Abstract
Immune reconstitution after HAART is incomplete, but no widely accepted method to quantify subclinical immune deficiency is available. We immunized 9 HIV-negative subjects and 29 HIV-infected patients with CD4 ≥450 cells/μL and undetectable HIV RNA levels with 2 doses of diphtheria/tetanus toxoid (TT) and KLH, a presumed neoantigen. We quantified the response by lymphoproliferative assay, delayed-type hypersensitivity (DTH), and antibody titers up to 59 days after enrollment. We assessed T cell proliferative capacity using anti-Vβ3 and anti-Vβ5 antibody stimulation, which we herein show induces predominant proliferation of naïve T cells. Subjects with detectable responses to KLH tended to exhibit greater proliferative responses to anti-Vβ3/Vβ5 stimulation; no such pattern was seen with response to TT. Several measures of in vitro T cell proliferative capacity correlated significantly with DTH and antibody responses to KLH, but not with TT responses; this association was independent of naïve T cell numbers. Our results indicate that naïve T cell proliferation predicts response to neo-, but not recall antigens, and suggest that it may be a meaningful reflection of in vivo immune competence in HIV-infected persons.
Keywords: Naïve T cells, HIV, HAART, immune reconstitution, T cell proliferation, neoantigens, vaccine responses
HIV infection is associated with progressive immunodeficiency, as evidenced by the occurrence of opportunistic infections and neoplasms in the late stages of HIV disease. Although the most clinically recognizable defect of advanced HIV disease is a decline in the number and percentage of CD4+ T cells, many other impairments can be demonstrated, including CD8+ T cell[1; 2], B cell[3; 4; 5; 6], natural killer cell[7; 8; 9], and dendritic cell[10; 11; 12] function derangements. Treatment with combination antiretroviral therapy suppresses viral replication and increases CD4+ T cell numbers to levels associated with a minimal risk of opportunistic complications[13], but does not fully reconstitute immune function[14; 15; 16]. Beyond decreased frequencies, CD4+ T cells in HIV disease exhibit a limited ability to proliferate in response to T cell receptor (TCR) stimulation[17; 18], a lesion that may underlie their limited ability to respond to HIV or antigens derived from other clinically significant pathogens. We have previously shown that this lesion may be partly due to a restricted ability of CD4+ T cells to progress through the cell cycle[17], despite normal expression of early activation markers.
Attempts to elucidate the clinical relevance of these defects, and to quantify the extent to which they can be corrected by antiretroviral therapy, however, have been hampered by the lack of a reproducible test of CD4+ T cell functional competence. We have previously proposed that the ability to respond to immunizations may be a physiologically relevant model of the integrity of the capacity to mount an effective immune response. Using this readout, we have found that in HIV-infected persons who have achieved a satisfactory response to HAART, immune activation and nadir CD4+ T cell counts, but not current CD4+ T cell counts, predict response to a vaccination challenge[19], suggesting that quantitative restoration of CD4+ T cells is not sufficient to ensure recovery of immune function. Similarly, dramatic increases in CD4+ T cell counts after administration of interleukin-2 are not associated with significant enhancement of responses to immunization [20].
Because the capacity of T cells to expand upon T cell receptor engagement, a process that is significantly impaired in HIV infection, is central in the ability to mount an effective response to immunization, we sought to investigate whether naïve T cell proliferation could predict in vivo responses to immunization in HIV disease. Our findings indicate that naïve T cell proliferative capacity is associated with response to immunization with neoantigen, but not recall antigens, in HIV-infected individuals who achieved sustained control of viral replication and replenishment of CD4+ T cell counts after successful antiretroviral treatment.
METHODS
Subjects, materials and methods
Between January and June, 2001, we enrolled 29 consecutive HIV-infected adult patients followed at either the John T. Carey Special Immunology Unit of University Hospitals of Cleveland or the Infectious Disease clinic at MetroHealth Medical Center in Cleveland, Ohio and 9 HIV-uninfected controls. Patients receiving stable highly active antiretroviral therapy (HAART), defined as 3 or more antiretroviral agents, who had a CD4+ T cell count of over 450 cells/μL at the time of most recent measurement and plasma HIV RNA levels consistently below 400 copies/ml during at least the previous 12 months were considered eligible. Exclusion criteria included acute febrile illnesses, history of adverse events after immunizations, history of cytokine therapy or antineoplastic chemotherapy and pregnancy or breast-feeding. The study was approved by the institutional review boards of University Hospitals of Cleveland and the MetroHealth Medical Center, and all subjects provided written informed consent in accordance with US Department of Health and Human Services guidelines.
Immunization schedule and assessment of immune response
All subjects were evaluated on days 1, 3, 17, 31 and 59, and all received a uniform panel of immunizations on days 3 and 31, consisting of 0.5 mL of diphtheria/tetanus toxoid intramuscularly as recall antigens (Aventis Pasteur, Swiftwater, PA) and 0.1 mg of keyhole limpet hemocyanin (KLH) intradermally as a neoantigen (Intracel Corp., Rockville, MD). Measurements obtained on days 1 and 3 were averaged and used as baseline.
Lymphocyte subsets were enumerated in freshly obtained whole blood using directly labeled murine monoclonal antibodies against CD3, CD4, CD8, CD28, CD45RA, CD45RO, CD62L, CD95, HLA-DR and CD38 (BD Pharmingen, San Diego, CA) by three-color flow cytometry. Naïve CD4 phenotype was defined as CD4+CD45RA+CD62L+. CD4+ and CD8+ T cells expressing both CD38 and HLA-DR were considered to be activated. Absolute lymphocyte counts were derived from complete blood counts and leukocyte differential counts.
Lymphoproliferative assays (LPA) were performed using peripheral blood mononuclear cells (PBMCs) in response to Candida albicans (Greer Laboratories, Lenoir, NC, 20 μg/ml), Cytomegalovirus (BioWhittaker, Walkersville, MD, 1:40 dilution), diphtheria toxoid (DT, Wyeth-Ayerst, Marieta, PA, 2 limiting flocculation units (LFU)/ml), HIV p24 (Protein Sciences Corporation, Meriden, CT, 3.35 μg/m), KLH (Intracel Corp., 10 μg/ml) Mycobacterium avium complex (MAC, kindly provided by R. Wallis, UMDNJ, Newark, NJ, 5 μg/ml,), tetanus toxoid (TT, Wyeth-Ayerst, 2 LFU/ml), streptokinase (SK, Sigma, St. Louis, MO, 200 μg/ml), and pokeweed mitogen (PWM, Sigma, 5 μg/ml). Controls included culture supernatants from baculovirus cell lines (Protein Sciences Corp.) for HIV p24 and uninfected control cell lysates (BioWhittaker) for CMV antigen. All experiments were performed in quadruplicate. Results are expressed as stimulation indices (SI), defined as the ratio of median counts per minute (cpm) in the presence of antigen and median background cpm of medium alone; SI values <1 were imputed a value of 1. To be classified as a responder on LPA, a subject was required to have both a post-immunization SI ≥10 and an increase of ≥0.67 log10 cpm over the baseline value.
Delayed-type hypersensitivity (DTH) responses were measured 48–72 hours after intradermal injection of Candida albicans antigen (Allermed, San Diego, CA, 1:5000/0.1 ml), TT (Aventis Pasteur, 0.08 LFU/0.1 ml), and KLH (Intracel Corp., 0.05 mg/0.1 ml). A positive DTH response was defined as a post-immunization induration that was both ≥10 mm and ≥6 mm larger than baseline. We chose this cutoff as an arbitrary but conservative value, based on previous observations suggesting that DTH responses tend to vary over time, even in the absence of a known intervention [19]. No defined cutoff has been previously shown to be consistently associated with clinical immunization responses.
Anti-TT, anti-DT and anti-KLH IgG antibodies were measured by enzyme immunoassay as previously described; responders were identified by an antibody level of >0.1 U/mL and a fourfold or greater increase from baseline.
Assessment of naïve T cell proliferation
PBMCs were obtained from peripheral blood samples by centrifugation over a Ficoll-Histopaque cushion and immediately frozen at −70°C. Frozen PBMCs were thawed and stained with 5, 6-carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR) tracking dye. Cells were incubated in RPMI 1640 supplemented with 10% fetal bovine serum (Sigma), glutamine and antibiotics. Anti-Vβ3 antibody, which we have previously characterized for its ability to induce T cell proliferation [17; 18; 21], or antiVβ5-phycoerythrin conjugated antibody (BD Pharmingen, 100 ng/ml) was used to stimulate cells. Following 4 days of incubation, CD4+Vβ3+ or CD4+Vβ5+ cells were assessed by flow cytometric analysis for tracking dye stain. Cells incubated without stimulation were used for comparison.
To determine the cell type primarily involved in anti-Vβ proliferation responses, PBMCs were depleted of CD45RA+ or CD45RO+ cells by magnetic bead separation and the resulting cells were labeled with CFSE and stimulated with anti-Vβ3 antibodies. Proliferation of CD4+Vβ3+ cells was assessed by flow cytometric analysis 4 days later. In additional studies, CD45RA+ cells were depleted from PBMCs and 2 d post stimulation with anti-Vβ3 or anti-Vβ5 antibody, CD4+CD27+ or CD8+CD27+ cells were assessed for expression of the nuclear antigen Ki67, a marker of cycling cells. These responses were compared to Ki67 induction in whole PBMC or CD45RA-positively selected cells.
Statistical analysis
We compared continuous variables by Student’s T test or a non-parametric test as required by the distribution of the data, and categorical variables by Pearson’s Chi-square or Fisher’s exact test when required. Associations between continuous variables was assessed by Spearman’s rank order correlation, and the effect of potential confounders was addressed by multiple regression analysis; extensive regression diagnostic procedures were undertaken to choose models with ideal fit to the data. Analyses were done with SPSS for Windows, version 11.0 (SPSS, Inc., Chicago IL) and Intercooled Stata, version 6.0 (Stata Corp., College Station, TX). All significance tests were two-tailed, and p-values of 0.05 or lower were considered significant.
RESULTS
Demographics and baseline characteristics of the study subjects are summarized in table 1. The HIV-uninfected controls were younger, and more often female, than the HIV-infected patients. CD4+ T cell counts, as well as absolute lymphocyte counts, were comparable between patients and controls at baseline, reflecting the extent of HAART- induced immune reconstitution achieved by these patients, but even in this population of sustained HAART responders CD4+ T cell percentages were significantly lower and CD8+ T cell counts and percentages were significantly higher among patients. Patients also had a significantly higher numbers and percentages of activated CD8+ T cells (defined as CD8+CD38+HLA-DR+), but the proportion and absolute number of activated CD4+ T cells was similar in patients and controls. Patients had lower numbers and percentages of CD4+ T cells that coexpressed CD28. All subjects, regardless of HIV status, had detectable antibody levels to DT and TT at baseline, validating the assumption that these are prevalent antigens in this population; conversely, none had detectable antibodies to the presumed neoantigen KLH. Antibody levels to both DT and TT, however, were significantly lower among patients; similarly, the magnitude of LPA responses to both of these antigens was diminished in patients compared to controls.
Table 1.
Characteristics of study subjects at baseline.
| HIV-negative controls N=9 | HIV-infected patients N=29 | pa | |
|---|---|---|---|
| Age, years | 31 (25–36) | 41 (38–46) | 0.004 |
| % male | 22.2 | 93.1 | <0.001b |
| CD4+ T cells/μL | |||
| Absolute | 811 (713–1023) | 730 (570–841) | 0.11 |
| Percent | 46 (45–50) | 33 (27–39) | 0.001 |
| CD8+ T cells/μL | |||
| Absolute | 494 (472–712) | 950 (691–1193) | 0.003 |
| Percent | 28 (22–34) | 44 (39–48) | <0.001 |
| Naïve CD4+ T cells/μL | |||
| Absolute | 456 (413–552) | 259 (218–394) | 0.003 |
| Percent | 55 (50–64) | 44 (31–54) | 0.03 |
| Activated CD4+ T cells/μL | |||
| Absolute | 24 (17–31) | 21 (15–32) | 0.88 |
| Percent | 3 (2–4) | 3 (2–5) | 0.47 |
| Activated CD8+ T cells/μL | |||
| Absolute | 21 (17–30) | 93 (60–203) | <0.001 |
| Percent | 6 (3–6) | 11 (6–16) | 0.001 |
| CD4+ CD28+ T cells/μL | |||
| Absolute | 811 (698–1013) | 625 (447–723) | 0.01 |
| Percent | 99 (96–99) | 94 (86–98) | 0.06 |
| Baseline Antibody level, U/mL | |||
| DT | 1.1 (0.6–1.5) | 0.3 (0.2–0–6) | 0.03 |
| TT | 3.5 (2.5–6.1) | 1.3 (0.5–2.4) | 0.002 |
| Baseline LPA, SI | |||
| DT | 7.9 (3.9–53.6) | 2.3 (1.3–5.1) | 0.03 |
| TT | 92.9 (51.6–207.4) | 6.4 (1.8–52.9) | 0.01 |
All data are presented as median (interquartile range), unless otherwise specified. DT, diphtheria; TT, tetanus; KLH, keyhole limpet hemocyanine; LPA, lymphoproliferative assay; SI, stimulation index.
Mann-Whitney’s U test, unless otherwise specified
Pearson’s χ2
Responses to immunization
Overall post- immunization findings and phenotypic predictors of response to immunization have been reported elsewhere[19]. Briefly, controls exhibited more robust antibody responses to DT and KLH, LPA responses to TT, DT and KLH, and DTH responses to KLH compared with the HIV-infected patients. There were no significant changes in response to the irrelevant antigens Candida albicans, MAC, SK or to PWM after immunization. Nadir CD4+ T cell count and the proportion of CD4+ T cells coexpressing CD28 were previously found to predict the magnitude of response to immunization in this population[19].
Cells that expand after Vβ3 and Vβ5 stimulation are predominantly naïve T cells
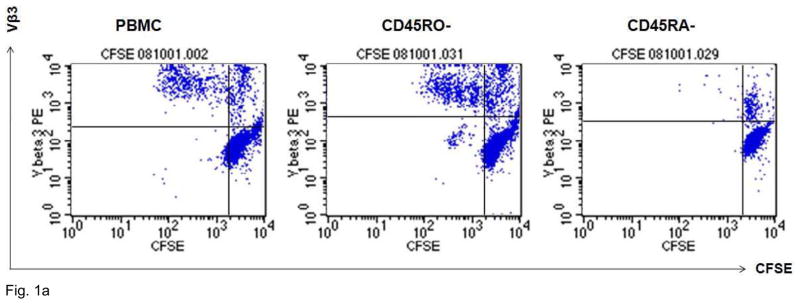
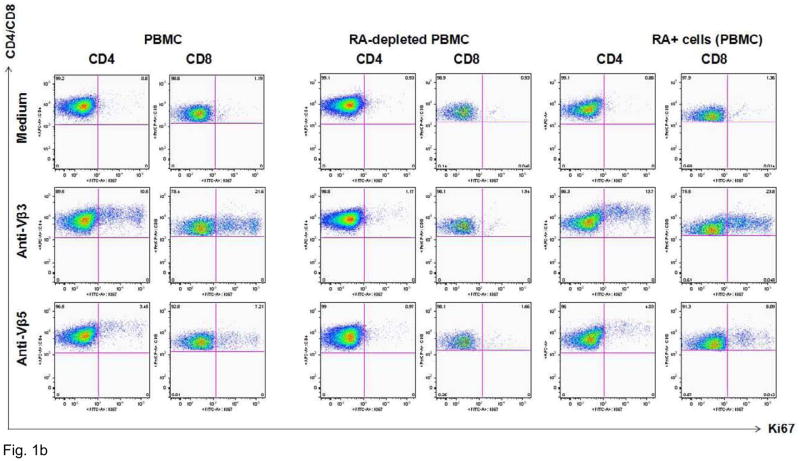
Figure 1 shows typical responses to Vβ3 and Vβ5 stimulation of CD4+ and CD8+ T cells in representative HIV-infected patients. Proliferative responses to TCR stimulation, as assessed by 4-day CFSE dye dilution assay, were observed mostly in CD45RA-depleted T cells. To confirm the naïve phenotype of the proliferating cells, we further assessed the expression of Ki67, a marker of cell cycle progression, among CD4+CD27+ and CD8+CD27+ cells 2 d after Vβ3 or Vβ5 stimulation. We compared Ki67 induction among whole PBMC, CD45RA-depleted PBMC and CD45RA-positively selected cells. Cells that were induced to express Ki67 were virtually all CD27 positive (not shown) and consistent with the requirement for naïve T cells, were absent in PBMC cultures depleted of CD45RA+ cells but recovered in CD45RA-positively selected populations. Thus, these findings suggest that proliferation responses to Vβ3 or Vβ5 antibody stimulation are largely restricted to naïve T cells.
Figure 1. Vβ3+ cells that expand after agonistic antibody stimulation have a predominantly naïve phenotype.
A. PBMCs from a healthy control donor were depleted of CD45RO+ or CD45RA+ cells by magnetic bead depletion. Remaining cells were greater than 97% depleted of the respective subsets. CD4+ cells were labeled with CFSE tracking dye and incubated with anti-Vβ3 antibodies for 4 d. CFSE concentration is on the X-axis and Vβ3 positivity is on the Y-axis. Virtually all the proliferative activity is accounted for by the CD45RO-depleted cells: CD45RA-depleted cells show virtually no proliferative response to Vβ3 stimulation. B. Entry into cell cycle in response to Vβ3 and Vβ5 stimulation, as assessed by Ki67 expression, is also largely restricted to cells with a naïve phenotype. Ki67 expression is plotted along the X-axis. CD45RO-depleted cells (two middle columns) are virtually indistinguishable from unfractionated PBMCs (two leftmost columns), showing robust cell cycle progression after 2-day Vβ3 and Vβ5 stimulation, whereas RA-depleted cells (two rightmost columns) show minimal response.
Naïve T cell proliferation is associated with response to immunizations
We hypothesized that, if a defect in the ability of T cells to expand after TCR engagement could lead to impaired cellular immune responses in HIV infection, then the proliferative capacity of naïve T cells would be predictive of the magnitude of response to neoantigens, a reflection of naïve T cell function. Since we showed that TCR stimulation through Vβ3 and Vβ5 led to proliferation of a population predominantly composed of naïve T cells, we analyzed the association of in vitro Vβ3 and Vβ5 response with the frequency and magnitude of response to TT and KLH immunization among HIV-infected patients, as an in vivo readout of cellular immune competence, to test this hypothesis.
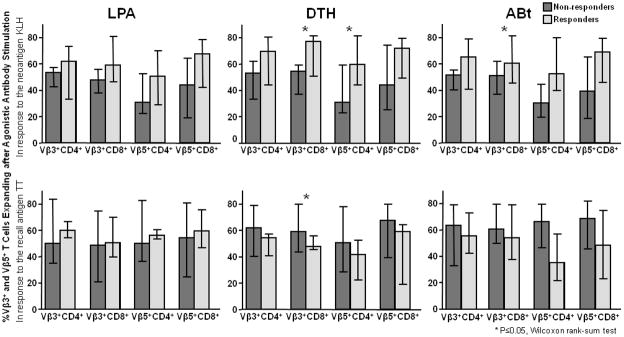
Figure 2 summarizes the magnitude of Vβ3 and Vβ5-induced T cell expansion in responders and non-responders to KLH and TT immunization. Subjects who were capable of mounting a response to KLH immunization, as assessed by LPA, DTH or antibody titers, tended to exhibit greater naïve T cell proliferation after Vβ3 and Vβ5 stimulation at baseline, across all combinations of stimulus and T cell population. These differences were statistically significant for the magnitude of CD8+ proliferation in response to Vβ3 stimulation among responders to KLH by DTH and antibody titer, as well as for CD4+ T cell response to Vβ5 stimulation among responders to KLH by DTH. Conversely, no similar pattern was discernible among responders and non-responders to TT immunization; instead, DTH non-responders to TT had a higher naïve CD8+ proliferative response to Vβ3 stimulation than responders. To further analyze the association between naïve T cell proliferation and response to immunizations, we then correlated the magnitude of Vβ3 and Vβ5-induced T cell expansion with the magnitude of LPA, DTH and antibody response to immunization with KLH or prevalent antigens. The correlation matrix for KLH and TT is shown in Table 2. There was a significant positive correlation between in vitro expansion of naïve CD4+ T cells after anti-Vβ5 stimulation and of naïve CD8+ T cells after Vβ3 stimulation with DTH response to KLH (correlation coefficients, 0.39, p=0.04 and 0.41, p=0.03, respectively), but not to tetanus toxoid. Expansion of naïve CD4+ T cells and naïve CD8+ T cells in response to Vβ3 and Vβ5 stimulation also tended to predict KLH DTH (correlation coefficient, 0.33, p=0.07 and 0.31, p=0.1, respectively), but did not reach statistical significance. There were also significant positive correlations between in vitro naïve CD4+ T cell proliferation after Vβ3 and Vβ5 stimulation with post-immunization KLH antibody titers (correlation coefficients, 0.37, p=0.04 and 0.37, p=0.03, respectively). Conversely, there was no detectable association between in vitro naïve T cell proliferation and any marker of TT immunization response, with correlation coefficients approaching zero in most cases. Because we have previously demonstrated that naïve CD4+ T cell count and proportion of CD4+ T cells that coexpress CD28 are independently associated with response to immunization in these subjects[19], we then used linear regression analysis, in which we forced those two variables to be included in the model, to determine whether the associations between naïve T cell proliferation and neoantigen response remained after accounting for the effect of these additional variables. On these multivariate analyses, the association held for KLH DTH response (regression coefficient [95% confidence interval] = 0.19 [0.002–0.39], p=0.048 for CD+ Vβ5 response, 0.2 [0.005–0.34], p=0.009 for CD8+ Vβ3 response), but did not reach statistical significance for KLH antibody titers, indicating that CD4+ T cell expansion after Vβ5 stimulation and CD8+ T cell expansion after Vβ3 stimulation predicted DTH responses to the neoantigen KLH even after accounting for the effect of naïve T cell count and CD28 coexpression on CD4+ T cells. Because we found during exploratory analyses an inverse association between age and naïve T cell proliferative capacity, and a direct association between CD4+ T cell count and T cell proliferative capacity (data not shown), we additionally fitted multivariable models including both age and CD4+ T cell count as covariates. In these analyses, the relationships described above remained statistically significant (adjusted p-values for CD+ Vβ5 = 0.018 an for CD8+ Vβ5 = 0.004), suggesting that the findings are independent of age or CD4 count as possible mediating mechanisms. Similar multivariable analyses, adding different sets of phenotypic markers, failed to demonstrate an association between in vitro naïve T cell proliferation and any readout of response to vaccination with the recall antigen TT.
Figure 2. In vitro naïve T cell proliferation is greater in HIV-infected patients who respond to immunization with neoantigen, but not recall antigen.
Boxes represent the median, and error bars represent the interquartile range, of percent Vβ3+ or Vβ5+ T cells that proliferate after TCR stimulation in responders (light boxes) or non-responders (dark boxes) to the neoantigen KLH (top row) or the recall antigen tetanus toxoid (bottom row). Responses are consistently greater in all assays among responders to KLH, but not tetanus toxoid.
Table 2. In vitro naïve T cell proliferation correlates with magnitude of response to immunization with neoantigen, but not prevalent antigen.
The table represents the correlation matrix between in vitro T cell proliferation and response to immunization; data are Spearman’s correlation coefficients (P value). Note lack of correlation with response to TT (a recall antigen), but significant correlations with DTH and antibody response to KLH (a neoantigen).
| In vitro T cell proliferation | Response to KLH immunization |
Response to TT immunization |
||||
|---|---|---|---|---|---|---|
| LPAa | DTHb | ABtc | LPAa | DTHb | ABtc | |
| Vβ3+ CD4+d | 0.167 (NS) | 0.334 (NS) | 0.373 (0.04) | 0.229 (NS) | −0.087 (NS) | −0.102 (NS) |
| Vβ5+ CD4+d | 0.236 (NS) | 0.387 (0.04) | 0.371 (0.05) | 0.243 (NS) | −0.098 (NS) | 0.064 (NS) |
| Vβ3+ CD8+d | 0.312 (NS) | 0.401 (0.03) | 0.279 (NS) | 0.195 (NS) | 0.014 (NS) | 0.275 (NS) |
| Vβ5+ CD8+d | 0.152 (NS) | 0.305 (NS) | 0.336 (NS) | 0.124 (NS) | −0.109 (NS) | 0.089 (NS) |
LPA, lymphoproliferative assay; DTH, delayed-type hypersensitivity; ABt, antibody titer.
As stimulation index
As mm of induration at maximum diameter
As U/mL
As % of Vβ3 or Vβ5+ cells that expand after stimulation with the corresponding agonistic antibody
DISCUSSION
Mounting an effective response to immunization requires intact afferent and efferent immune pathways, making evaluation of vaccine responsiveness a desirable tool to assess overall immune competence. Because immunization against clinically relevant pathogens is a routinely recommended intervention for HIV-infected persons, understanding the mechanisms that underlie response to various antigenic stimuli could help formulate strategies to maximize the effectiveness of routine preventive immune-based interventions. The latter may be particularly important in HIV disease, where viral replication, current and historical CD4 cell counts [18; 19; 21] and effectiveness of HAART therapy may all influence immune function and potentially responsiveness to immunization. Thus, there is no general consensus on an optimal time to vaccinate HIV-infected persons in order to achieve the most effective response to prophylactic or therapeutic immunization. Establishing immune correlates of vaccine protection, therefore, represents one approach to help address this issue.
Moreover, a detailed understanding of the correlates of a robust immune response in HIV infection, particularly in the setting of effective antiretroviral therapy, may help establish more meaningful goals of immune reconstitution after treatment that go beyond a target CD4+ T cell count, and could inform decisions about the timing of initiation of antiretroviral treatment. Whereas the immune defects that underlie impaired vaccine responsiveness and overall immune deficiency in HIV disease are likely to be much more pronounced in the absence of antiretroviral therapy, we focused on fully suppressed patients in this study because this population is particularly relevant in the current era of widely available potent anti-HIV therapy, at least in the developed world. Emerging data indicate that the magnitude of HIV replication, as reflected on plasma HIV RNA levels, may be inaccurate as a predictor of the rate of CD4+ T cell loss[22]. Additionally, several lines of evidence suggest that subclinical immune deficiency, even after successful HAART-induced CD4+ T cell replenishment, is associated with ongoing adverse consequences, including a modestly but measurably decreased survival[23; 24; 25] and a heightened risk of malignant diseases[26; 27; 28]. Therefore, a functional marker of immune competence that provides a more accurate reflection of immune restoration after treatment than routine CD4+ T cell quantification may be of particular interest in treated, stable patients.
Our previous studies have established several immune correlates of vaccine efficacy in HIV-infected persons. For example, we have determined that CD28 expression on CD4+ T cells and the absolute numbers of naïve CD4+ T cells predict responsiveness to neoantigens [19; 29]. Moreover, CD4 nadir is also associated with neoantigen immunization responses[19]. Since we have described a similar relationship between historical CD4 cell counts and in vitro proliferation function of T cells from HIV-infected persons [18], we hypothesized that in vitro proliferation function would also predict vaccine responsiveness. Our results confirm this relationship as we show here that in vitro baseline CD4+ and CD8+ T cell proliferation responses to TCR agonistic antibodies are directly related to antibody titers and delayed-type hypersensitivity responses that develop after administration of the neoantigen KLH. In every combination of proliferating T cell subset and immunization readout, we observed a trend towards a positive correlation between the two, and several of those associations reached statistical significance; we failed to detect a similar trend in analyses of the response to the recall antigen TT. Table 2 shows that in each case, there was a modest positive association between naïve T cell proliferation and KLH immunization response, whereas correlations approached zero in the case of tetanus toxoid immunization. The most likely explanation for this difference rests in the nature of the stimulus, as we have utilized an anti-Vβ3 or anti-Vβ5 agonistic antibody for our in vitro assays and these antibodies primarily induce expansion of naïve T cells, as demonstrated by our results. Thus, the ability of naïve T cells to expand in vitro may be related to the ability of the host to mount an immune response against neoantigens, but not recall antigens.
Since we have previously established a relationship between naïve T cell counts and vaccine responsiveness to neoantigens and since naïve T cell numbers also could conceivably influence the outcome of the in vitro proliferation assays, it was important to account for the potential influence of naïve T cell numbers on the relationships that we had observed between proliferation function and vaccine responsiveness. Unfortunately, our initial attempts to separate naïve T cells from frozen PBMC samples by magnetic bead depletion led to inconsistent purity levels. Therefore, we tested whole PBMC samples for cellular proliferation function and we relied on multivariable statistical tests to ascertain if the relationship between T cell proliferation and vaccine responsiveness was independent of naïve T cell numbers. Our results demonstrate that delayed-type hypersensitivity responses were related to CD4 and CD8+ T cell proliferation function, independent of naïve T cell numbers. The inclusion of both CD4 and CD8 cells in this relationship is not entirely surprising since we have shown previously that in vitro CD4 and CD8 T cell proliferation function is tightly correlated [18] and since CD8 cells can mediate delayed-type hypersensitivity reactions [30]. Thus, our results provide the first indication that measurements of naïve T cell function in vitro have clinical implications for vaccine responsiveness.
T cell dysfunction in HIV disease has been recognized since the earliest reports of rare individuals suffering from acquired immune deficiency syndrome[31]. Early studies indicated that poor T cell proliferation responses to anti-CD3 antibody were associated with rapid disease progression[32; 33; 34; 35]. Moreover, the loss of T cell function in vitro was defined as progressive and sequential with impairments first being observed with antigen recall responses, then with alloantigen responses and finally with mitogen-induced responses[36]. More recent observations have largely confirmed these initial findings and have refined the analyses of T cell function to examine specific T cell subsets, including memory T cells with HIV specificity[37; 38; 39; 40; 41], memory T cell subsets [42]and naïve T lymphocytes [43]. These observations provide compelling evidence that both cell numbers and function are disrupted during the course of HIV infection. The observations reported here confirm and expand on the clinical implications of these perturbations in HIV disease.
Acknowledgments
This work was supported in part by the Center for AIDS Research (CFAR, AI 36219) at Case Western Reserve University and University Hospitals of Cleveland and grant # AI 38858 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gamberg J, Pardoe I, Bowmer MI, Howley C, Grant M. Lack of CD28 expression on HIV-specific cytotoxic T lymphocytes is associated with disease progression. Immunol Cell Biol. 2004;82:38–46. doi: 10.1111/j.1440-1711.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- 2.Gulzar N, Copeland KF. CD8+ T-cells: function and response to HIV infection. Curr HIV Res. 2004;2:23–37. doi: 10.2174/1570162043485077. [DOI] [PubMed] [Google Scholar]
- 3.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 4.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sonnerborg A, Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–6. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 5.Malaspina A, Moir S, Kottilil S, Hallahan CW, Ehler LA, Liu S, Planta MA, Chun TW, Fauci AS. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J Immunol. 2003;170:5965–72. doi: 10.4049/jimmunol.170.12.5965. [DOI] [PubMed] [Google Scholar]
- 6.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–99. [PubMed] [Google Scholar]
- 7.Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6:621–9. doi: 10.2174/156652406778195035. [DOI] [PubMed] [Google Scholar]
- 8.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 9.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, Fauci AS. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–6. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 11.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 12.Quaranta MG, Mattioli B, Giordani L, Viora M. The immunoregulatory effects of HIV-1 Nef on dendritic cells and the pathogenesis of AIDS. Faseb J. 2006;20:2198–208. doi: 10.1096/fj.06-6260rev. [DOI] [PubMed] [Google Scholar]
- 13.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 14.Lederman HM, Williams PL, Wu JW, Evans TG, Cohn SE, McCutchan JA, Koletar SL, Hafner R, Connick E, Valentine FT, McElrath MJ, Roberts NJ, Jr, Currier JS. Incomplete immune reconstitution after initiation of highly active antiretroviral therapy in human immunodeficiency virus-infected patients with severe CD4+ cell depletion. J Infect Dis. 2003;188:1794–803. doi: 10.1086/379900. [DOI] [PubMed] [Google Scholar]
- 15.Lederman MM. Immune restoration and CD4+ T-cell function with antiretroviral therapies. Aids. 2001;15(Suppl 2):S11–5. doi: 10.1097/00002030-200102002-00003. [DOI] [PubMed] [Google Scholar]
- 16.Lange CG, Lederman MM. Immune reconstitution with antiretroviral therapies in chronic HIV-1 infection. J Antimicrob Chemother. 2003;51:1–4. doi: 10.1093/jac/dkg071. [DOI] [PubMed] [Google Scholar]
- 17.Sieg SF, Harding CV, Lederman MM. HIV-1 infection impairs cell cycle progression of CD4(+) T cells without affecting early activation responses. J Clin Invest. 2001;108:757–64. doi: 10.1172/JCI12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieg SF, Mitchem JB, Bazdar DA, Lederman MM. Close link between CD4+ and CD8+ T cell proliferation defects in patients with human immunodeficiency virus disease and relationship to extended periods of CD4+ lymphopenia. J Infect Dis. 2002;185:1401–16. doi: 10.1086/340509. [DOI] [PubMed] [Google Scholar]
- 19.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, Valdez H. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. Aids. 2003;17:2015–23. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 20.Valdez H, Mitsuyasu R, Landay A, Sevin AD, Chan ES, Spritzler J, Kalams SA, Pollard RB, Fahey J, Fox L, Namkung A, Estep S, Moss R, Sahner D, Lederman MM. Interleukin-2 Increases CD4+ lymphocyte numbers but does not enhance responses to immunization: results of A5046s. J Infect Dis. 2003;187:320–5. doi: 10.1086/346056. [DOI] [PubMed] [Google Scholar]
- 21.Lange CG, Valdez H, Medvik K, Asaad R, Lederman MM. CD4+ T-lymphocyte nadir and the effect of highly active antiretroviral therapy on phenotypic and functional immune restoration in HIV-1 infection. Clin Immunol. 2002;102:154–61. doi: 10.1006/clim.2001.5164. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, Whalen CC, Sieg S, Yadavalli S, Deeks SG, Lederman MM. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. Jama. 2006;296:1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 23.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, Vaeth M, Obel N. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 24.Jensen-Fangel S, Pedersen L, Pedersen C, Larsen CS, Tauris P, Moller A, Sorensen HT, Obel N. Low mortality in HIV-infected patients starting highly active antiretroviral therapy: a comparison with the general population. Aids. 2004;18:89–97. doi: 10.1097/00002030-200401020-00011. [DOI] [PubMed] [Google Scholar]
- 25.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, Pellegrin JL, Katlama C, Dabis F, Leport C. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 26.Monforte A, Abrams D, Pradier C, Weber R, Reiss P, Bonnet F, Kirk O, Law M, De Wit S, Friis-Moller N, Phillips AN, Sabin CA, Lundgren JD. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. Aids. 2008;22:2143–53. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverberg MJ, Neuhaus J, Bower M, Gey D, Hatzakis A, Henry K, Hidalgo J, Lourtau L, Neaton JD, Tambussi G, Abrams DI. Risk of cancers during interrupted antiretroviral therapy in the SMART study. Aids. 2007;21:1957–63. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 28.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 29.Valdez H, Smith KY, Landay A, Connick E, Kuritzkes DR, Kessler H, Fox L, Spritzler J, Roe J, Lederman MB, Lederman HM, Evans TG, Heath-Chiozzi M, Lederman MM. Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. ACTG 375 team. AIDS Clinical Trials Group. Aids. 2000;14:11–21. doi: 10.1097/00002030-200001070-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kalish RS, Askenase PW. Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: implications for allergies, asthma, and autoimmunity. J Allergy Clin Immunol. 1999;103:192–9. doi: 10.1016/s0091-6749(99)70489-6. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425–31. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 32.Roos MT, Miedema F, Koot M, Tersmette M, Schaasberg WP, Coutinho RA, Schellekens PT. T cell function in vitro is an independent progression marker for AIDS in human immunodeficiency virus-infected asymptomatic subjects. J Infect Dis. 1995;171:531–6. doi: 10.1093/infdis/171.3.531. [DOI] [PubMed] [Google Scholar]
- 33.Roos MT, Miedema F, Meinesz AP, De Leeuw NA, Pakker NG, Lange JM, Coutinho RA, Schellekens PT. Low T cell reactivity to combined CD3 plus CD28 stimulation is predictive for progression to AIDS: correlation with decreased CD28 expression. Clin Exp Immunol. 1996;105:409–15. doi: 10.1046/j.1365-2249.1996.d01-794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roos MT, Prins M, Koot M, de Wolf F, Bakker M, Coutinho RA, Miedema F, Schellekens PT. Low T-cell responses to CD3 plus CD28 monoclonal antibodies are predictive of development of AIDS. Aids. 1998;12:1745–51. doi: 10.1097/00002030-199814000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Schellekens PT, Roos MT, De Wolf F, Lange JM, Miedema F. Low T-cell responsiveness to activation via CD3/TCR is a prognostic marker for acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus-1 (HIV-1)-infected men. J Clin Immunol. 1990;10:121–7. doi: 10.1007/BF00918194. [DOI] [PubMed] [Google Scholar]
- 36.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boritz E, Palmer BE, Wilson CC. Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cells that proliferate in vitro detected in samples from most viremic subjects and inversely associated with plasma HIV-1 levels. J Virol. 2004;78:12638–46. doi: 10.1128/JVI.78.22.12638-12646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeil AC, Shupert WL, Iyasere CA, Hallahan CW, Mican JA, Davey RT, Jr, Connors M. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc Natl Acad Sci U S A. 2001;98:13878–83. doi: 10.1073/pnas.251539598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–25. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 41.Wilson JD, Imami N, Watkins A, Gill J, Hay P, Gazzard B, Westby M, Gotch FM. Loss of CD4+ T cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J Infect Dis. 2000;182:792–8. doi: 10.1086/315764. [DOI] [PubMed] [Google Scholar]
- 42.Elrefaei M, McElroy MD, Preas CP, Hoh R, Deeks S, Martin J, Cao H. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173:2184–9. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 43.Sieg SF, Bazdar DA, Lederman MM. Impaired TCR-mediated induction of Ki67 by naive CD4+ T cells is only occasionally corrected by exogenous IL-2 in HIV-1 infection. J Immunol. 2003;171:5208–14. doi: 10.4049/jimmunol.171.10.5208. [DOI] [PubMed] [Google Scholar]