Abstract
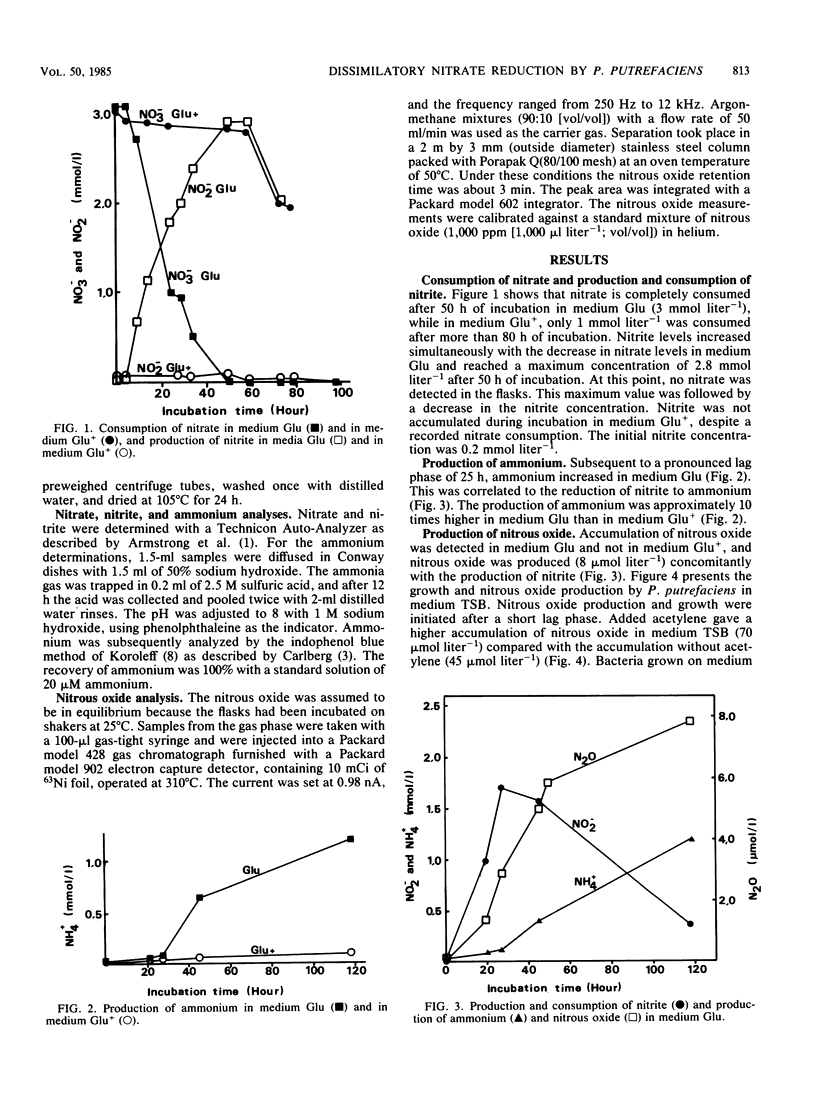
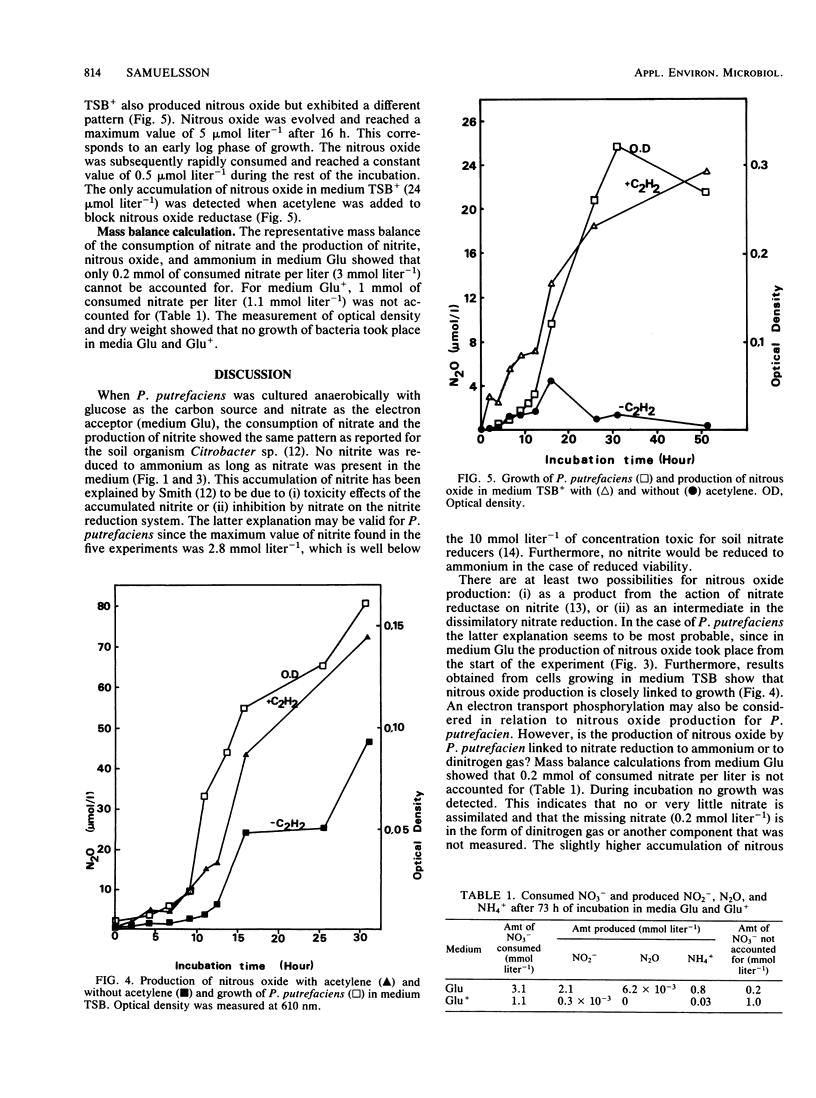
The influence of redox potential on dissimilatory nitrate reduction to ammonium was investigated on a marine bacterium, Pseudomonas putrefaciens. Nitrate was consumed (3.1 mmol liter-1), and ammonium was produced in cultures with glucose and without sodium thioglycolate. When sodium thioglycolate was added, nitrate was consumed at a lower rate (1.1 mmol liter-1), and no significant amounts of nitrite or ammonium were produced. No growth was detected in glucose media either with or without sodium thioglycolate. When grown on tryptic soy broth, the production of nitrous oxide paralleled growth. In the same medium, but with sodium thioglycolate, nitrous oxide was first produced during growth and then consumed. Acetylene caused the nitrous oxide to accumulate. These results and the mass balance calculations for different nitrogen components indicate that P. putrefaciens has the capacity to dissimilate nitrate to ammonium as well as to dinitrogen gas and nitrous oxide (denitrification). The dissimilatory pathway to ammonium dominates except when sodium thioglycolate is added to the medium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caskey W. H., Tiedje J. M. The reduction of nitrate to ammonium by a Clostridium sp. isolated from soil. J Gen Microbiol. 1980 Jul;119(1):217–223. doi: 10.1099/00221287-119-1-217. [DOI] [PubMed] [Google Scholar]
- Hasan S. M., Hall J. B. The physiological function of nitrate reduction in Clostridium perfringens. J Gen Microbiol. 1975 Mar;87(1):120–128. doi: 10.1099/00221287-87-1-120. [DOI] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol. 1981 Mar;41(3):705–709. doi: 10.1128/aem.41.3.705-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Grant M. A., Shapleigh J., Hoffman P. Nitrogen oxide reduction in Wolinella succinogenes and Campylobacter species. J Bacteriol. 1982 Nov;152(2):915–918. doi: 10.1128/jb.152.2.915-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson M. O., Rönner U. Ammonium production by dissimilatory nitrate reducers isolated from baltic sea water, as indicated by N study. Appl Environ Microbiol. 1982 Nov;44(5):1241–1243. doi: 10.1128/aem.44.5.1241-1243.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S. Dissimilatory Reduction of NO(2) to NH(4) and N(2)O by a Soil Citrobacter sp. Appl Environ Microbiol. 1982 Apr;43(4):854–860. doi: 10.1128/aem.43.4.854-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S. Nitrous oxide production by Escherichia coli is correlated with nitrate reductase activity. Appl Environ Microbiol. 1983 May;45(5):1545–1547. doi: 10.1128/aem.45.5.1545-1547.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari T., Knowles R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem Biophys Res Commun. 1976 Apr 5;69(3):705–710. doi: 10.1016/0006-291x(76)90932-3. [DOI] [PubMed] [Google Scholar]
- Yoshinari T. N2O reduction by Vibrio succinogenes. Appl Environ Microbiol. 1980 Jan;39(1):81–84. doi: 10.1128/aem.39.1.81-84.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


