Abstract
Angiotensin-II (Ang-II) is an autacoid generated as part of the pathophysiology of cardiac hypertrophy and failure. In addition to its role in cardiac and smooth muscle contraction and salt retention, it was shown to play a major role in the cardiac interstitial inflammatory response and fibrosis accompanying cardiac failure. In this study, we examined a model of Ang-II infusion to clarify the early cellular mechanisms linking interstitial fibrosis with the onset of the tissue inflammatory response. Continuous infusion of Ang-II resulted in increased deposition of collagen in the heart. Ang-II infusion also resulted in the appearance of distinctive small, spindle-shaped, bone marrow-derived CD34+/CD45+ fibroblasts that expressed collagen type I and the cardiac fibroblast marker DDR2 while structural fibroblasts were CD34-/CD45-. Genetic deletion of monocyte chemoattractant protein (MCP)-1 (MCP-1-KO mice) prevented the Ang-II-induced cardiac fibrosis and the appearance of CD34+/CD45+ fibroblasts. Real-time PCR in Ang-II-treated hearts revealed a striking induction of types I and III collagen, TGF-β1, and TNF mRNA expression; this was obviated in Ang-II-infused MCP-1-KO hearts. In both wild-type and MCP-1-KO mice, Ang-II infusion resulted in cardiac hypertrophy, increased systolic function and hypertension which were not significantly different between the WT and MCP-1-KO mice over the 6 week course of infusion. In conclusion, the development of Ang-II-induced non-adaptive fibrosis in the heart required induction of MCP-1, which modulated the uptake and differentiation of a CD34+/CD45+ fibroblast precursor population. In contrast to the inflammatory and fibrotic response, the hemodynamic response to Ang-II was not affected by MCP-1 in the first 6 weeks.
Keywords: fibroblasts, collagen, inflammation, angiotensin, monocyte chemoattractant protein-1, monocytes, progenitor cells
Introduction
Augmented interstitial or non-adaptive fibrosis in the heart is invariably associated with ventricular remodeling and subsequent cardiac dysfunction and is therefore a common pathological feature of many types of heart failure [1,2]. The development of fibrosis is also often associated with inflammation [3,4]. However, the molecular and cellular mechanisms of non-adaptive fibrosis itself, as well as the contribution of the inflammatory system to the development of fibrosis in the heart are still not well understood.
Traditionally, resident cardiac fibroblasts were thought to be activated by pro-inflammatory processes to proliferate and synthesize collagen that is secreted and deposited in the interstitial space [5,6]. However, recent studies by our laboratory and others describe an important role for the uptake of fibroblast precursor cells of blood-borne, bone marrow-derived origin in pathological interstitial fibrosis [7-10]. We have developed a model in which daily 15-min occlusions of the left anterior descending coronary artery resulted in a fibrotic cardiomyopathy (I/RC) that developed in the absence of myocardial infarction [11]. I/RC depended upon the appearance of a unique population of small, spindle-shaped fibroblasts in the heart that arose from bone marrow-derived (hematopoietic), blood-borne, monocytic fibroblast precursors that expressed CD34 and CD45 [7]. When cultured in vitro, these cells were morphologically different than fibroblasts isolated from sham hearts that lacked this cell population [7]. Because there was no myocardial infarction and therefore no myocyte death, I/RC was associated with minimal change in cytokines and chemokines other than a distinctive prolonged rise in monocyte / macrophage chemoattractant protein 1 (MCP-1) expression [11]. MCP-1 is upregulated in inflammatory and fibrotic processes and plays a critical role in the pathogenesis of many fibrotic diseases including cardiac diseases [12]. We extended our observations by subjecting mice with genetic deletion of MCP-1 (MCP-1-KO mice) to I/RC and showed that in these mice the development of the fibrotic cardiomyopathy was obviated [13]. Together with further in vitro studies, we demonstrated an obligate role for MCP-1 in the development of non-adaptive fibrosis that resides in its chemoattractive effect on monocytic fibroblast precursors [14]. We have suggested that this mechanism might constitute a potential link between interstitial fibrosis and inflammation in the heart [7,14].
Since Ang-II generation is associated with almost all occurrences of heart failure and hypertrophy [15-18], and is associated with both inflammation and interstitial fibrosis [19,20], we chose to investigate the involvement of monocytic fibroblast precursor cells in the development of cardiac fibrosis in response to Ang-II. In this study we show that Ang-II infusion induced MCP-1 and the concurrent uptake of monocytes and CD34+/CD45+ fibroblast precursor cells along with the appearance of marrow-derived CD45+ fibroblasts in the heart. Genetic deletion of MCP-1 obviated the appearance of CD45+ fibroblasts in the heart and markedly reduced cardiac fibrosis. By contrast, deletion of MCP-1 did not alter the appearance of Ang-II-induced hypertension nor cardiac hypertrophy. Our data indicate that the development of Ang-II-mediated fibrosis required induction of MCP-1 and uptake of myeloid fibroblast precursor cells into the heart, but that fibrosis was not the dominant factor in producing the early cardiovascular functional responses to Ang-II.
Methods
Animals
B6.129S4-Ccl2tm1Rol/J (MCP-1-KO) mice (backcrossed to C57BL/6 for >10 generations) and C57BL/6J wild-type (WT) mice (both from Jackson Laboratory) were infused with 1.5 μg/kgBW/min Ang-II via subcutaneously implanted osmotic pumps for 1, 2 or 6 wks. Control animals were implanted with sterile saline-filled pumps. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US NIH. All animals were treated in accordance with the guidelines of the Baylor College of Medicine Animal Care and Research Advisory Committee.
Cardiac Fibrosis
Hearts were embedded in paraffin and sectioned as described earlier [11]. To measure collagen deposition, sections were stained with picrosirius red. Images were scanned and collagen stained areas were calculated as percentages of the total myocardial area. Alpha-smooth muscle actin (α-SMA)+ myofibroblasts and Mac-2+ macrophage densities were determined by antibody staining [11].
Immunostaining
After trypsin-induced antigen retrieval and cell permeabilization, heart sections were stained with anti- MCP-1, α-SMA, Mac-2, and CD31 specific antibodies, followed by IgG specific, peroxidase- or fluorescence-conjugated secondary antibodies as described previously [7,11,13].
Identification of Fibroblast Populations
Cardiac fibroblasts were isolated and cultured as described previously [7]. Freshly isolated cells were incubated with calceinAM, PE-conjugated anti-CD34, and PE/Cy-5-conjugated anti-CD45. Fluorescence intensities were measured on a Beckman Coulter Epics XL.MCL. Proliferation of cultured cardiac fibroblasts was determined by BrdU incorporation [7]. To normalize data from different experiments, enhanced proliferation in response to serum was expressed as the fold increase compared to cells maintained in serum-free medium. Isolated cardiac fibroblasts were grown on glass slides [7]. Cells were stained with PE-conjugated anti-CD34 or anti-CD45, fixed, permeabilized, then stained with anti-DDR2 or anti-collagen type I, followed by DyLight™ 488-conjugated secondary antibody. Glass slides were mounted with SlowFade Gold containing DAPI. Images were digitally photographed and analyzed.
mRNA Expression
Total RNA was isolated from whole heart with TRIzol reagent and purified via columns (Quiagen RNeasy kit) and cDNA was synthesized. Real-time PCR amplification reactions were performed with iQ SYBR Green Super mix on an iQ5 cycler (BioRad). Gene expression was measured by the ΔΔCT method and was normalized to 18s ribosomal RNA levels. The data are presented as the fold expression relative to the 1 wk control group. Primer sequences: MCP-1: sense 5′-TCCACAACCACCTCAAGCACTTC-3′ and antisense 5′-GGCATCACAGTCCGAGTCACAC-3′; type I collagen: sense 5′-TGTTGGCCCATCTGGTAAAGA-3′ and antisense 5′-CAGGGAATCCGATGTTGCC-3′; type III collagen: sense 5′-TGGTCCTCAGGGTGTAAAGG-3′ and antisense 5′-GTCCAGCATCACCTTTTGGT-3′; TNF: sense 5′-CCAGTGTGGGAAGCTGTCTT-3′ and antisense 5′-AAGCAAAAGAGGAGGCAACA-3′; TGF-β1: sense 5′-CACTGGAGTTGTACGGCAGT-3′ and antisense 5′-AGAGCAGTGAGCGCTGAATC-3′; 18s RNA: sense 5′-ACCGCAGCTAGGAATAATGGA-3′ and antisense 5′-GCCTCAGTTCCGAAAACCA-3′.
Cardiovascular Parameters
Cardiac function was obtained by 2D-directed M-mode echocardiography (Vevo770; Visual Sonics) and Doppler Ultrasound (Model DSPW, Indus Instruments) as previously described before and after 6 wks of Ang-II infusion [11]. Functional data were stored and analyzed offline. Blood pressure measurements were obtained by the tail-cuff method (Visitech BP2000) before, and after 1, 2, and 6 wks of Ang-II infusion.
Statistical Analysis
All data are expressed as mean ± SEM. One-way ANOVA was used to evaluate differences between treatment groups and post-hoc testing (Tukey-Kramer Method) was performed when appropriate (Fig. 1-5, Table 1). Student's t-test was used to evaluate the differences in the % change of cardiovascular parameters (Table 1). A P-value <0.05 was considered statistically significant.
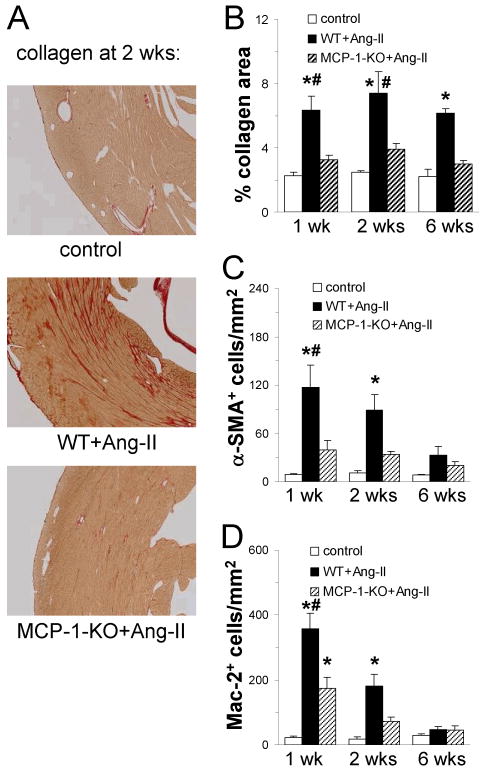
Fig. 1. Continuous Ang-II infusion results in interstitial fibrosis in the heart that is dependent on MCP-1 expression.
Tissue sections were stained for (A and B) collagen deposition (image magnification: ×100), and (C) alpha-smooth muscle actin (α-SMA) and (D) Mac-2 expressing cells. Control mice received saline (n=2 WT + n=2 MCP-1-KO; no difference between groups was observed); WT and MCP-1-KO mice (n= 5-6/group) received Ang-II for the indicated time. In contrast to WT mice, MCP-1-KO mice are protected from Ang-II infusion: interstitial collagen deposition and amount of myofibroblasts are lower than in WT mice and are comparable to control values. Deletion of MCP-1 reduces the number of macrophages, but do not completely inhibit macrophage infiltration. *P<0.05 between Ang-II-treated and control groups; #P<0.05 between Ang-II-treated WT and MCP-1-KO groups.
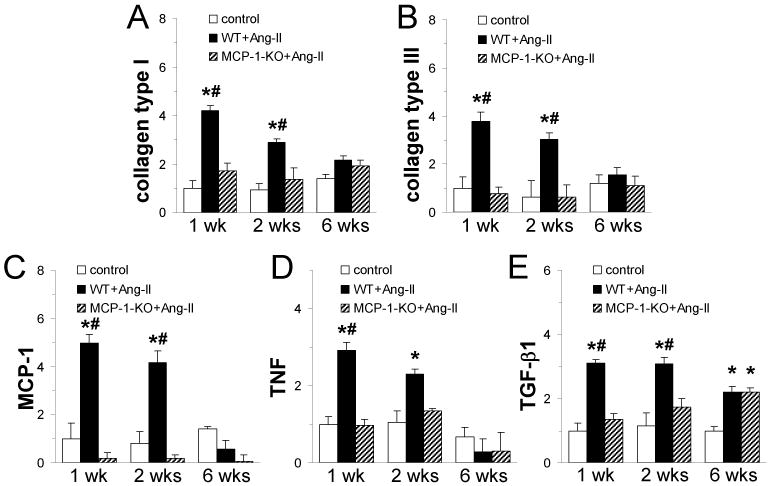
Fig. 5. Continuous Ang-II infusion induces collagen and cytokine expression in the heart that is dependent on MCP-1 expression.
Total myocardial RNA was isolated and subjected to real-time PCR using specific primers for (A) collagen type I, (B) collagen type III, (C) MCP-1, (D) TNF, and (E) TGF-β1 and SYBR Green. mRNA expression for each protein was calculated as fold induction compared to the control 1 wk group. Control mice received saline (n=3 WT + n=2 MCP-1-KO; no difference between groups was observed); WT and MCP-1-KO mice (n= 6/group) received Ang-II for the indicated time. *P<0.05 between Ang-II-treated and control groups; #P<0.05 between Ang-II-treated WT and MCP-1-KO groups.
Table 1. Cardiovascular Parameters.
The hemodynamic and anatomic responses to Ang-II infusion were not affected by deletion of MCP-1. There was no significant difference between the changes in WT and MCP-1-KO mice in the parameters measured (t-test; P< 0.05; for each individual animal, the % change was calculated with parameters measured before and after 6 wks Ang-II infusion). BW: body weight, HR: heart rate, LVEDV: left ventricular end diastolic volume; LVEDD: LV end diastolic diameter, LVAW-d: LV anterior wall thickness during diastole, LVPW-d: LV posterior wall thickness during diastole, EF: ejection fraction; E-Peak V: early transmitral filling velocity; IVRT / R-R: isovolumic relaxation time corrected by the time per heartbeat.
| WT | MCP-1-KO | |||||
|---|---|---|---|---|---|---|
| pre | 6 wks | % change | pre | 6 wks | % change | |
| Global Parameters | ||||||
| n | 13 | 13 | 13 | 14 | 14 | 14 |
| BW, g | 23.1 ± 0.3 | 27.9 ± 0.4* | 21 ± 2 | 23.8 ± 0.4 | 27.4 ± 0.5* | 15 ± 2 |
| HR, bpm | 404 ± 13 | 417 ± 13 | 4 ± 4 | 382 ± 9 | 411 ± 13 | 8 ± 3 |
| Cardiac Remodeling Parameters | ||||||
| LVEDV, ul | 78 ± 1 | 77 ± 5 | -1 ± 7 | 79 ± 3 | 68 ± 4 | -12 ± 7 |
| LVEDD, mm | 4.18 ± 0.03 | 4.13 ± 0.12 | -1 ± 3 | 4.20 ± 0.06 | 3.92 ± 0.10 | -6 ± 3 |
| LVAW-d, mm | 0.79 ± 0.04 | 1.05 ± 0.04* | 38 ± 8 | 0.90 ± 0.05 | 1.06 ± 0.05 | 22 ± 9 |
| LVPW-d, mm | 0.75 ± 0.04 | 0.87 ± 0.04 | 21 ± 8 | 0.64 ± 0.02 | 0.93 ± 0.05* | 49 ± 11 |
| Systolic Function Parameters | ||||||
| EF, % | 46 ± 2 | 55 ± 3* | 23 ± 7 | 44 ± 1 | 63 ± 3* | 45 ± 8 |
| Diastolic Function Parameters | ||||||
| E-Peak V, cm/s | 72 ± 1 | 82 ± 5 | 13 ± 6 | 66 ± 2 | 74 ± 2 | 14 ± 5 |
| IVRT / R-R, ×100 | 13.5 ± 0.4 | 13.6 ± 1.0 | 4 ± 10 | 13.4 ± 0.4 | 12.2 ± 0.7 | -9 ± 6 |
P< 0.05 versus pre group (same genetic background) (one-way ANOVA, Tukey-Kramer post hoc)
Results
Ang-II infusion resulted in interstitial fibrosis in the heart that is dependent on MCP-1 expression
Compared to saline-treated mice (control), interstitial cardiac fibrosis as determined by histological staining in WT mice was prevalent after 1 wk of Ang-II infusion, increased further after 2 wks, and was still highly prevalent after 6 wks of Ang-II exposure (Fig. 1A and B). In the Ang-II-treated WT mice, myofibroblast (interstitial α-SMA+ cells) density peaked after 1 wk (Fig. 1C), as did the Mac-2+ macrophage number (Fig. 1D). When MCP-1-KO mice were infused with Ang-II, the induction of both fibrosis and the accompanying cellular response was obviated. Interstitial collagen deposition in the left ventricle in the Ang-II infused MCP-1-KO mice was comparable to values from untreated control mice (Fig. 1A and B), as was the amount of α-SMA+ cells (Fig. 1C). The number of Mac-2+ macrophages in MCP-1-KO hearts was increased by Ang-II infusion compared to control, but was significantly lower than the amount found in Ang-II-treated WT hearts. These data indicate that Ang-II infusion induced the deposition of collagen in the heart and that MCP-1 was necessary for this induction.
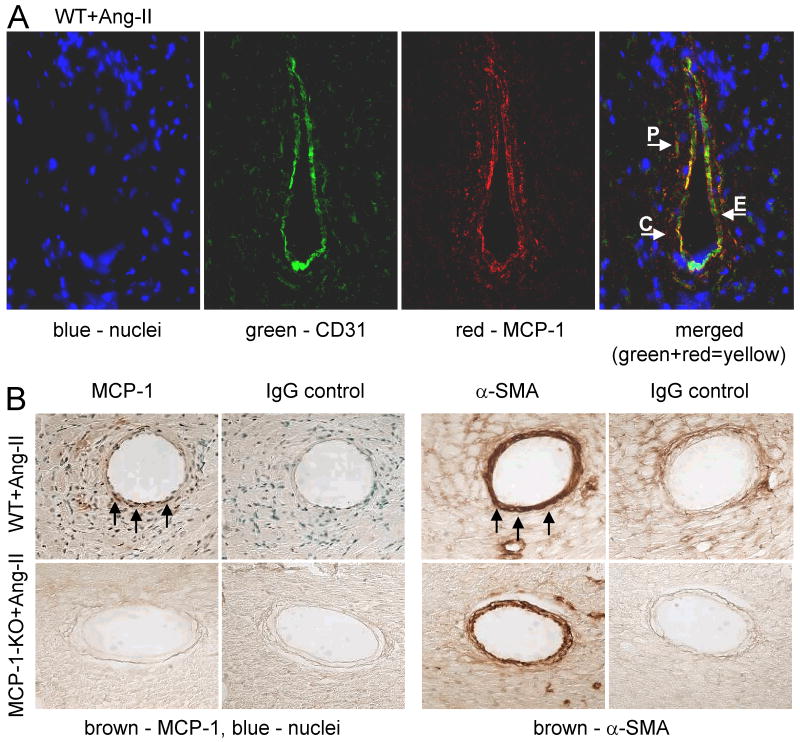
Immunochemical examination of 2 wks Ang-II-infused WT hearts indicated that MCP-1 was predominantly expressed around small vessels (Fig. 2). Specifically, MCP-1 was found largely on the surface of CD31 (Fig. 2A) expressing cells and scattered amid the media (Fig. 2A and B) within small vessels, indicating that MCP-1 was present on endothelial and smooth muscle cells. Positive MCP-1 staining was also found on the surface of CD31- cells within the immediate proximity of small vessels (Fig. 2A), however, the identity of these cells is unknown. No Mac-2+ macrophages were found in areas associated with positive MCP-1 staining. MCP-1+ cells were rarely, if at all, found interstitially. No MCP-1+ cells were detected in Ang-II-infused MCP-1-KO mice (Fig. 2B) or in saline-treated mice.
Fig. 2. MCP-1 expression is found around small vessels.
(A) 2 wks Ang-II-infused WT heart tissue was simultaneously stained for MCP-1 (red) and CD31 (green) to identify endothelial cells (the overlap of both colors in the merged image results in yellow). Blue represents DAPI-stained cell nuclei (image magnification: ×400). MCP-1 was detected on the surface of endothelial cells (arrow E) and pericytes (arrow P), as well as on CD31- cells in close proximity to the vessel (arrow C). Positive MCP-1 staining was not associated with Mac-2 (images not shown), indicating that CD31-/MCP-1+ cells were not macrophages. MCP-1 was not detected in areas distant from vessels and/or interstitially. (B) Adjacent perfusion-fixed heart tissue sections were stained for either MCP-1 or α-SMA using a peroxidase-based color (brown; arrows) development system (image magnification: ×400). Selected slides were counterstained with methyl green that identifies cell nuclei (blue). MCP-1+ cells were found within the media (α-SMA+ region) of small vessels of Ang-II-infused WT, but not MCP-1-KO mice (or saline-treated mice – not shown).
Ang-II-induced appearance of CD34+/CD45+ fibroblasts in the heart is dependent on MCP-1 expression
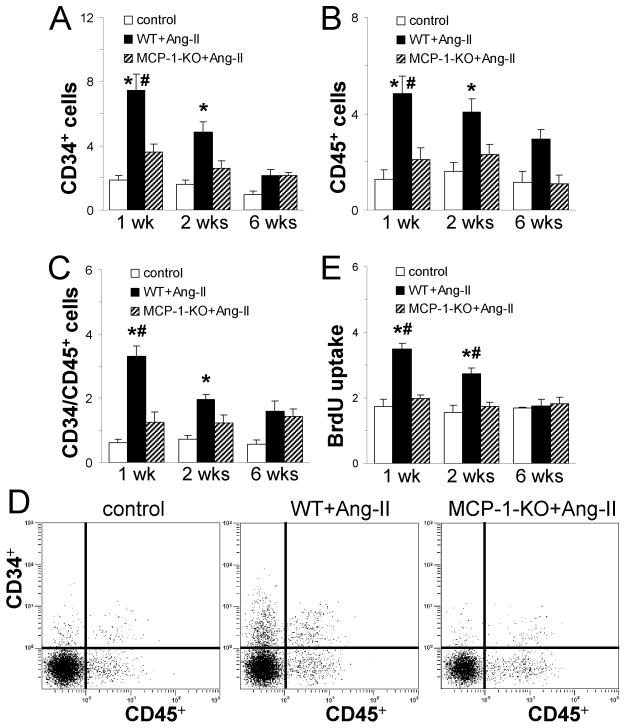
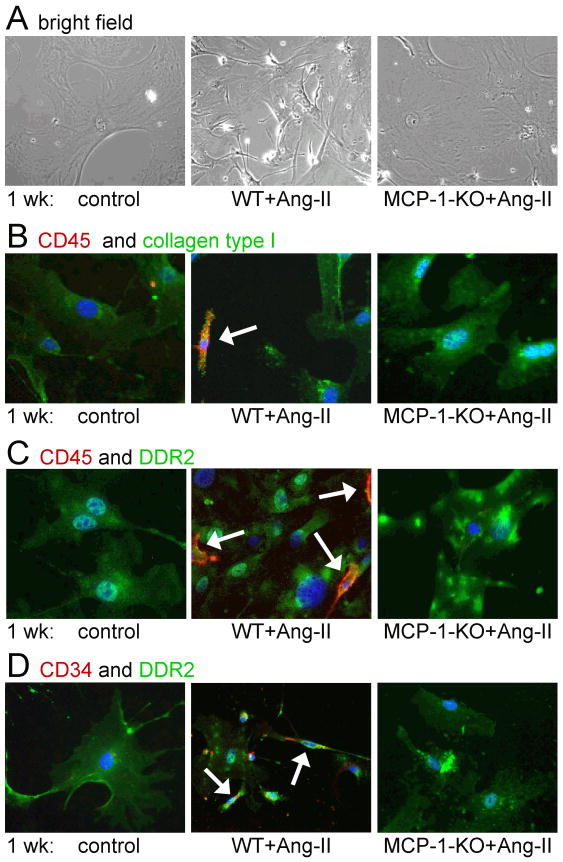
In Fig. 3, we demonstrate that Ang-II infusion resulted in a marked increase in CD34+, CD45+, and CD34+/CD45+ cells peaking at 1 wk of infusion and returning to normal levels by 6 wks of infusion as determined by flow analysis of freshly isolated cardiac non-myocyte cells (Fig. 3A-D). In animals with genetic deletion of MCP-1, CD34+/CD45+ cells were not found in the heart (Fig. 3A-D). When isolated cells from Ang-II-exposed WT hearts were cultured, they were shown to be highly proliferative as measured by uptake of BrdU compared to cells from control hearts (Fig. 3E). However, cells from Ang-II-exposed MCP-1-KO hearts proliferated comparable to cells from control hearts (Fig. 3E). Further characterization of these cells is demonstrated in Fig. 4. Cultured cells from control hearts consisted mainly of large flat cells that differed significantly from the small, spindle-shaped population obtained from Ang-II-treated WT hearts (Fig. 4A). Small, spindle-shaped cells expressed CD34, CD45, collagen type I, and the cardiac fibroblast marker DDR2 (Fig. 4B-D), indicating they were fibroblasts. Structural fibroblasts did not express CD34 and/or CD45. All cells were positive for α-SMA and collagen type I [7]. In Ang-II-treated mice with genetic deletion of MCP-1, the presence of the small, spindle-shaped cells was not observed (Fig. 4A). Taken together, these data indicate that in the absence of MCP-1, spindle-shaped CD34+/CD45+ fibroblasts were not present in the heart.
Fig. 3. Continuous Ang-II infusion induces the appearance of primitive, hematopoietic cells in the heart that is dependent on MCP-1 expression.
Hearts were removed and non-myocytes isolated. (A-C) Dispersed cells were analyzed for (A) CD34, (B) CD45, and (C) both CD34 and CD45 expression on viable (calcein+) cells by flow cytometry. (D) Representative cytometric diagrams showing the CD34+ and CD45+ distribution of all viable non-myocytes. Ang-II infusion results in the uptake of hematopoietic cells in WT mouse hearts. Deletion of MCP-1 reduces the number of CD34 and CD45 positive cells compared with WT. (E) The same cells were cultured in vitro. Ang-II infusion in WT mice results in increased cell proliferation (BrdU uptake) compared to control cultures. Cells isolated from Ang-II-treated MCP-1-KO mice proliferate at similar rates as isolations from control groups. Control mice received saline (1 and 2 wks: n=6 WT + n=2 MCP-1-KO; 6 wks: n=4 WT + n=1 MCP-1-KO; no difference between groups was observed); WT and MCP-1-KO mice (1 and 2 wks: n= 8/group; 6 wks: n=5/group) received Ang-II for the indicated time. *P<0.05 between Ang-II-treated and control groups; #P<0.05 between Ang-II-treated WT and MCP-1-KO groups.
Fig. 4. Isolated cardiac fibroblasts after Ang-II infusion are small and spindle-shaped and are not observed in MCP-1-KO hearts.
(A) Morphology of cultured cell isolations was determined by phase contrast microscopy (image magnification: ×200). After 1 wk of Ang-II infusion, cell cultures from MCP-1-KO mice show similar morphologic characteristics (large, flat) as cultures from control mice. Cultures from WT hearts also contain small and spindle-shaped cells. Cells were further stained with either (B) CD45 (red), collagen type I (green), DAPI (blue), (C) CD45 (red), DDR2 (green), DAPI (blue), and (D) CD34 (red), DDR2 (green), DAPI (blue), and were identified by fluorescence microscopy (image magnification: ×200). Only small, spindle-shaped cells (arrows) found in Ang-II-treated WT hearts stain positive for precursor cell markers CD34 and CD45, whereas all cells (small, spindle-shaped and large, flat) are positive for collagen type I and DDR2 indicating their fibroblast phenotype.
Ang-II infusion induces collagen, MCP-1, TNF and TGF-β1 mRNA expression in the heart
We examined the transcriptional activation of critical factors associated with fibrotic cardiomyopathies. As shown in Fig. 5A-E, compared with control animals, we found marked increases in collagen types I and III, as well as in MCP-1, TNF and TGF-β1 mRNA expression that were maximal after 1 wk of Ang-II infusion. Deletion of MCP-1 prevented the increased expression of collagen type I, type III, TNF and TGF-β1; the levels in MCP-1-KO mice were not different than control group levels. However, in MCP-1-KO mice, after 6 wks of Ang-II infusion, there was a significant rise in TGF-β1 mRNA expression, indicating MCP-1-independent TGF-β1 induction. These data demonstrate that Ang-II infusion induced a MCP-1-dependent increase in collagen and inflammatory cytokine mRNA in the mouse heart.
Ang-II infusion results in hypertension and cardiac hypertrophy that is independent of MCP-1
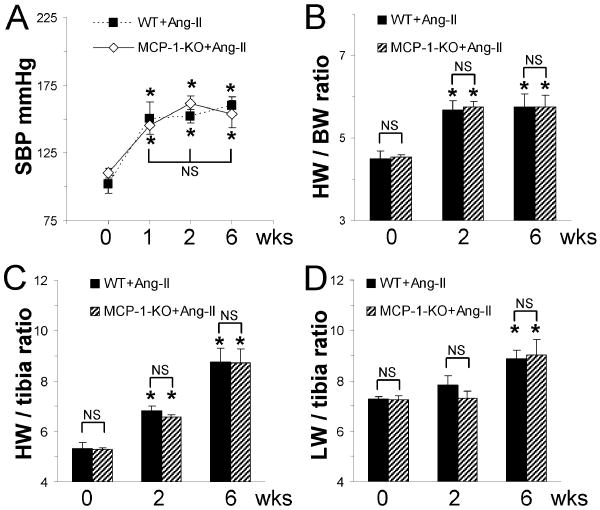
Before Ang-II infusion, WT and MCP-1-KO animals did not exhibit significant differences in blood pressure (Fig. 6A). Ang-II infusion in both WT and MCP-1-KO mice resulted in increased systolic blood pressure within 1 wk that stayed elevated until the end of experiment (6 wks) and was not different between groups at any time point measured (Fig. 6A). Thus, WT and MCP-1-KO mice developed comparable hypertension. Ang-II infusion also induced comparable cardiac hypertrophy in WT and in MCP-1-KO mice as indicated by the increased heart to body weight ratio (Fig. 6B) and heart weight to tibia length ratio (Fig. 6C) after 2 and 6 wks of infusion that were not different between groups. Both mouse groups also developed similar lung failure in response to 6 wks Ang-II infusion as indicated by the increased lung weight to tibia length ratios (Fig. 6D).
Fig. 6. Continuous Ang-II infusion results in hypertension and cardiac hypertrophy in both WT and MCP-1-KO mice.
(A) Systolic blood pressure (SBP) was determined by repeated tail cuff measurements before (0 wk) and after Ang-II infusion (n=10/group). (B) Heart weight to body weight (HW/BW) ratio, (C) heart weight to tibia length (HW/tibia) ratio, and (D) lung weigh to tibia length (LW/tibia) ratio were obtained either without (0 wk) or after the indicated Ang-II infusion time (n=4-6/group). *P<0.05 between Ang-II-treated and untreated groups; NS= not significant.
Genetic deletion of MCP-1 does not alter cardiovascular parameters after Ang-II infusion
In Table 1, we examined indices of systolic and diastolic function and adverse remodeling after 6 wks of Ang-II infusion. As might be expected, there was clear evidence of a positive inotropic effect from Ang-II infusion with significantly increased ejection fractions. While there was a trend for a higher ejection fraction in the treated MCP-1-KO mice, these were not statistically different from the WT mice. Likewise, indices of ventricular remodeling and diastolic function did not show significant differences between WT and MCP-1-KO mice; both hypertrophied similarly from the Ang-II exposure. These data indicate that MCP-1 was not necessary for the hypertrophic and functional effects of Ang-II on the mouse heart.
Discussion
The authors wish to emphasize that this report does not speak to, nor does it suggest, that the experiments presented elucidate all cellular and molecular effects of Ang-II that might be pertinent to adverse remodeling in cardiac failure and hypertrophy. The study was designed to specifically investigate the potential role of MCP-1 induction, monocyte accumulation, and the genesis of CD34+/CD45+ fibroblasts in the formation of non-adaptive cardiac fibrosis. Our data suggest that Ang-II infusion rapidly induced MCP-1 along with hypertension, cardiac hypertrophy, and interstitial fibrosis. Ang-II infusion in congenic MCP-1-KO mice produced similar amounts of hypertrophy and hypertension, but with almost no interstitial cardiac fibrosis. Thus, the expression of MCP-1 appeared to be necessary for the induction of Ang-II-mediated non-adaptive fibrosis in the myocardium. The data do not address the influence of other “downstream” factors, such as members of the extracellular matrix protein and RhoA/ROCK families, that might also be important in modulating this response [21,22].
Substantial evidence suggests a role for the inflammatory and immune systems in adverse remodeling of the hypertrophic and/or failing heart [2-4,23,24]. Previous studies from our laboratory examined the relationship between interstitial fibrosis and immunologic and inflammatory factors in a murine ischemic cardiomyopathy model (I/RC) involving daily 15 min occlusions separated by 24 h reperfusion periods. This study demonstrated, that daily episodes of ischemia were associated with an elevated macrophage infiltration (peak 5 days), fibroblast number (peak 5 days) and interstitial fibrosis (peak 7 days) [11]. This non-infarctive protocol elicits very little inflammatory responses with the exception of a striking increase in MCP-1 (peak 3 days), which remained elevated for up to 14 days, and a modest induction of TGF-β1 after 3 days [11]. In subsequent studies, we showed that genetic deletion of MCP-1 (MCP-1-KO mice) or administration of monoclonal antibodies to MCP-1 markedly reduced the monocytic and fibrotic responses; importantly, there was no evidence of a “compensatory” elevation of other chemokines [13]. As in the present study, MCP-1-dependent fibrosis in I/RC was accompanied by the appearance of distinctive small, spindle-shaped cells with primitive (CD34) and hematopoietic (CD45) markers as well as fibroblast markers (collagen type I, DDR2). Association of a hematopoietic marker with these fibroblasts and the apparent integral role of the monocyte specific chemokine MCP-1 in I/RC suggested that these small, spindle-shaped fibroblasts arose from the bone marrow monocyte population, which we confirmed by bone-marrow irradiation and rescue studies [7]. Utilizing an in vitro model, we have also demonstrated that the generation of monocyte-derived fibroblasts required monocyte transendothelial migration and was modulated by Fcγ receptors, as well as by Rho-associated kinase-1 [14,25]. These studies exposed additional mechanisms to prevent the genesis of monocyte-derived fibroblasts, all of which also inhibited the appearance of fibrosis [7,14,25].
Role of MCP-1 in adverse remodeling
The studies described above suggested a critical modulatory role for MCP-1 induction and monocyte transendothelial migration into the myocardium, thus providing a potential causal link between an inflammatory response and cardiac fibrosis. Our studies suggested that, under conditions of ischemia and reperfusion, the generation of reactive oxygen was critical to both induction of MCP-1 (“upstream”) and the development of interstitial fibrosis (“downstream”) [11,26,27]. By contrast, agonists activating the Fcγ receptors, such as serum amyloid P, inhibited interstitial fibrosis without altering MCP-1 induction [7,14]. We now wished to examine the potential importance of MCP-1 and monocyte-derived fibroblasts in a model in which multi-factorial combinations of cardiac overload, hypertrophy, failure, and a variety of systemic autocoids influence the generation of adverse remodeling and interstitial fibrosis. Thus, the present study aimed to approach this problem with a model more pertinent to cardiovascular disease. For that reason, we chose to examine the effects of Ang-II, which is a constant modulator in virtually all models of ventricular hypertrophy and failure [15-18].
Importance of MCP-1 in early cellular and inflammatory response
Our early work demonstrated a causal link between the induction of MCP-1 and reactive oxygen species (ROS) [11,26,27]. The association of high Ang-II levels with ROS and stimulation of NADPH oxidase has also been reported [28,29]. Likewise, the ability of ACE inhibitors and AT-1 receptor antagonists to block fibrosis in aging is thought to result from reduction of ROS production [30,31]. The association of Ang-II with inflammation is also well-described [19], and is confirmed in the current study. The mechanisms by which Ang-II modulates and increases inflammation may involve several cell types and effector cascades [19]. In our study, however, we noted that, in the absence of MCP-1 expression, fewer Mac-2+ macrophages appeared in the heart, and that TNF mRNA was not induced within the heart. In previous publications, we have also shown that MCP-1 alone did not induce fibroblast differentiation or collagen production, but that its mechanism was due to in its chemoattractant effects [13,14]. This would suggest that the MCP-1-driven transendothelial migration and activation of monocytes was an early critical step in Ang-II-associated inflammation. Although these observations did not belie the potential role of downstream pro-inflammatory cytokines in modulating and activating interstitial fibroblasts for extracellular matrix formation, they point to a critical early role for MCP-1 in the development of inflammation and fibrosis in response to Ang-II.
Source of MCP-1
Our present data show that following Ang-II exposure, MCP-1 was predominantly present in close proximity of small vessels; specifically on the surface of endothelial and other CD31+ cells within the media. We also found CD31- cells close to small vessels that were surrounded by MCP-1, but we were unable to identify these cells except that they were not Mac-2+ macrophages. MCP-1+ cells were not found interstitially, indicating that MCP-1 expression was localized to the immediacy of vessels. However, our immunohistochemical data is inconclusive as to which cell types produce and secrete MCP-1 in response to systemic Ang-II exposure. In previous work involving brief myocardial ischemia and reperfusion, we have localized MCP-1 production to the vascular endothelium by in situ hybridization [27]. Other groups have demonstrated that Ang-II induced MCP-1 expression in endothelium and vascular smooth muscle cells [32,33]. In a model of hypertensive nephrosclerosis, MCP-1 was shown to be localized to glomerular endothelial, epithelial and vascular smooth muscle cells which was inhibited by Ang-II type 1 receptor blockage [34]. In these studies, several intracellular signaling pathways for Ang-II-induced MCP-1 production in the endothelium have been identified, with the production of ROS and activation of NFκB among them. Therefore, in our model, production of MCP-1 by the activated endothelium is highly likely.
Time course of MCP-1
Another unusual finding of the current study was the rapid onset of MCP-1 induction and the putative cellular and molecular response to it. Perhaps even more surprising, however, was the fact that despite continued Ang-II infusion, MCP-1 induction disappeared over 6 wks, as did the cellular response, defined as a decrease in CD34+/CD45+ cells in the heart. A similar phenomenon was observed in our studies of ischemia and reperfusion [7]. We had considered that this merely represented a loss of CD34 and CD45 markers from these fibroblasts. However, there was a concurrent reduction in α-SMA+ myofibroblasts, suggesting again that the appearance of these fibroblast precursor cells in the heart was MCP-1-dependent and that the absence of MCP-1 resulted in absence of this population. Despite the disappearance of the fibroblasts, interstitial fibrosis remained in both the I/RC study and in the present study as long as Ang-II infusion continued [7,11]. While these studies have not addressed the mechanisms by which MCP-1 expression was reduced, it has been noted that TGF-β1 inhibits MCP-1 (and other chemokine) expression as part of its role in suppression of inflammation [35,36]. TGF-β1 is also critical in perpetuating ongoing fibrosis [37,38]. It is, therefore, possible that the induction of TGF-β1, observed in both the I/RC model and after Ang-II infusion, was responsible for MCP-1 suppression while supporting continued fibrosis. As discussed above, this experimental model was designed to examine the initial response to Ang-II in an otherwise normal mouse and did not define the entire role of Ang-II or hypertension in interstitial fibrosis. It did suggest, however, that MCP-1 induction plays both a critical and obligate role in the onset of interstitial fibrosis.
Hemodynamic consequences of MCP-1 deletion
In the I/RC model of non-adaptive fibrosis, reduction of non-adaptive fibrosis by several different means resulted in a significant improvement of cardiac function [7,11,13,14,25]. In the current study, we confirmed our initial hypothesis that genetic deletion of MCP-1 would reduce interstitial fibrosis that resulted from Ang-II infusion. However, the hemodynamic and anatomic responses to Ang-II infusion were not significantly affected by deletion of MCP-1 over the 6 wk period in which these experiments were examined. There was a trend toward an increased ejection fraction and decreased end diastolic volume in the MCP-1-KO at 6 wks, but these differences were not statistically significant. It is possible that a difference in adverse remodeling might be detected if the Ang-II infusion experiments were prolonged. However, it was not the purpose of our studies to examine the long term hemodynamic consequences of Ang-II infusion; the pathophysiologic effects of Ang-II infusion are complex and involve many variables. The data do suggest, however, that in the presence of a high systolic afterload and strong inotropic and hypertrophic stimulation, the effects of interstitial fibrosis on cardiac function are not readily detected.
Fibroblast precursors and their origin
While resident tissue fibroblasts have been believed to be the primary source of myofibroblasts responding to tissue injury, it is now clear that fibroblasts are also derived from a variety of precursor cell sources [2,4]. Studies proposing a role for epithelial mesenchymal transition have suggested that this mechanism utilizes processes similar to those observed in embryonic organ development under circumstances of chronic inflammation or neoplasia [39]. Another potential source of fibroblasts is mesenchymal progenitors, which are best characterized as originating in bone marrow where they occupy selective niches [40]. These multi-potent mesenchymal cells give rise to the more restricted self-renewing progenitors that gradually lose differentiation potential until a complete restriction to fibroblast lineage is reached [40,41]. Mesenchymal stem cells have also been observed to reside endogenously within a variety of organs generally associated with blood vessels and connective tissues [40].
As described in this report, another source of fibroblast precursor appeared to be of hematopoietic origin and was found in the circulating monocyte pool [8-10]. In many laboratories, these cells are designated as “fibrocytes” because they develop a distinct cell surface phenotype when cultured. These cells have been associated with fibrosis in a variety of models of wound healing and interstitial fibrosis in the lung [42-44]. In our previous in vivo studies [7,14,25], as well as in the present report, we demonstrated that marrow-derived, blood-borne fibroblasts of hematopoietic origin play an obligate role in interstitial fibrosis associated with models of ischemic cardiomyopathy and Ang-II infusion. Our studies utilizing an in vitro model of monocyte-to-fibroblast transition have demonstrated the necessity for transendothelial migration of monocytes [14]. In particular, the monocyte-to-fibroblast transition was modulated by Fcγ receptors, suggesting an important role for the immunologic system in the development of fibroblasts of hematopoietic origin [7,14]. The present study suggests that these fibroblasts play an integral role, at least, in the inception of non-adaptive interstitial cardiac fibrosis from varying pathological entities in which Ang-II is a significant autacoid. It is important to point out that we do not exclude a role for fibroblasts of other origins in adverse remodeling. Separating the role of various fibroblast precursors in long-standing, pathological, interstitial fibrosis also must consider that both temporal and regional dispersion of fibrotic stimuli within an organ occur over a chronic course and that, unlike in experimental models, chronic diseases do not arise from a uniform monotonic event such as that used in our experimental model.
In conclusion, the present data elucidate a novel role for CD34+/CD45+ fibroblast precursor cells in the development of non-adaptive fibrosis in response to Ang-II. These cells were attracted to the heart by Ang-II-induced upregulation of MCP-1, a potent chemoattractant for monocytes and macrophages. In the absence of MCP-1, this cell population did not appear in the heart and cardiac fibrosis did not develop. Inhibition of myocardial fibrosis was insufficient to reduce Ang-II-mediated cardiac remodeling and systolic dysfunction in a 6 wk experimental model.
Supplementary Material
Acknowledgments
We thank Geoffrey Bender, Taiya Williams, Thuy Pham and Jennifer Pocius for expert technical assistance.
Funding Sources: This work was supported by the NIH [R01HL076661, R01HL089792]; the Medallion Foundation; and The Methodist Hospital Foundation.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 2.Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 7.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 9.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–1350. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG, Entman ML. Chemokines in myocardial ischemia. Trends Cardiovasc Med. 2005;15:163–169. doi: 10.1016/j.tcm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 14.Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci USA. 2008;105:10179–10184. doi: 10.1073/pnas.0804910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciarretta S, Paneni F, Palano F, Chin D, Tocci G, Rubattu S, et al. Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond) 2009;116:467–477. doi: 10.1042/CS20080390. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, et al. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 17.Brunner HR. Experimental and clinical evidence that angiotensin II is an independent risk factor for cardiovascular disease. Am J Cardiol. 2001;87:3C–9C. doi: 10.1016/s0002-9149(01)01538-7. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez A, Lopez B, Querejeta R, Diez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol. 2002;34:1585–1593. doi: 10.1006/jmcc.2002.2081. [DOI] [PubMed] [Google Scholar]
- 19.Mann DL. Angiotensin II as an inflammatory mediator: evolving concepts in the role of the renin angiotensin system in the failing heart. Cardiovasc Drugs Ther. 2002;16:7–9. doi: 10.1023/a:1015355112501. [DOI] [PubMed] [Google Scholar]
- 20.Sekiguchi K, Li X, Coker M, Flesch M, Barger PM, Sivasubramanian N, et al. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc Res. 2004;63:433–442. doi: 10.1016/j.cardiores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103:319–327. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- 22.Chorianopoulos E, Heger T, Lutz M, Frank D, Bea F, Katus HA, et al. FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol. 2010;105:301–313. doi: 10.1007/s00395-009-0046-y. [DOI] [PubMed] [Google Scholar]
- 23.Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Role of inflammation in the progression of heart failure. Curr Cardiol Rep. 2007;9:236–241. doi: 10.1007/BF02938356. [DOI] [PubMed] [Google Scholar]
- 24.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95:3C–8C. doi: 10.1016/j.amjcard.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–518. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nossuli TO, Frangogiannis NG, Knuefermann P, Lakshminarayanan V, Dewald O, Evans AJ, et al. Brief murine myocardial I/R induces chemokines in a TNF-a- independent manner: role of oxygen radicals. Am J Physiol Heart Circ Physiol. 2001;281:H2549–H2558. doi: 10.1152/ajpheart.2001.281.6.H2549. [DOI] [PubMed] [Google Scholar]
- 27.Lakshminarayanan V, Lewallen M, Frangogiannis NG, Evans AJ, Wedin KE, Michael LH, et al. Reactive oxygen intermediates induce monocyte chemotactic protein-1 in vascular endothelium after brief ischemia. Am J Pathol. 2001;159:1301–1311. doi: 10.1016/S0002-9440(10)62517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 30.Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, Rosario Lores AM, et al. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Mattson MP, Maudsley S. Live longer sans the AT1A receptor. Cell Metab. 2009;9:403–405. doi: 10.1016/j.cmet.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, et al. Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-kappaB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;294:H2879–H2888. doi: 10.1152/ajpheart.91406.2007. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimoto T, Gochou N, Fukai N, Sugiyama T, Shichiri M, Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens Res. 2005;28:165–172. doi: 10.1291/hypres.28.165. [DOI] [PubMed] [Google Scholar]
- 34.Hilgers KF, Hartner A, Porst M, Mai M, Wittmann M, Hugo C, et al. Monocyte chemoattractant protein-1 and macrophage infiltration in hypertensive kidney injury. Kidney Int. 2000;58:2408–2419. doi: 10.1046/j.1523-1755.2000.00424.x. [DOI] [PubMed] [Google Scholar]
- 35.Feinberg MW, Shimizu K, Lebedeva M, Haspel R, Takayama K, Chen Z, et al. Essential role for Smad3 in regulating MCP-1 expression and vascular inflammation. Circ Res. 2004;94:601–8. doi: 10.1161/01.RES.0000119170.70818.4F. [DOI] [PubMed] [Google Scholar]
- 36.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol. 2008;103:60–68. doi: 10.1007/s00395-007-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, et al. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 39.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 40.Esposito MT, Di Noto R, Mirabelli P, Gorrese M, Parisi S, Montanaro D, et al. Culture conditions allow selection of different mesenchymal progenitors from adult mouse bone marrow. Tissue Eng Part A. 2009;15:2525–2536. doi: 10.1089/ten.tea.2008.0509. [DOI] [PubMed] [Google Scholar]
- 41.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 44.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.