by an inherent plasticity of naturally occurring or converted Tregs that can lead to their reversion to pro-inflammatory cells post-transfer [7, 8].
As part of a large multi-molecular complex, the transcription factor FOXP3 down-regulates Treg expression of the pro-inflammatory genes, IL-2, IL-4 and IFN-γ [9, 10], and up-regulates expression of CTLA-4 (CD152), CD25 and other Treg-associated genes [11]. FOXP3 is also subject to various post-translational modifications [11-15]. Of relevance to the current study, the reversible acetylation and deacetylation of the ε-amino groups of lysine located in histones and many non-histone proteins (e.g. p53, GATA-1, STAT3, estrogen and androgen receptors, HSP90, α-tubulin and FOXP3) is controlled by histone acetyltransferases (HATs) and histone/protein deacetylases (HDACs), respectively [16, 17]. Usually, histone acetylation correlates with increased transcriptional activity and histone deacetylation correlates with gene silencing.
There are four classes of HDACs [16, 17]. The class I HDACs are HDAC1, 2, 3, and 8; the class II HDACs include HDAC4, 5, 7, 9 (subclass IIa) and HDAC 6, 10 (subclass IIb); the class III HDACs are structurally unrelated to either class I or class II HDACs and are homologs of yeast Sir2 proteins; currently the sole class IV HDAC is HDAC11. Class I HDACs are detected in the nucleus and are expressed ubiquitously, whereas class II HDACs shuttle between the nucleus and cytoplasm and are expressed in a tissue-specific manner [16, 17]. The activities of Zn-dependent class I and II HDACs are inhibited by “classical” HDAC inhibitors (HDACi), typically leading to activation of gene expression and increased protein function.
Many HDACi are under investigation as anticancer agents since they are potent inducers of cancer cell growth arrest, differentiation and/or apoptotic cell death [18]. HDACi also have anti-inflammatory effects, as shown for SAHA, Trichostatin-A (TsA) and butyrate [19]. Indeed, bufexamac, a non-steroidal anti-inflammatory drug used for many years, was recently identified as an HDACi with activity against class I HDAC and HDAC6 [20]. Historically, the anti-inflammatory effects of HDACi were attributed to their inhibitory effects on class I HDAC [21], but recent studies have shown direct effects of HDACi on FOXP3+ Tregs and implicated class IIa HDACs in Tregs as key targets of HDACi therapy [19]. Therapy with a panHDACi such as TsA or SAHA can stimulate thymic production of FOXP3+ Tregs and promote the peripheral conversion of murine and human T cells into Tregs [13, 22]. HDACi use also increased expression of FOXP3 in murine Tregs and enhanced their suppressive function in vitro and in vivo [13], pointing to the potential benefit of HDACi for therapy of autoimmunity and transplant rejection [19].
However, there are significant differences between human and murine Tregs that may limit the extrapolation of data generated in one species to the other. For example, while murine Treg cells are mainly generated in the thymus, peripheral homeostasis in humans involves increased proliferation and does not necessarily reflect thymic production [23]. Second, unlike murine cells, human CD4+CD25- (and CD8+) effector cells transiently express FOXP3+ upon T cell activation [24]. Third, FOXP3-transduced murine T cells develop suppressive function [25], whereas corresponding transduction or transfection of human cells led to conflicting data [26-28]. Fourth, while murine Treg preferentially produce the novel immunosuppressive cytokine, IL-35 [29], data concerning human Tregs and IL-35 are conflicting [30]. Hence, caution is required when extrapolating data from murine models to humans. The current study investigated the in vitro effects of various HDACi on human freshly isolated and expanded Tregs.
MATERIALS AND METHODS
HDACi
We purchased BML-210 (N-(2-aminophenyl)-N′-phenyl-octanediamide) from Biomol; bufexamac (p-butoxyphenylacethydroxamic acid), MS-275 (Entinostat, SNDX-275), SAHA (suberoylanilide hydroxamic acid, Vorinostat) and sodium butyrate from Axxora; valproic acid from Sigma; and obtained tubacin as a gift from Dr. Stuart Schreiber (Harvard University).
Cell isolation and culture
Mononuclear cell-enriched apheresis product was obtained by leukapheresis of healthy volunteer donors (n=24, 20 male, 4 female) by the University of Pennsylvania Human Immunology Core. Specimens were collected under a University Institutional Review Board-approved protocol and informed consent was obtained from each donor.
Treg expansion
CD4+ T cells were purified from apheresis product using RosetteSep human CD4+ T cell enrichment cocktail (Stemcell Technologies), and CD25hi Treg cells isolated using a MoFlo high-speed cell sorter (DakoCytomation). K64.86 cells, an artificial antigen-presenting cell (APC) line [31], were washed and re-suspended in serum-free culture medium (X-VIVO 15, LONZA) 24 h prior to antibody loading. Cells were irradiated with 100 Gy and washed, followed by addition of OKT-3 anti-CD3 mAb (1 μg/ml). Cells were rotated at 4 °C for 30 min, after which unbound antibody was removed by washing three times. Ab-loaded K64.86 cells were re-suspended in serum-free culture medium at a density 1×106 cells/ml, and combined with CD4 cells (also in serum-free medium) at a final ratio of 1 K64.86 cell: 2 CD4 cells. After 24 hrs of culture, human AB serum (10% final concentration) and human IL-2 (CHIRON Therapeutics, final concentration of 300 U/ml) were added. Cultures were monitored for cell volume and cell density using a Coulter Multisizer 3 (Beckman Coulter) on days 5, 8, 12 and 15 of culture. Following counting, the culture was adjusted to 3×105 cells/ml and IL-2 (300 U/ml) was added at 5, 8, 12 and 15 d.
Quantitative real-time PCR (qPCR)
Tregs were isolated using a CD4+CD25+CD127dim/− Treg Isolation Kit using the manufacturer's instructions (Miltenyi). CD4+CD25- T effectors (Teff) were isolated from the same donor, using a CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi). Tregs and Teffs were stimulated with CD3/CD28 beads (Dynabeads, Invitrogen) for 2, 4, 6, 21 or 24 h, 3 d or 5 d in the presence or absence of HDACi. Cells were cultured in RPMI-1640 (Gibco) supplemented with 10% FBS, 100 U/ml penicillin/streptomycin and 50 μM 2-ME (Invitrogen), termed as “culture medium”. Total RNA was isolated using TRIzol (Invitrogen) and RNeasy kits (QIAGEN), and specific primer and probe sequences for target genes (Applied Biosystems) were used for qPCR amplification of total cDNA (TaqMan, Applied Biosystems). Relative quantitation was determined using a control value of 1, with normalization to 18S rRNA.
Toxicity testing
CD4+CD25- Teffs were isolated from PBMC using CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Irradiated autologous PBMC were used as APC at 1:1 ratio. In some experiments, fresh or cryopreserved PBMC were used as Teffs and APC. PBMC or Teffs were CFSE-labeled [32], resuspended in culture medium (1×106 cells/ml), 100 μL of PBMC or 50 μL of Teffs and 50 μL APC placed in each well in a 96-well plate, and CD3 beads (OKT3 mAb-coated Invitrogen beads) added at a ratio of 3 beads/cell. CFSE-labeled cells without CD3 beads were used as negative controls (no divisions). CFSE-labeled CD3-stimulated cells without HDACi were used as positive controls (maximum divisions). After 3-4 days of incubation, CD4+ cell divisions were determined by CFSE dilution. Levels of each drug that had minimal or no toxic effect on T cell proliferation were then tested in the presence of Tregs in suppression assay. Toxicity testing was performed at least twice with cells from different donors.
Treg suppression assays
Teffs, APC and PBMC were prepared as described above. CD4+CD25+ Tregs were isolated from fresh PBMC using CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). Freshly isolated or expanded Tregs were resuspended in culture medium (1×106 cells/ml) and added to 96-well plates in serial dilutions, giving Treg/Teff ratios of 1/1, ½, 1/4, 1/8 and 1/16. Each well contained 50×103 APC, 50×103 Teff (or 100×103 PBMC) and Tregs ± HDACi (as indicated). Wells without drugs served as positive controls in preparation of suppression curves. Wells with HDACi but without Tregs served as additional toxicity controls, and concentrations of HDACi that impaired cell divisions were excluded from further analysis. Cells without CD3 beads served as negative controls. After 3 or 4 d of incubation, CD4+ cell divisions were determined by CFSE dilution.
To evaluate proliferation of Tregs, expanded or fresh isolated Tregs were CFSE-labeled, and added to wells in serial dilutions and in the presence or absence of differing concentrations of HDACi. CD3 mAb-coated beads, Teffs and APC were added, and each suppression assay was performed as usual except that Treg divisions were determined by CFSE dilution. To study HDACi activities specifically for Tregs only, suppression assay with pre-incubated Tregs was performed. For that assay expanded Tregs were put into the 96-well plate in serial dilutions ± different concentration of HDACi. On the following day, Tregs were washed twice to remove residual HDACi from culture media, and anti-CD3 beads, CFSE-labeled Teffs and APC added as described for the standard suppression assay.
Flow cytometry
We purchased anti-CD4-APC, anti-CD25-PE and -APC, anti-IL-2-PE and anti-CTLA-4 Pe-Cy5 (BD Biosciences); anti-FOXP3-AlexaFluor 647 antibodies (clone 259D, Biolegend); anti-CD4-Pacific blue, anti-FOXP3-PE, anti-FOXP3-Alexa Fluor 647, anti-FOXP3-Pacific blue and anti-FOXP3-FITC (clones PCH101, 236A/E7) (eBioscience). Recombinant human IL-2 was purchase from Roche. Intracellular FOXP3 staining was performed using the FOXP3 Fix/Perm Buffer set (Biolegend or eBioscience respectively) according to each manufacturer's recommendations. Intracellular CTLA-4 and IL-2 stainings were performed using the same conditions as FOXP3 staining (eBioscience kit). In case of Il-2 staining, cells were stimulated in the presence of BD GolgiStop ™ (BD Biosciences). The cell fluorescence was measured using Cyan (Dako) and data were analyzed using Flowjo software (TreeStar). In addition, we studied by flow cytometry expanded or fresh isolated Tregs, which were stimulated with anti-CD3/CD28 beads for 1-5 d, or after 3 and 4 days of a Treg suppression assay.
Statistics
Data were analyzed using GraphPad Prism software. First, all sampling were tested for normality (D'Agostino-Pearson test). A nonparametric Mann-Whitney test was used for qPCR data. For flow cytometry data, raw data, calculated as % of divisions of CD4+ Teff, were standardized using min-max normalization, and the % of standardized suppression was calculated as (100 - % of dividing cells) to make suppression curves from different donors comparable (Fig. S1). Comparative suppression was then calculated as the ratio of area under standardized suppression curves (AUC) with or without drugs; this approach is illustrated for freshly isolated and expanded Tregs (Fig. S2 and Fig. S3, respectively). Kruskal-Wallis test with Dunn's post-test were used to test significance between control suppression and suppression with HDACi. To test for a correlation between suppressive capacity of Tregs and FOXP3 or CTLA-4 expression, a Pearson test was used given that all data were normal. Linear regression ± 95% predictive value were used in graphs only for visualization. To check the relationship between different linear correlations, partial correlation analyses were performed. For all data, a value of p<0.05 was regarded as significant.
RESULTS
Differing expression of HDACs by CD4+CD25+CD127- Tregs and CD4+CD25- Teffs
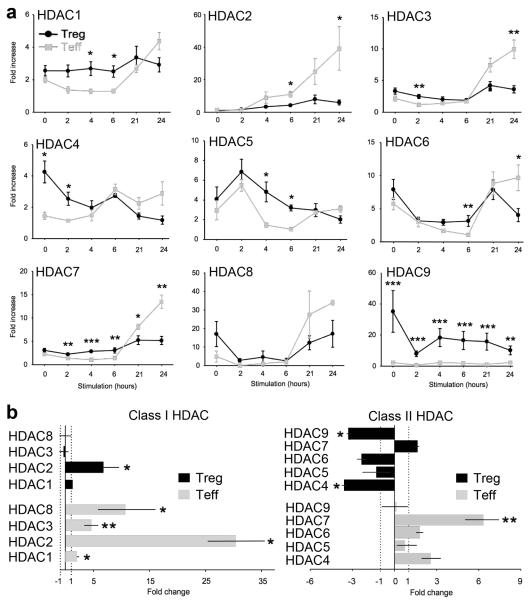
There are no data, to our knowledge, concerning the expression of individual HDAC isoenyzmes by resting and activated T cells, including Tregs. Hence, in 3 donors, we used qPCR to assess HDAC mRNA levels in freshly isolated Tregs and Teffs and after stimulation with CD3/CD28 mAb-coated beads for 2, 4, 6, 21 or 24 h (Fig. 1a). Baseline levels of 3 of the 4 class I HDACs (HDAC1, 2 and 3) were comparable in Tregs and Teffs, whereas Tregs showed higher baseline expression than Teffs of the remaining class I HDAC (HDAC8). Following stimulation, the expression of class I HDACs increased markedly in Teffs (p<0.05), whereas only minor changes were noted in Tregs except for HDAC8, whose levels initially fell rapidly but rose again after 6 h of cell activation. In contrast to class I HDACs, expression of class II HDACs differed in Tregs vs. Teffs both before and after stimulation. At baseline, Tregs showed higher levels than Teffs of all 5 class II HDACs (HDAC4, 5, 6, 7 & 9), with HDAC9 showing the most notable difference, consistent with murine Treg data [13]. After stimulation, levels of HDAC4 and HDAC9 expression decreased significantly in Tregs (p<0.05). Compared to baseline expression, 24 h of cell activation led to significantly increased expression of all class I HDAC but only a single class II HDAC (HDAC7) in Teff cells, whereas in Tregs only a single class I HDAC (HDAC2) was increased and class II HDAC genes, except for HDAC7, were generally decreased (Fig. 1b). While mRNA expression of course does not necessarily indicate HDAC protein level or predict catalytic activity, these data illustrate substantial differences in regulation of HDAC mRNA by human Tregs vs. Teffs under steady state and activating conditions.
Figure 1.
Serial expression of HDAC mRNA by human Tregs (black) or Teffs (grey) before and after CD3/CD28 stimulation. Freshly isolated CD4+CD25+CD127- Tregs and CD4+CD25- Teffs from the same donor were stimulated for the periods shown and HDAC gene expression determined by qPCR. (a) Relative quantitation was determined separately for each HDAC using a control value of 1, with normalization to 18S rRNA; data (mean ± SEM) are from three male donors. Significant differences in the levels of HDAC mRNA expression in Tregs vs. Teff cells are noted with asterisks (*p<0.05, **p<0.01, ***p<0.001). (b) HDAC mRNA expression in Tregs and Teffs after CD3/CD28 stimulation for 24h compared to unstimulated cells (n=3). Data showing statistical analysis (mean ± SEM) for values for each HDAC at 24 h vs. baseline for the specified cell population (*p<0.05, **p<0.01). The mRNA level of each HDAC after stimulation was divided by the level before stimulation (right x axes), and, in cases of values less than 1, 1/value was performed (left x axes).
Multiple HDACi enhanced the suppression function of Tregs
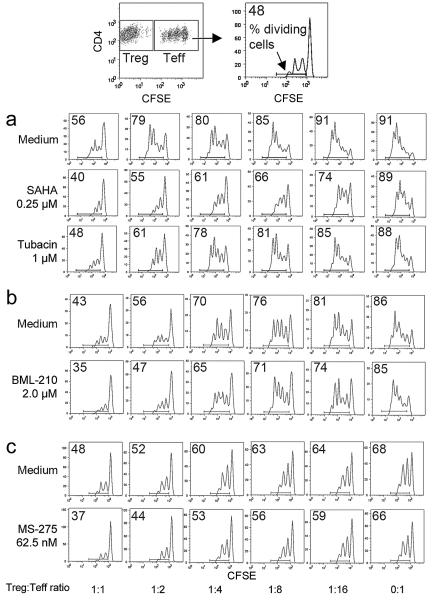
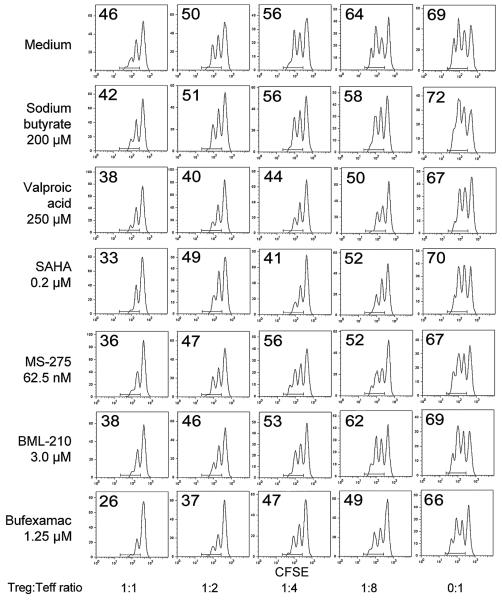
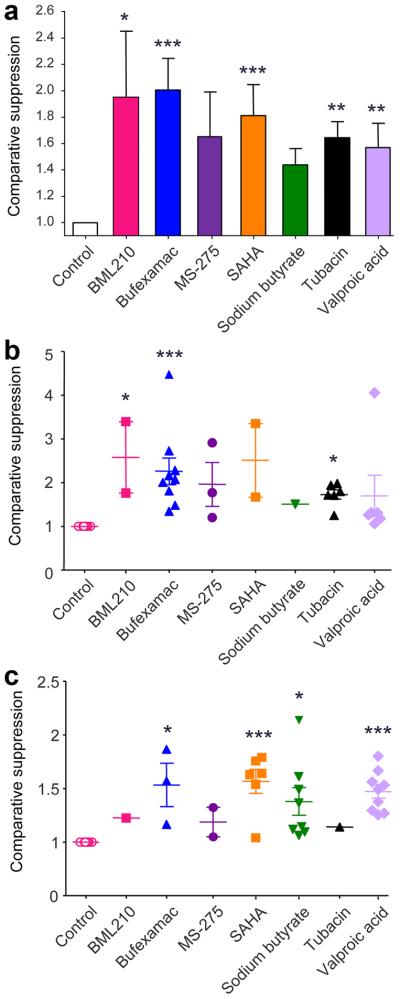
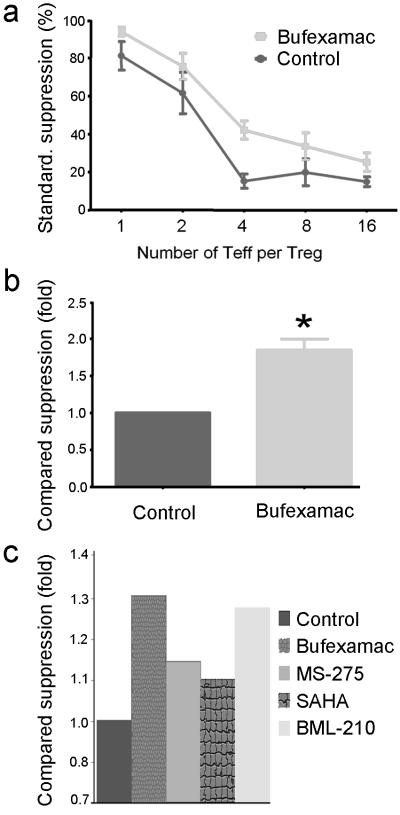
Using fresh isolated (n=20) and expanded (n=4) Tregs from 24 healthy donors (20 male, 4 female), we performed Treg suppression assays (n=60) with varying concentrations of SAHA, sodium butyrate, valproic acid, bufexamac, MS-275, BML-210 and tubacin. These agents were chosen based upon their long-standing clinical use (butyrate, valproate and bufexamac), specific clinical approval as an HDACi (SAHA), class or sub-class selectivity (MS-275, tubacin), or potential special therapeutic interest (BML-210). As HDACi can induce lymphocyte cell-cycle arrest, differentiation or apoptosis in vitro [33], we first assessed the toxicity of varying concentrations of each drug on Teffs and APC. The concentration of each drug that had negligible effect on T cell proliferation over 3 d was determined, and then tested in conjunction with Tregs in standard suppression assays (Table 1). Effects of HDACi were determined by assessing the division of CFSE-labeled CD4+ Teff at varying Treg to Teff cell ratios; representative data are shown for freshly isolated Tregs (Fig. 2) and expanded Tregs (Fig. 3). Each compound was tested 4-15 times with cells from different donors. As Tregs from different donors had differing degrees of suppression, raw data were standardized using min-max normalization, and the % of standardized suppression was calculated as (100-% of dividing cells). Comparative suppression was calculated as the ratio of area under the curve (AUC) with or without each drug; this approach is summarized in Fig. S1 and representative data are shown for freshly isolated Tregs (Fig. S2) and expanded Tregs (Fig. S3). HDACi compounds enhanced Treg suppression to varying extents, with SAHA, bufexamac and BML-210 showing the greatest potencies (Fig. 4 and Table 1). This effect of HDACi exposure was stable for at least 5 d, as evaluated in additional 12 experiments (data not shown).
Table 1.
Summary of the specificities, optimal concentrations and suppression achieved using HDACi
| Name | HDAC specificity | Optimal concentration (μM) |
Number of donors |
Fold increase in suppression | P value | ||
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean ± SEM | |||||
| BML-210 | Pan-HDACi except for HDAC6 [50] |
2-3 | 4 | 1.2 | 3.4 | 2.0 ± 0.5 | p<0.05 |
| Bufexamac | HDAC1 & 6 [20] | 1-1.25 | 13 | 1.1 | 4.5 | 2.0 ± 0.2 | p<0.001 |
| MS-275 | Class I HDACs 1-3 [51] |
0.03-0.06 | 5 | 1.1 | 2.9 | 1.7 ± 0.3 | p>0.05 |
| SAHA | Pan-HDACi [52] | 0.2-0.25 | 8 | 1.1 | 3.4 | 1.8 ± 0.2 | p<0.001 |
| Sodium butyrate |
Pan-HDACi [53] | 200-300 | 8 | 1.1 | 2.2 | 1.4 ± 0.1 | p>0.05 |
| Tubacin | HDAC6-specific inhibitor [54] |
1-1.5 | 7 | 1.1 | 2.0 | 1.6 ± 0.1 | p<0.01 |
| Valproic acid |
Pan-HDACi except HDAC6 & 10 [55] |
200-250 | 15 | 1.1 | 4.1 | 1.6 ± 0.2 | p<0.01 |
Figure 2.
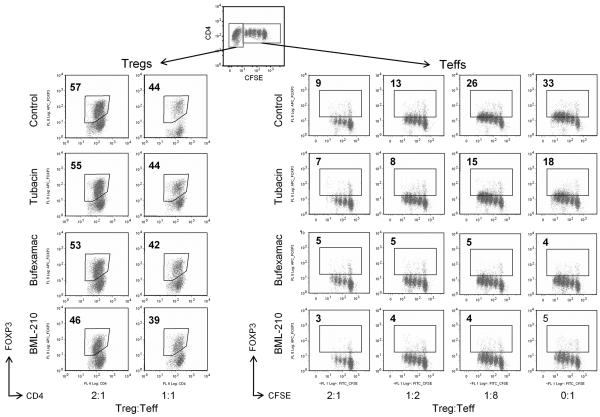
Effects of HDACi on suppression assays using freshly isolated Tregs and CFSE-dilution of Teff cells. Top-most panels show how Tregs and Teffs were gated and CFSE peaks evaluated. Assays were performed using concentrations of HDACi that did not affect Teff proliferation directly as determined in preliminary toxicity assays and also monitored in each experiment by assessing Teff proliferation in the absence of added Tregs (0:1 Treg:Teff ratio). The proportion of dividing cells in each well is indicated in the top left of each CFSE-dilution plot; 3 representative experiments (from 29) with cells from different donors and use of (a) SAHA and tubacin, (b) BML-210, and (c) MS-275.
Figure 3.
Effects of HDACi on suppression assays using expanded Tregs. Assays were performed in the presence of HDACi concentrations that did not affect Teff proliferation directly as determined in preliminary assays and as monitored in each experiment by assessing Teff proliferation in the absence of added Tregs (0:1 Treg:Teff ratio). The proportion of dividing cells in each well is indicated in the top left of each CFSE-dilution plot. One experiment, representative of 31, is shown.
Figure 4.
Comparative suppression of freshly isolated or expanded Treg with HDACi based on data from 60 experiments with cells from 24 donors; all comparisons were determined using Dunn's multiple comparison test (*p<0.05, **p<0.01 and ***p<0.001). (a) Overall pooled data from all assays, with the effect of each drug on suppression of proliferation as compared to control wells receiving medium alone. (b) Data using freshly isolated Tregs plus HDACi and involving 29 experiments with cells from 20 donors. (c) Data using expanded Tregs plus HDACi and involving 31 experiments with cells from 4 donors. For all assays, comparative suppression (mean ± SEM) was calculated as ratio of area under the standardized suppression curves with or without each drug.
Though we used levels of each HDACi that did not impair divisions of Teffs in the absence of Tregs, HDACi use might increase the sensitivity of Teffs or APC to suppression without enhancing the suppressive function of Tregs. We therefore also incubated Tregs with or without HDACi for 24 h, washed Tregs twice to remove residual HDACi from culture media, and added Teffs and APC for 3 days of suppression assay as usual. Tregs that were pre-incubated with HDACi and washed had lesser though still significant effects on Teff proliferation, due to shorter time of incubation or removal of compounds, than Tregs continuously exposed to HDACi for 3 d (Fig. 5). These effects were unlikely to result from residual HDACi presence since each compound was ineffective when tested at 3-5 fold lower concentrations than usual (data not shown).
Figure 5.
Tregs pre-incubated with HDACi showed increased suppressive function. Tregs were incubated with HDACi for 24 h, washed, CFSE-labeled Teffs and APC added, and suppression assays performed as usual over 3 d (see Methods). Upper panel shows standardized suppression curves (a), and compared suppression using area-under-curve ratios (b) for 5 independent experiments with bufexamac (3 donors, p=0.036, Wilcoxon Signed Rank Test). Panel (c) shows data from one experiment with other tested drugs; cumulative difference of HDACi vs control is significant (p=0.025).
Varying effects of HDACi on FOXP3 expression
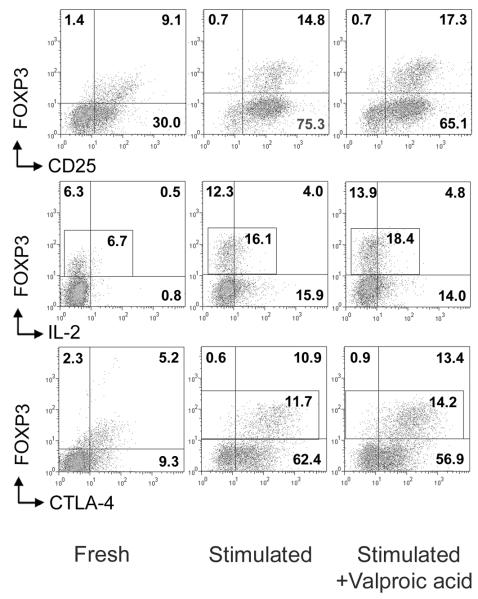
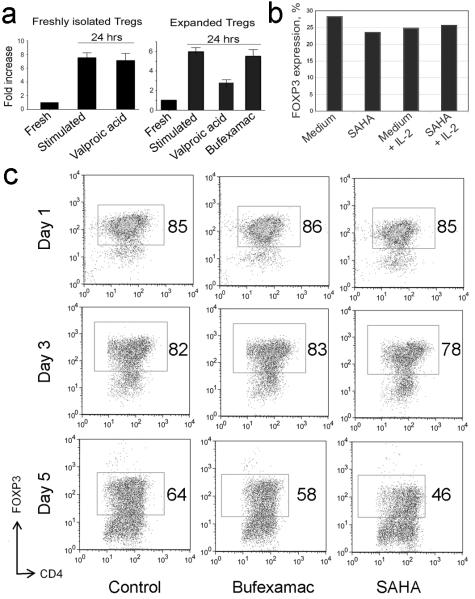
We analyzed several potential mechanisms for the increased suppressive ability of HDACi-treated Tregs, beginning with use of peripheral blood mononuclear cells (PBMC). When human PBMC were stimulated for 24 h with CD3/CD28 beads ± HDACi, HDACi use moderately increased the CD25+FOXP3+ and CTLA-4+FOXP3+ (especially CTLA-4hi) populations of CD4+ cells, but decreased FOXP3-CD25+ and FOXP3-CTLA-4+ subsets (Fig. 6), suggesting enhanced expression of FOXP3 in cells likely to be natural Tregs rather than activated Teff cells. Additionally, HDACi slightly decreased IL-2 production (Fig. 6). However, unlike with murine Tregs [13], HDACi use in vitro did not enhance FOXP3 mRNA or protein expression by purified human Tregs, as observed by qPCR (5 experiments, 1 with expanded Tregs and 4 with fresh isolated Tregs) or flow cytometric analysis of freshly isolated or expanded Tregs (1 experiment with each population); in these studies Tregs were stimulated with CD3/CD28 mAb-coated beads and analyzed at day 1, day 3 or day 5 of culture (Fig. 7). Moreover, in some cases we observed a variable decrease of FOXP3 expression over several days, regardless HDACi exposure; this effect was highly variable between donors. HDACi addition did not significantly affect mRNA expression of Bcl-2, Bcl-XL, CTLA4, GARP, or that of several cytokines (IL-2, IL-6, IL-10 or TNF-α), and did not change cells viability according to FS-SS gating or DAPI staining (data not shown). Reasoning that FOXP3 levels might reflect lack of access to IL-2 in these cultures, we performed 2 additional experiments. First, we activated freshly isolated Tregs for 6 h in the presence of IL-2 (100 U/ml) and SAHA, and found that addition of IL-2 prevented loss of FOXP3 expression by Tregs incubated with SAHA (Fig. 7). Second, we stimulated expanded and fresh isolated Tregs for 24 h in the presence of IL-2 (300 U/ml) and each HDACi; again there was no significant change in FOXP3 expression (Table 2).
Figure 6.
FOXP3, CD25, CTLA4 expression and IL-2 production in CD4+ cells after stimulation with or without HDACi. Human PBMC were stimulated for 24 h with CD3/CD28 mAb-coated beads ± addition of HDACi (two experiments with valproic acid and bufexamac were performed). Expression of specified markers as % of positive cells showed for CD4+ gated cells, left column before stimulation, stimulated cells (middle column) and stimulated cells plus valproic acid (right column).
Figure 7.
HDACi exposure does not significantly increase expression of FOXP3 in human Tregs, and FOXP3 expression depends upon level of IL-2. (a) qPCR analysis of FOXP3 mRNA in fresh isolated (left) or expanded (middle) Tregs stimulated with CD3/CD28 mAb-coated beads for 24 h in the presence of bufexamac or valproic acid or control (no HDACi). Data of two experiments shown; 5 experiments with cells from 5 donors (4 fresh isolated, 1 expanded Tregs) were obtained with comparable data. (b) Flow cytometric analysis of FOXP3 expression by freshly isolated Tregs that were stimulated with CD3/CD28 mAb-coated beads for 6 h in the presence or absence of human IL-2 (100 U/ml) and SAHA. (c) Flow cytometric analysis of FOXP3 expression by freshly isolated Tregs that were stimulated with CD3/CD28 mAb-coated beads for 1, 3 or 5 d in the presence of bufexamac or SAHA or control (no HDACi); comparable data were seen using expanded Tregs (not shown). Two experiments were performed, and the percentage of FOXP3+ cells is shown in each panel.
Table 2.
Flow cytometric expression of FOXP3 in Tregs, stimulated with CD3/CD28 beads for 24 h with IL-2 (300 U/ml) and standard concentrations of HDACi
| Agent | % FOXP3 expression |
|---|---|
| Medium | 89.0 |
| BML210 | 88.7 |
| Bufexamac | 89.9 |
| MS275 | 88.1 |
| SAHA | 87.5 |
| Tubacin | 88.9 |
| Valproic acid | 88.2 |
Suppressive capability of Tregs correlates with expression of CTLA-4 rather than FOXP3
We have shown that HDAC9 deletion by homologous recombination can promote murine Treg survival and proliferation in vitro, resulting on average in a 2-fold increase in the percentage of Treg by the end of a standard 3 d Treg suppression assay [14]. We therefore performed suppression assays using CFSE-labeled human Tregs to test whether HDACi use affected proliferation of human Tregs. We found that each HDACi tested, including BML-210, MS-275, SAHA, sodium butyrate, valproic acid and bufexamac, caused mild to moderate impairment of Treg division whether evaluated at 3 or 5 d (Fig. S4). Moreover, HDACi therapy did not increase FOXP3 expression within either the Treg or the Teff populations after 3 (Fig. 8) or 5 d (data not shown) of a suppression assay. However HDACi increased the proportion of CTLA4hi Tregs by up to 2-fold in suppression assays (Fig. 9). This phenomenon was observed with freshly isolated (from 4 donors) as well as expanded (from 2 donors) Tregs at 3 d (for all HDACi except bufexamac) and at 5 d of suppression assay for bufexamac along with all other tested drugs.
Figure 8.
Flow cytometry with intranuclear staining for FOXP3 after 3 days of suppression assay. HDACi did not increase FOXP3 expression in either Treg or CFSE-labeled Teff subsets; data are representative of 11 experiments with cells from different donors, freshly isolated as well as expanded Tregs, examined at day 3 or day 5 of suppression assay. Teffs and APC were used in standard Treg suppression assay, followed by intranuclear staining for FOXP3; Tregs were gated according to CD4 and CFSE properties (top panel) and expression of FOXP3 in cells exposed to HDACi was compared with control cells (no HDACi, upper row). Percentages of labeled FOXP3+ Tregs (left panel) and Teffs (right panel) are shown.
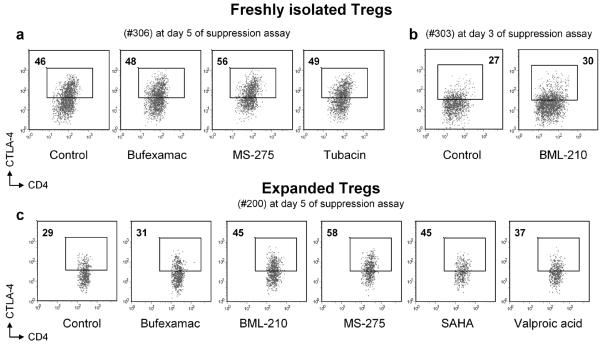
Figure 9.
All HDACi tested increased CTLA-4 intracellular expression in human Tregs. Flow cytometry of freshly isolated Tregs from two donors (a, b) and expanded (c) Tregs during suppression assays at day 3 (b) or day 5 (a, c). Teffs and APC were used in standard Treg suppression assay, followed by intracellular staining for CTLA-4; Tregs were gated according to CD4 and CFSE properties and expression of CTLA-4hi in cells exposed to HDACi was compared with control cells (no HDACi). Percentages of labeled CTLA-4hi Tregs shown. Data are representative of 10 experiments with cells from different donors, freshly isolated (from 4 donors) as well as expanded (from 2 donors) Tregs, examined at day 3 or day 5 of suppression assay.
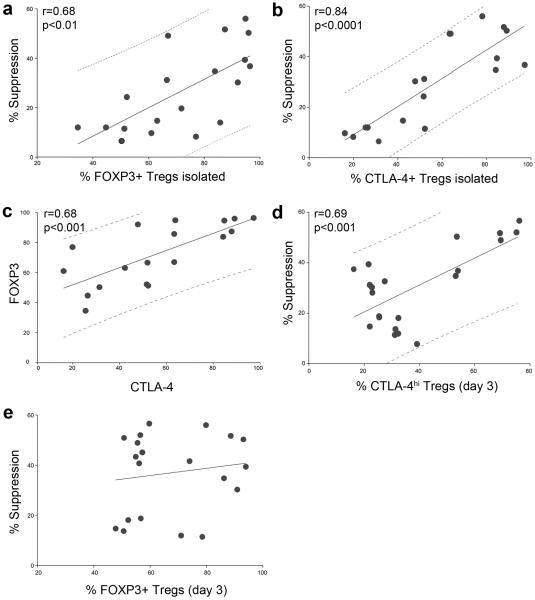
Given the effect of HDACi exposure on CTLA-4 expression by Tregs, we analyzed further the expression of FOXP3 vs. CTLA-4 in Treg suppression assays. We observed a strong correlation between the purity of Treg after isolation or expansion (calculated as % of FOXP3+ cells) and suppressive capability of these cells (Fig. 10a). Likewise, we showed that CTLA-4 expression in Treg after isolation or expansion also correlated with suppressive activity of these cells (Fig 10b), and that levels of FOXP3 and CTLA-4 expression in Tregs after isolation or expansion correlated with each other (Fig 10c). However, to distinguish real correlations from false ones within these 3 connected factors (FOXP3, CTLA-4 and suppressive ability), we performed partial correlation analysis. As a result, when we excluded CTLA-4, FOXP3 expression lost any connection with suppressive capability (Table 3). These data showed that the correlation between FOXP3 expression after isolation of Tregs and suppressive function was observed only due to a correlation between CTLA-4 and FOXP3 expression, and the correlation of CTLA-4 with Treg suppressive function. In addition to the strong correlation between CTLA-4 at day 0 and following inhibition of Teff proliferation, we observed that suppressive function correlated highly with the proportion of CTLA-4hi (Fig. 10d) but not FOXP3+ (Fig. 10e) after 3 d of suppression assays. All collected data were analyzed together or separately for expanded and for freshly isolated Tregs, for experiments without HDACi or with HDACi, and the same patterns were observed (data not shown). Thus, CTLA-4 expression, especially CTLA-4hi, but not FOXP3 expression, is an important contributor to human Treg suppression, and use of HDACi increases the proportion of CTLA-4hi Treg during Treg suppression assays.
Figure 10.
Statistical analysis of relationships between Treg suppression and expression of FOXP3 or CTLA-4. Suppression of Teff cell proliferation was calculated as % of divisions of Teffs in wells without Tregs minus % of divisions of Teffs in wells with 1:1 Treg:Teff ratios; Pearson tests with linear regression ± 95% predictive values shown. (a) Correlation of % FOXP3+ cells, isolated with magnetic beads (14 experiments) or expanded (5 experiments) and suppression function; data from 15 donors. (b) Correlation of % CTLA-4+ cells, isolated with magnetic beads (14 experiments) or expanded (4 experiments) and suppression function; data from 14 donors. (c) Correlation of FOXP3+ and CTLA4+ expression in cells isolated with magnetic beads (14 experiments) or expanded (4 experiments); data from 14 donors. (d) Correlation of high CTLA-4 expression by Tregs at day 3 of suppression assay and suppression; data from 10 experiments with 6 donors, with and without HDACi. (e) No correlation of % FOXP3+ cells in Treg subset at day 3 of assay and suppression observed with these cells; data from 11 experiments with 7 donors, with and without HDACi.
Table 3.
Partial correlation analysis
| Pairs of variables | Excluded variable |
Correlation coefficient, r |
P value |
|---|---|---|---|
| CTLA4 and FOXP3 |
no | 0.73 | 0.001 |
| Suppression excluded | 0.43 | 0.085 | |
| CTLA4 and suppression |
no | 0.78 | <0.0001 |
| FOXP3 excluded | 0.57 | 0.017 | |
| FOXP3 and suppression |
no | 0.68 | 0.002 |
| CTLA4 excluded | 0.26 | 0.312 |
DISCUSSION
HDACi small molecules can promote cell-cycle arrest and the differentiation or apoptosis of cancer cells, suggesting their promise as a new class of anticancer drugs [18]. However, much less is known about their effects on the immune system, including human lymphocyte functions [19]. The current work was stimulated by our finding that HDACi use can promote the development and suppressive function of murine FOXP3+ Tregs [13], and we now provide the first data on the expression of HDACs and the effects of HDACi treatment on the functions of FOXP3+ human Tregs.
We analyzed the expression of HDACs in resting vs. activated human Tregs and Teffs. Class I HDACs (HDAC1-3 & HDAC8) are ubiquitously expressed and localized in the nucleus, where they are central to the regulation of gene expression. Thus, HDAC1 and HDAC2 are present in the Sin3A and NuRD co-repressor complexes [34] and HDAC3 is present in the NCoR/SMRT co-repressor complex [35]. The importance of class I HDACs is underlined by the finding that in each case, including HDAC1 [36], HDAC2 [37], HDAC3 [38] and HDAC8 [39], global deletion results in pre-natal or peri-natal mortality. In the current study, we found that the expression of class I HDACs was fairly similar in resting human Tregs and Teffs but differed upon CD3/CD28 activation. Activation induced increased expression of multiple class I HDACs in Teffs but not Tregs, except for a modest increase in HDAC2 expression. Increased expression of class I HDACs in conventional T cells undergoing activation was reported previously [40]. Such increases are consistent with roles for induction of HDAC1 and HDAC2 in the regulation of transcriptional repression in dividing cells [41], and that of HDAC3 [42] and HDAC8 [43] in promoting suppression of apoptosis. While there are no previous data, to our knowledge, concerning changes in HDAC expression upon human Treg activation, the lack of upregulation of class I HDAC expression in Tregs upon activation is consistent with the very limited capacity of Tregs to divide under standard culture conditions in vitro [5] and their marked resistance, as compared to Teffs, to the development of apoptosis [44].
In contrast to class I HDACs, class II HDACs (HDAC4-7 & HDAC9) are primarily expressed in muscle, neural tissues and thymocytes, and exhibit tissue-specific repression by shuttling between the nucleus and cytoplasm [16, 17]. Their global deletion is lethal only in the cases of HDAC4 [45] and HDAC7 [46], reflecting involvement in skeletal and vascular development, respectively. Under resting conditions, human Tregs had higher levels of class II HDACs than Teffs, including a 20-fold difference in the case of HDAC9. However, upon CD3/CD28 activation, levels of class II HDACs except HDAC7 were down-regulated by about 2-3-fold in Tregs, whereas in Teffs all class II HDACs except HDAC9 were upregulated. HDAC7 play a central role in thymic selection through regulation of Nur77 expression [47], and is present in a multi-component complex in Tregs that also contains FOXP3 [12, 44], but involvement of HDAC7 in Treg development and peripheral functions is not yet understood. Levels of HDAC9 remained >10-fold higher in Tregs than that of Teffs at all times, suggesting the relative unimportance of HDAC9 to Teff functions. By contrast, the decrease in HDAC9 expression upon Treg activation is of interest given data from murine studies. Murine Tregs require TCR activation for optimal FOXP3-dependent functions [11], and HDAC9 is an inhibitor of FOXP3 that is exported from the nucleus upon TCR signaling [13]. The current data showing that HDAC9 is rather selectively expressed by human Tregs suggest that HDAC9 may play a similar role in controlling human Treg functions. Overall, the observed differences in HDAC expression suggest the potential for future preferential targeting of human Tregs using class II HDAC-specific HDACi or inhibitors of individual class II HDAC isoforms.
Various types of HDACi are currently being developed for use in oncology or considered for potential application as anti-inflammatory agents [19]. The current studies showed that incubation with HDACi of varying types enhanced the suppressive capability of freshly isolated or expanded human Tregs, consistent with murine data [13, 14]. Beneficial effects were also seen using Tregs that were pre-incubated with HDACi and washed, indicating that increased suppression can be attributed at least in part to a direct effect of HDACi on Tregs, though optimal enhancement of suppression required continuous exposure in Treg suppression assays. Our findings are encouraging with regard to future considerations of HDACi for control of inflammation and autoimmunity, given that much is already known regarding the clinical pharmacokinetics, toxicity and side-effects of HDACi, and some, such as valproic acid, have been widely used in many patients.
HDACi can directly increase human and murine FOXP3 acetylation and chromatin binding [12, 13], leading to increased expression of FOXP3-regulated genes, including CTLA-4 [11]. While the identification of the key HDAC or HDACs involved remains to be determined, we did achieve some progress with regard to the mechanisms by which HDACi use can potentiate human Treg function. Enhanced suppressive function was not associated with obvious increases in FOXP3 expression or protein stability, or with increased conversion of naïve T cells into induced Tregs. Thus, treatment with 7 different HDACi (BML-210, bufexamac, MS-275, SAHA, sodium butyrate, tubacin, valproic acid) led to modest and variable decreases in FOXP3 mRNA and proportions of FOXP3+ cells in Tregs, stimulated alone or stained after suppression assay. However, the loss of FOXP3 expression in these experiments was prevented when exogenous IL-2 was added. At the same time, stimulation of human PBMC with HDACi led to moderate increase of FOXP3+CD25+ and FOXP3+CTLA-4+ subsets in CD4+ cells. However, since these phenotypic markers are not unique for Tregs and can be expressed by activated Teffs, it is currently not possible to clearly separate the effect of HDACi on Tregs versus Teff cells under these conditions.
Our studies also showed impaired conversion of CD4+CD25- Teff cells to CD25+FOXP3+ cells during suppression assays performed in the presence of HDACi. In the absence of Tregs, activation of Teff cells is associated with their induction of FOXP3, whereas Teff cell induction of FOXP3 is decreased by the addition of Tregs. This suppressive effect on Teff cell induction of FOXP3 was increased by HDACi addition to cultures. Since the most pronounced conversion of Teff into FOXP3+ cells and maximal cell division was observed in the wells without Tregs, human Teff cell induction of FOXP3 expression is associated with immune activation rather than with acquisition of any suppressive function.
HDACi use was not associated with increased proliferation of Tregs. In contrast to these negative data, our analysis did show that HDACi use can increase CTLA4 expression under conditions of the Treg suppression assay, and that such expression, unlike that of FOXP3, is highly correlated with human Treg suppression. Thus, we found a significant direct correlation between expression of CTLA-4 by Tregs after isolation or during suppression assays with Treg suppressive activity. These data are consistent with the impaired Treg suppression and development of systemic autoimmunity seen in mice with a selective deficiency of CTLA-4 in their Tregs [48]. Moreover, human CD4+CD25- T cells transfected with CTLA-4 did not express FOXP3 but potently suppressed Teff activation, suggesting that suppressive function relates to CTLA-4 expression rather than to FOXP3 expression [28], similarly to the current study. Our observations also agree with recent evidence that the main suppressive mechanism of Tregs is related to their expression of CTLA-4 [49]. CTLA-4 expression by Tregs could well reflect the epigenetic status of FOXP3 working in suppressive complexes, such that an absence of direct correlation between level of mRNA or protein expression of FOXP3 and suppressive function may be explained. Lastly, additional considerations may influence the outcomes of studies involving HDACi and Tregs. Our data suggest that IL-2-deficient conditions such as occur with stimulation of Tregs alone, or in suppression assays, can complicate assessment of the mechanisms of action of HDACi on Tregs. Lastly, a potential requirement for Treg/APC interactions and possible overgrowth of Tregs by activated FOXP3+ Teffs may also mask assessment of the effects of HDACi.
Further studies are required to assess whether HDACi use can complement therapies being developed that involve Treg expansion and adoptive transfer, given that these agents were recently shown to stabilize the human Treg phenotype and prevent their conversion to Th17 cells [7]. This stabilization and enhancement of suppressive function may ultimately prove of benefit clinically in the contexts of autoimmunity and transplantation. Such applications may also benefit from the ongoing development by various groups of new HDACi that block class II HDACs or individual HDAC proteins [19].
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by National Institutes of Health Grant AI073489
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Buckner JH, Ziegler SF. Functional analysis of FOXP3. Ann N Y Acad Sci. 2008;1143:151–69. doi: 10.1196/annals.1443.014. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev. 2009;23:1270–82. doi: 10.1101/gad.1791009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 5.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–6. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 8.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–55. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A. 2005;102:5138–43. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem. 2006;281:36828–34. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, Saouaf SJ, Greene MI. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–6. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 14.de Zoeten EF, Wang L, Sai H, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010:583–94. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Zoeten EF, Lee I, Wang L, Chen C, Ge G, Wells AD, Hancock WW, Ozkaynak E. Foxp3 processing by proprotein convertases and control of regulatory T cell function. J Biol Chem. 2009;284:5709–16. doi: 10.1074/jbc.M807322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–18. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–99. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3(+) regulatory T cells. Nat Rev Drug Discov. 2009;8:969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang H, Wang XS, Huang XP, Roth BL, Butler KV, Kozikowski AP, Jung M, Tropsha A. Novel inhibitors of human histone deacetylase (HDAC) identified by QSAR modeling of known inhibitors, virtual screening, and experimental validation. J Chem Inf Model. 2009;49:461–76. doi: 10.1021/ci800366f. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard F, Chipoy C. Histone deacetylase inhibitors: new drugs for the treatment of inflammatory diseases? Drug Discov Today. 2005;10:197–204. doi: 10.1016/S1359-6446(04)03309-4. [DOI] [PubMed] [Google Scholar]
- 22.Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257:97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Tuovinen H, Laurinolli TT, Rossi LH, Pekkarinen PT, Mattila I, Arstila TP. Thymic production of human FOXP3(+) regulatory T cells is stable but does not correlate with peripheral FOXP3 expression. Immunol Lett. 2008;117:146–53. doi: 10.1016/j.imlet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 25.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 26.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 27.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, Orban PC, Ziegler SF, Roncarolo MG, Levings MK. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–84. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O, Walker LS, Sansom DM. Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol. 2008;181:1683–91. doi: 10.4049/jimmunol.181.3.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 30.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 31.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH. Engineering artificial antigen-presenting cells to express a diverse array of costimulatory molecules. Mol Ther. 2007;15:981–8. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X, Liu R, Balcarcel RR, Fisher N, Levine BL, Carroll RG, Warner N, Blazar BR, June CH, Riley JL. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–68. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreira JM, Scheipers P, Sorensen P. The histone deacetylase inhibitor Trichostatin A modulates CD4+ T cell responses. BMC Cancer. 2003;3:30. doi: 10.1186/1471-2407-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grozinger CM, Schreiber SL. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. 2002;9:3–16. doi: 10.1016/s1074-5521(02)00092-3. [DOI] [PubMed] [Google Scholar]
- 35.Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000;14:1048–57. [PMC free article] [PubMed] [Google Scholar]
- 36.Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. Embo J. 2002;21:2672–81. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest. 2008;118:3588–97. doi: 10.1172/JCI35847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haberland M, Mokalled MH, Montgomery RL, Olson EN. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 2009;23:1625–30. doi: 10.1101/gad.1809209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dangond F, Gullans SR. Differential expression of human histone deacetylase mRNAs in response to immune cell apoptosis induction by trichostatin A and butyrate. Biochem Biophys Res Commun. 1998;247:833–7. doi: 10.1006/bbrc.1998.8891. [DOI] [PubMed] [Google Scholar]
- 41.Ma P, Schultz RM. Histone deacetylase 1 (HDAC1) regulates histone acetylation, development, and gene expression in preimplantation mouse embryos. Dev Biol. 2008;319:110–20. doi: 10.1016/j.ydbio.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escaffit F, Vaute O, Chevillard-Briet M, Segui B, Takami Y, Nakayama T, Trouche D. Cleavage and cytoplasmic relocalization of histone deacetylase 3 are important for apoptosis progression. Mol Cell Biol. 2007;27:554–67. doi: 10.1128/MCB.00869-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balasubramanian S, Ramos J, Luo W, Sirisawad M, Verner E, Buggy JJ. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia. 2008;22:1026–34. doi: 10.1038/leu.2008.9. [DOI] [PubMed] [Google Scholar]
- 44.Tao R, Hancock WW. Resistance of Foxp3+ regulatory T cells to Nur77-induced apoptosis promotes allograft survival. PLoS ONE. 2008;3:e2321. doi: 10.1371/journal.pone.0002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–66. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–34. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 47.Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG, Verdin E. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity. 2003;18:687–98. doi: 10.1016/s1074-7613(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 48.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 49.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–11. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 50.Wong JC, Hong R, Schreiber SL. Structural biasing elements for in-cell histone deacetylase paralog selectivity. J Am Chem Soc. 2003;125:5586–7. doi: 10.1021/ja0341440. [DOI] [PubMed] [Google Scholar]
- 51.Hildmann C, Wegener D, Riester D, Hempel R, Schober A, Merana J, Giurato L, Guccione S, Nielsen TK, Ficner R, Schwienhorst A. Substrate and inhibitor specificity of class 1 and class 2 histone deacetylases. J Biotechnol. 2006;124:258–70. doi: 10.1016/j.jbiotec.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 52.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 53.Hess-Stumpp H, Bracker TU, Henderson D, Politz O. MS-275, a potent orally available inhibitor of histone deacetylases--the development of an anticancer agent. Int J Biochem Cell Biol. 2007;39:1388–405. doi: 10.1016/j.biocel.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–94. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–86. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.