Abstract
Rabbit does nurse their litter once every 24 h during the night. We hypothesized that corticosterone, ghrelin, leptin and metabolites like glucose, liver glycogen and free fatty acids could be affected in the pups by the time at which does nurse them. Therefore, we measured these parameters in pups nursed at 02:00 h (nighttime for the doe) to compare them with results from a previous study where does nursed at 10:00 h, during daytime. From postnatal day 7, pups were sacrificed either just before their scheduled time of nursing or at 4, 8, 12, 16 or 20 h after nursing (n = 6 at each time point); additional pups were sacrificed at 4 h intervals between 48 and 72 h after nursing to study the persistence of oscillations during fasting. All pups developed locomotor anticipatory activity to nursing. Corticosterone, ghrelin and free fatty acids exhibited a rhythm that persisted in fasted pups. Glucose concentrations were lower in fasted than in nursed pups, and glycogen was only detected in nursed subjects. Leptin values were stable and low in nursed subjects but increased significantly in fasted subjects up to 72 h after the expected nursing time. The rhythm of ghrelin persisted during fasting, contrary to our previous findings in pups nursed during daytime (i.e. outside the natural time of nursing for this species). Therefore, in 7-day-old rabbit pups, night nursing is a strong zeitgeber for corticosterone, ghrelin, free fatty acids and energy metabolites but not for leptin.
Keywords: corticosterone, ghrelin, leptin, development, circadian rhythms, food-entrainable oscillator, food-anticipatory-activity
Introduction
The rabbit pup is considered a good model for food entrainment because studies in the field, laboratory and chronobiological conditions indicate that this species nurses its pups just once a day for a brief period of 3-5 min (Broekhuizen and Mulder, 1983; Caba and González-Mariscal, 2009; González-Mariscal et al., 1994; Jilge, 1993; Zarrow et al., 1965). On postnatal day (P) 7, pups ingest up to 35% of their body weight in milk in less than 5 min (Caba et al., 2003). In addition, pups increase their locomotor behavior before daily nursing both in light/dark (Jilge, 1993) and in constant light conditions (Jilge, 1995). This anticipatory activity (AA) before the single daily nursing bout is similar to the food anticipatory activity showed by rats maintained under a schedule of restricted feeding when they increase their wheel-running activity two to three hours before mealtime (Antle and Silver, 2009; Mistlberger, 1994; Stephan, 2002).
In addition to anticipatory behavior, physiological parameters also show synchronization with food. Thus, core body temperature, corticosterone (Cort) and energy metabolites are entrained by once-daily food episode both in rats (Díaz-Muñoz et al., 2000; Honma et al., 1984; Krieger, 1974) and rabbit pups under light/dark cycle (Escobar et al., 2000; Jilge et al., 2000; Rovirosa et al., 2005). These behavioral and physiological parameters synchronized with mealtime are controlled by a putative food-entrainable oscillator (FEO; Stephan, 2002). Recently, we reported that Cort and free fatty acids (FFA) also seemed to be entrained by nursing scheduled at 10:00 h, in rabbit pups housed in constant dark conditions, but whose mothers were exposed to a 12h:12h light/dark cycle with lights on at 07:00 h. On the contrary, stomach weight and liver glycogen followed an exogenous rhythm as values increased only after nursing, but not in fasted pups (Morgado et al., 2008). In the same study, we also found that ghrelin was low at nursing and reached a peaked 12 h later, whereas this pattern was absent in fasted pups. This pattern is different from that of adult rats, sheep and humans with fixed meal times, in which ghrelin is released into the blood just before feeding (Bodosi et al., 2004; Cummins et al., 2001; Sugino et al., 2002). Considering the particular rhythm of food intake in rabbit pups, we expected to find an elevation of ghrelin before nursing. The reason for this increased release of ghrelin 12 h after nursing rather than just before nursing is not clear, but data from other species suggest that it may be related with the time of the day at which nursing occurs. Thus, in rats and humans, the physiological consequences of food ingestion are critically dependent of the time of day at which feeding takes place (Belda et al., 2005; Goel et al., 2009; Yoshihara et al., 2005). In our previous study in the rabbit, pups were scheduled to suckle during the day, at 10:00 h (Morgado et al., 2008). However, rabbits mothers nurse their pups during the night, at around 02:00 h (González-Mariscal et al., 2009; Jilge, 1991, 1993; Szeto et al., 2004). Furthermore, the behavioral activity of pups differs, depending on whether they are nursed during the mother's day (10:00 h) or night (02:00 h). In 7-day-old pups, the anticipatory locomotor activity tripled when pups were nursed at 10:00 h, whereas it only doubled in pups fed at 02:00 h (lights on at 07:00 h = ZT0; lights off at 19:00 h; Caba et al., 2008). On this basis, we hypothesized that timing of nursing at 02:00 h could affect hormonal and metabolic parameters in comparison to our previous publication where pups were nursed at 10:00 h.
In the present study, we investigated the physiological consequences of nursing during the night. We measured ghrelin and leptin as indicators of the effects of food intake in pups. Ghrelin promotes hunger and meal initiation (Bodosi et al., 2004; Cummins et al., 2001; Sugino et al., 2002), whereas leptin decreases food intake and increase energy expenditure (Mercer et al., 1997; Sugino et al., 2002). Cort was measured as an indicator of physiological synchronization (Morgado et al., 2008; Rovirosa et al., 2005). FFA were also taken into account as they are proposed to have a relevant role in food entrainment, perhaps as part of the synchronizing signal of the putative FEO (Escobar et al., 2009; Martinez-Merlos et al., 2004). Finally, glucose and liver glycogen were measured to determine the energetic balance in response to nursing.
Materials and Methods
Animals and housing conditions
New Zealand white female rabbits bred in our colony in Xalapa, México, were housed under a controlled light cycle (12 h:12 h light/dark cycle, lights on at 07:00 h) and stable temperature conditions (23± 2 °C), and were provided with rabbit pellets (Purina) and water ad libitum. These females were mated and housed individually in circadian activity-recording cages and were monitored daily from day 28 of pregnancy until delivery. Each cage had two compartments, one for the doe and one for the nest, connected by a tunnel equipped with a sliding door on each end, permitting the experimenter to control the mother's access to the tunnel and nest. When the dam crosses one of the doors the other closes automatically. In this way light cannot reach the nest compartment and pups are in continuous darkness. The mother's and nest compartments were 0.60 m wide × 0.50 m long × 0.40 m high, and the tunnel was 0.25 m wide × 0.50 m long × 0.40 m high. Before parturition, the doe had free access to the nest compartment. On day 28 of pregnancy, each doe was provided with about 100 g of straw with which she built a nest. Then, just before parturition, she covered the nest with fur from her belly, as reported elsewhere (González-Mariscal et al., 1994). On the day of parturition (P0), which always occurred in the nest compartment, the litter was adjusted to 6-7 pups and the sliding door between the tunnel and the mother's cage was locked after the mother had left the nest. Thirteen litters were studied. The pups' cage was maintained in continuous darkness, but the mother remained exposed to the 12 h:12 h light/dark cycle. The locomotor behavior of the pups was monitored in the nest with a detector sensitive to infrared radiations (λ5-14 μm) located on the ceiling of the nest box. This device is a pyroelectric sensor coupled to a Fresnel lens which detects movements of animals through the infrared radiations emitted by their body. When subjects move a signal is generated by the sensor, transduced and stored in 15-s bins and converted to 5 min bins for analysis to obtain double-plotted actograms and periodograms with the circadian recording system SPAD9 (OMNIALVA, México). As the movement of any pup could activate the sensor, the measures can be regarded as indicative of the general activity of a litter. Additionally data of 13 litters were plotted and presented as waveform of total locomotor activity.
All experimental procedures were approved by the National Institutes of Health (NIH-USA) and were conducted according to their Statement of Assurance with Standards for Humane Care and Use of Laboratory Animals.
Experimental groups
Every day at 02:00 h, starting on P1, the sliding door was opened and the doe jumped in immediately to nurse the pups. Nursing lasted always less than 5 min, after which the mother jumped out of the nest and returned to her compartment. Then, the sliding door was locked immediately and not reopened until 24 h later.
To determine basal Cort, total ghrelin, leptin concentrations and metabolites, 36 pups were sacrificed at P7 at various time points relative to nursing, starting at 02:00 h, just before their scheduled time of nursing, and then every 4 h (n = 6/time point) at 4, 8, 12, 16 and 20 h after nursing, never including more than two pups from a same litter at each time point. To determine the persistence of oscillations, 42 other pups were fasted for two consecutive nursing bouts and sacrificed 48, 52, 56, 60, 64, 68 or 72 h after the last nursing. This strategy of fasting for two consecutive nursing bouts has been used previously in rabbit pups to study persistence of hormonal and metabolic parameters (Morgado et al., 2008) and circadian oscillations in temperature (Jilge et al., 2000). It has been established that this procedure does not lead to acute hypoglycemia; in our previous study the average glucose concentrations were 120-150 mg/dl 48-72 h after the last nursing (Morgado et al., 2008), well above the threshold of clinical hypoglycemia in this species (74 mg/dl; Suckow and Douglas, 1997).
Blood and organ removal
The pups were euthanized with an overdose of sodium pentobarbital (20 mg per pup intraperitoneal (i. p.). A volume of 3 ml of blood was collected from the left ventricle of the heart with a 21-g needle attached to a syringe and then transferred to microcentrifuge tubes placed on crushed ice. All procedures from anesthesia to blood collection lasted 2.5 to 3 min. Samples were centrifuged for 10 min at 2500 rpm at 4 °C. The serum was transferred to labeled 1.5 ml tubes and stored at -20 °C for further analysis of hormones and metabolites. Immediately after blood sampling, the stomach was dissected from the cardiac to pyloric sphincter and weighed with its milk content. The liver was removed and immediately frozen at -70 °C for later determination of glycogen.
Cort, ghrelin and leptin assays
Serum Cort, ghrelin and leptin were determined by radioimmunoassay (RIA), using same commercial kits as described previously in rabbits by our laboratory and others. For Cort, the intra-assay variation was 6.4 % and the sensitivity was 0.125 μl/dl (ICN BOMEDICALS, Costa Mesa, CA; Brecchia et al., 2006; Morgado et al., 2008; Rovirosa et al., 2005; Szeto et al., 2004). For ghrelin, the intra-assay variability was 9.5% and the sensitivity was from 7.8 pg/ml (LINCO RESEARCH, St. Charles, MO; Morgado et al., 2008). For leptin, the intra-assay variation was 3.9 % and the sensitivity was from 1ng/ml (LINCO RESEARCH, St.Charles, MO; Brecchia et al., 2006; Corino et al., 2002). For each assay, the protocol was run exactly as the manufacturers recommended without serum dilution; the standards were run in triplicate, and the samples in duplicate.
Metabolic measurements
Serum aliquots were thawed and processed using spectrophotometric methods, as previously described for rabbit pups (Escobar et al., 2000; Morgado et al., 2008). Glucose was estimated from a 10 μl sample using a commercial colorimetric kit (Diasys, Germany) which is based on enzymatic glucose oxidase reactions and measured at 500 nm. FFA were estimated from 100 μl samples incubated in the presence of Co (NO3)2 and α-nitroso-β-naphthol, following the method reported by Novák (1965) and measured at 500 nm. Liver glycogen was determined from a 1 g sample of liver dissolved in hot 30% KOH, and then precipitated with 95% ethanol and hydrolyzed with HCl, following the protocol by Hassid and Abraham (1957). The concentrations of metabolites were estimated from reaction-product absorbency using a spectrophotometer (JENWAY, U.K.) set to the appropriate wavelength for each metabolite.
Data analysis
Locomotor activity was analyzed by an Enright periodogram (SPAD9). We aimed to evaluate the effect of time by comparing the values of the different physiological parameters along the experimental time points under two feeding conditions, nursing and fasting. For this purpose, we did one-way ANOVA analyses in which the dependent variables were the various hormonal and metabolic parameters while the independent grouping factor was the time at which pups were sacrificed relative to nursing. The effect of nursing was tested on the time points from 0 to 20 h (6 times of sacrifice) whereas persistence of the effects of nursing during fasting was analyzed from 48-72 h (7 times of sacrifice). In addition, a two-ways ANOVA for the main effects of feeding condition and time was carried out, excluding the time point at 72 h of the fasted condition, which had no corresponding point in the nursed condition. In order to not violate the assumptions of homogeneity of variance, in all cases, data were rank-transformed before ANOVA (Conover and Iman, 1981). Post-hoc Fisher's least significant difference (LSD) tests were used for two by two comparison of equivalent time in both conditions. Statistical analyses were performed using Sigma Stat Statistical Software version 3.5. Data were expressed as the mean ± SE and statistical significance was set at P< 0.05, and they were plotted without transformation.
Results
Locomotor activity
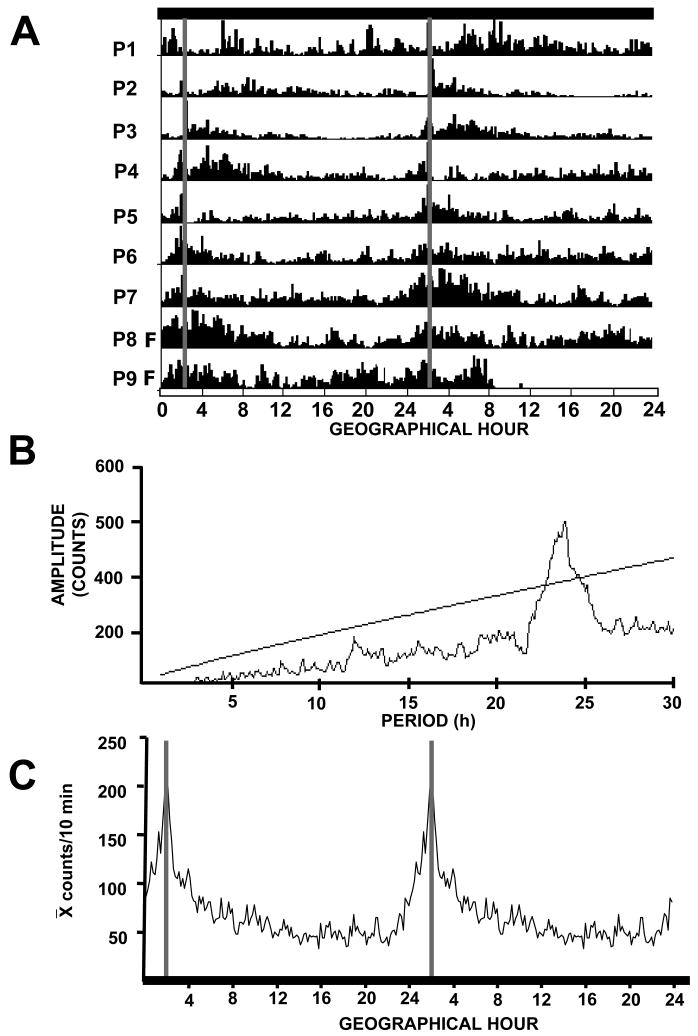
Actogram and periodogram results of locomotor activity revealed the presence of a significant increase in activity which started 2-3 h before nursing (Χ2 test, P<0.001; Fig. 1A & B). During P0-P2, the pups showed low nursing-related activity; from P3-P4 onwards, they developed an increase of locomotor activity prior to the scheduled nursing. This activity declined gradually after nursing. When the pups were left in fasting condition (P8-P9), they remained active for a longer interval after the expected time of nursing (Fig. 1A). All litters developed AA as shown in Fig. 1C.
Figure 1.
Locomotor activity of neonatal rabbits during the first 9 days of age. (A). Double plotted actogram from one representative litter (n=6 pups); nursing was scheduled to occur at 02:00 h (gray vertical line). Each horizontal line represents a postnatal day (P) of recording. The intensity of activity is indicated by the black vertical lines and represents number of times (bins) subjects perform locomotor behavior activity in 5 min. From P1-P4 synchronization to nursing develops and the anticipatory rhythm is well established at P5. At P8 and P9 the doe was not permitted to nurse (F, fasting). Black bar at top represents constant darkness for the pups. (B). Periodogram of activity indicates a circadian component (Χ2, P< 0.001). (C). Double plotted waveform from the 13 litters used. Data are mean counts per 10 min over 24 h from P5 to P7 when anticipatory activity is well established. Gray vertical line represents time of nursing.
Stomach weight
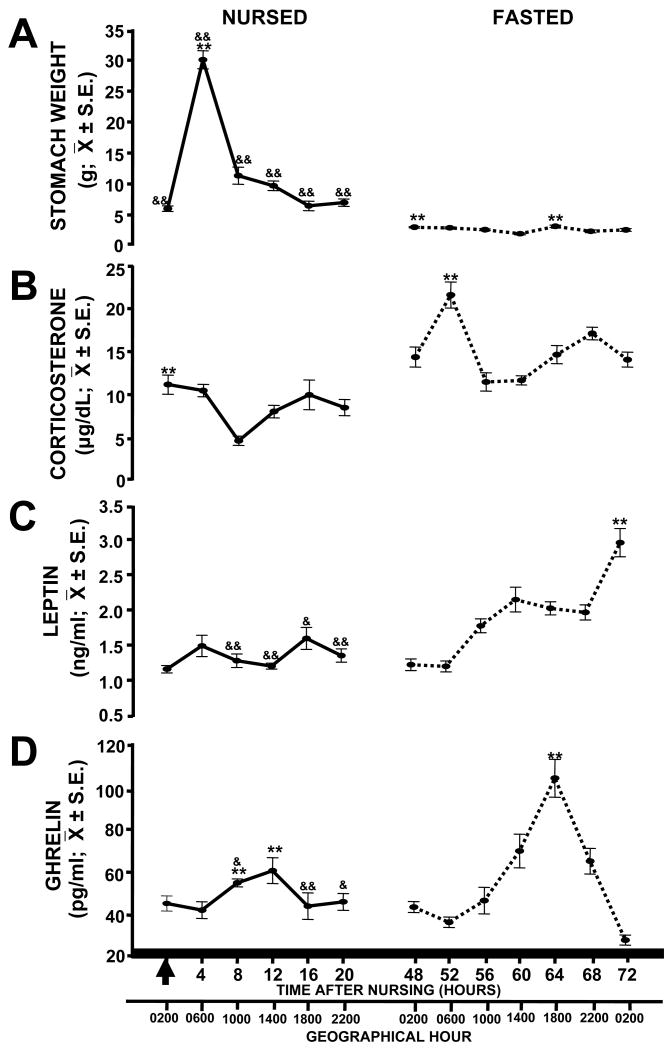
In nursed pups, stomach weight varied significantly with time (F (5, 35) =19.43, P<0.001; Fig. 2A). The highest stomach weight was observed 4 (P<0.001 against other time points) and 8 h (P=0.01) after nursing. The weight at 8 h differed significantly from the weight at 0, 16 and 20 h (P<0.001). The weight was significantly higher at 12 h than that at 0, 16 (P<0.001) and 20 h (P=0.003). In fasted pups, there was also a significant variation across time (F (6, 41) = 5.48, P<0.001); values at 48 and 64 h were higher than values at 60 (P<0.001), 68 (P<0.01), 56 and 72 h (P<0.05). In addition, the weight at 60 was lower than those at 52 (P<0.001), 56 and 72 h (P=0.02). State of feeding and time had a significant effect on stomach weight (F(1,71)=465.94; P<0.001; r2=0.90) and the time factor also contributes significantly on this parameter (F(1,71)=6.92; P<0.001). The interaction of state of feeding and time relative to nursing had an additive effect on stomach weight (F(1,71)=13.19; P<0.001).
Figure 2.
Temporal profiles of stomach weight (A), serum concentrations of corticosterone (B), leptin (C), and ghrelin (D) in nursed (P7) and fasted (P9) pups. Pups were scheduled to nurse at 02:00 h and blood samples were collected every 4 h starting at the time of nursing at P7. Fasted pups were not permitted to suck milk during two consecutive periods of scheduled nursing. Arrow represents time of nursing. Black bar at bottom represents constant darkness for the pups. Values are mean ± S.E. * represents significant difference between highest and lowest values in each group, **P<0.01. & denotes significant difference between corresponding time point of each group, &, P<0.05, && P<0.01.
Corticosterone
In nursed pups, serum values of Cort varied significantly with time (F(5,35) =5.34, P=0.001; Fig. 2B). Concentrations at 0, 4, 16 (P<0.001), 12 (P=0.022) and 20 h (P=0.009) were significantly higher than concentration at 8 hours after nursing. Additionally, the value at 0 h was significantly higher than the value at 12 h (P=0.036). In fasted pups, serum values of Cort also varied significantly with time (F(6,41) =7.31, P<0.001). The Cort value at 52 h was significantly higher than values at 56, 60 (P<0.001), 48, 72 (P<0.002) and 64 h (P=0.006). The value at 68 h was higher than those values at 56, 60 (P<0.001) and 72 h (P=0.044). In addition, the value at 64 h after nursing was higher than those at 56 and 60 h (P=0.02); the value at 48 was higher than that at 56 h (P=0.047). State of feeding and time of sacrifice within each feeding condition had a significant effect on Cort values (F(1,71)=99.95; P<0.001; r2=0.72; F(1,71)=8.78; P<0.001). There was no significant interaction between state of feeding and time factor (F(1,71)=2.01; P<0.090) on Cort concentrations.
Leptin
In nursed pups, leptin values did not vary significantly with time of sacrifice (F(5,35)=1.97, P=0.112; Fig. 2C). In fasted pups, concentration of leptin differed significantly with time of sampling (F(6,41)=22.31, P<0.001). The value at 72 hours after nursing was significantly higher than values at 48, 52, 56, 64, 68 (P<0.001) and 60 h (P=0.004). Concentrations of leptin started to increase from 56 h after nursing, being thereafter always significantly higher than at 48 and 52 h (P<0.01). The values at 60, 64 and 68 h were significantly higher than the values at 48 and 52 h (P<0.001 in all cases). In addition, the concentration at 56 h was lower than that at 60 h (P=0.018). There was a significant effect of state of feeding (F(1,71)=29.03; P<0.001; r2=0.63) and time relative to nursing (F(1,71)=8.44; P<0.001) on leptin concentrations. The interaction of state of feeding and time relative to nursing had an additive effect (F(1,71)=5.98; P<0.001) with significant difference in points at 8, 12, 20 (P<0.002) and 16 h (P=0.020).
Ghrelin
In nursed pups, ghrelin varied significantly across time (F(5,35)=3.11, P=0.022; Fig. 2D). Values at 8 and 12 h were significantly higher than values at 0 (P<0.05), 4 and 16 h (P=0.01). In fasted pups, concentrations of ghrelin varied significantly with time (F(6,41)=19.93, P< 0.001). Concentrations of ghrelin started to increase significantly at 60 hours after last nursing and reached their peak at 64 h, then decreased again to reach its lowest concentration at 72 h. Value at 64 h was significantly higher than values at 48, 52, 56, 72 (P<0.001), 60 (P=0.032) and 68 h (P=0.008). The value at 60 h was significantly higher than those values at 52, 72 (P<0.001), 48 and 56 h (P=0.003). Value at 68 h was significantly higher than at 52, 72 (P<0.001), 48 and 56 h (P=0.01). In addition, the value at 72 h was significantly lower than values at 48, 56 (P<0.001) and 52 h (P=0.035). There was not a significant effect of state of feeding on ghrelin values (F(1,71)=3.33; P=0.073; r2=0.55). However, the concentrations of this hormone differed significantly depending on the time of sacrifice (F(1,71)=7.23; P<0.001). State of feeding and time had a significant interaction (F(1,71)=6.86; P<0.001) with values significantly different at 8 (P=0.031), 16 (P<0.001) and 20 h (P=0.023).
Glucose
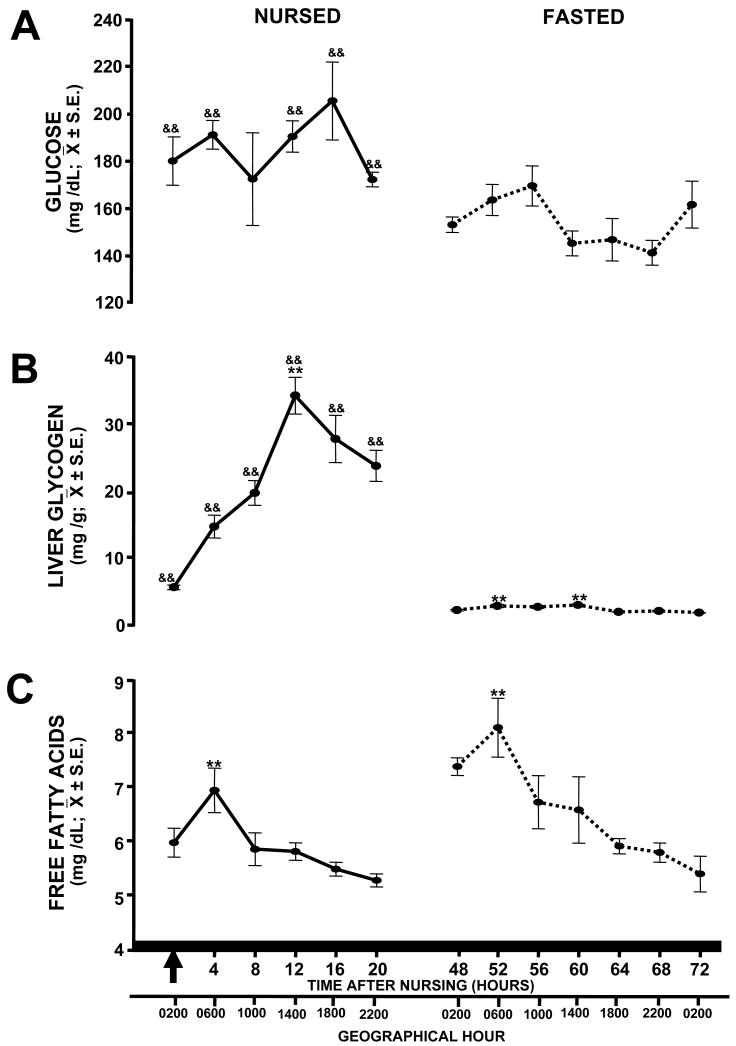
The serum concentration of glucose did not vary significantly with time neither in nursed pups (F(5,35)=1.62, P=0.185; Fig. 3A) nor in fasted pups (F(6,41)=2.18, P=0.068). The glucose values differed significantly depending on state of feeding (F(1,71)=43.04; P<0.001; r2=0.53) but not on time of sacrifice (F(1,71)=1.64; P=0.164). There was a significant interaction between state of feeding and time of sacrifice (F(1,71)=3.14; P=0.014) on glucose concentrations with values significantly different at 0, 4 (P=0.014), 12, 16 and 20 (P=0.001).
Figure 3.
Temporal profiles of serum concentration of glucose (A), liver glycogen (B) and serum free fatty acids (C) in nursed (P7) and fasted (P9) pups. Pups were scheduled to nurse at 02:00 h and blood samples were collected every 4 h starting at the time of nursing at P7. Fasted pups were not permitted to suck milk during two consecutive periods of scheduled nursing. Arrow represents time of nursing. Black bar at bottom represents constant darkness for the pups. Values are mean ± S.E. **P<0.01. & denotes significant difference between corresponding time point of each group, &, P<0.05, && P<0.01.
Liver glycogen
In nursed pups, liver glycogen varied significantly with time (F(5,35)=20.21, P<0.001; Fig. 3B). The value at 12 h was significantly higher than values at 0, 4, 8 and 20 h (P<0.001). The value at 0 h was significantly lower than values at 4 (P=0.021), 8, 16 and 20 h (P<0.001); the value at 4 h was significantly lower than values at 8 (P=0.03), 16 and 20 h (P<0.001). The value at 16 h was different than value at 8 h (P=0.032). In fasted pups, glycogen values were very low, but they still differed significantly with time (F(6,41)=10.51, P<0.001). Values at 52 and 60 h were significantly higher than those at 48 (P=0.014; P=0.005), 64, 68 and 72 h (P<0.001). The value at 56 h was higher than values at 64, 72 (P<0.001) and 68 h (P=0.006). The value at 48 was significantly different than the value at 72 h (P=0.008). State of feeding had a significant effect on liver glycogen concentrations (F(1,71)=503.07; P<0.001; r2=0.91) and the time factor also influenced significantly this parameter (F(1,71)=11.93; P<0.001). The interaction of state of feeding and time factor had an additive effect on glycogen concentration in liver (F(1,71)=11.52; P<0.001) which differed significantly between all experimental points (P<0.001).
Free fatty acids
In nursed pups, FFA concentrations varied significantly with time (F(5,35)=4.48, P=0.004; Fig. 3C). The concentration at 4 hours after last nursing was higher than concentrations at 8 (P=0.036), 16 (P=0.003) and 20 h (P<0.001). The value at 20 h was lower than those at 8 (P=0.035) and 12 h (P=0.017). In fasted pups, FFA also varied with time (F(6,41)=6.67, P<0.001). The maximum concentration occurred at 52 h, the value at this time point was significantly higher than the value at 56 (P=0.026), 60 (P=0.009), 64, 68 and 72 h (P<0.001). The value at 48 h was significantly higher than values at 60 (P=0.028), 64 (P=0.003), 68 and 72 h (P<0.001). In addition, value at 56 was higher than at 72 h (P=0.019). There was a significant effect of state of feeding on FFA values (F(1,71)=24.30; P<0.001; r2=0.56); in addition the time of sacrifice influenced significantly the values of those metabolites (F(1,71)=9.87; P<0.001). There was no significant interaction between state of feeding and time factor (F(1,71)=0.49; P=0.78) on concentrations of FFA.
Discussion
One of the main findings of the present study concerns the presence of a rhythmic pattern of ghrelin concentrations after nursing, which persists after fasting with much higher intensity in rabbit pups nursed during the night, contrary to our previous study in which pups were nursed during the day (Morgado et al., 2008). Indeed, the pattern of ghrelin was similar in both studies when pups were nursed, but markedly different after fasting. In fasted pups, persistence was only present when pups had been nursed during the night. This is the first report to our knowledge that ghrelin may present a circadian rhythm in mammalian neonates in relation with nursing and moreover depending on the time at which nursing takes place. We consider that the increased ghrelin observed both in nursed and fasted rabbit pups may be related to the next period of AA, for the following three reasons: (1) Ghrelin values increase when the stomach is empty (Bodosi et al., 2004; Cummins et al., 2001; Murakami et al., 2002). In rats, ghrelin increased during the day 6-10 h before onset of night when rats ate their main meal (Bodosi et al., 2004; Murakami et al., 2002). In our rabbits, when we compare the exogenous rhythm of the stomach with ghrelin concentrations, it is interesting to observe that the emptying of around 75% of the stomach volume coincided with a significant rise of ghrelin, both in our previous work (Morgado et al., 2008) and in the present study. (2) Ghrelin administration induces AA. In rats, intracerebroventricularly ghrelin increases spontaneous locomotor behavior (Jászberényi et al., 2006) and promotes arousal and wakefulness in rats infused either intracerebroventricularly or in the lateral hypothalamus, medial preoptic area and paraventricular nucleus (Szentirmai et al., 2006, 2007). In mice, i.p. administration of ghrelin during daytime at zeitgeber time 6, when subjects are resting, increases general locomotor activity and subsequent food intake (LeSauter et al., 2009). Additionally, systemic ghrelin induces foraging and food hoarding in Siberian hamsters (Keen-Rhinehart and Bartness, 2005). Conversely, FAA in mice with targeted mutations of the ghrelin receptor gene is significantly attenuated in comparison to wild-type mice (Blum et al., 2009; LeSauter et al., 2009). In the rabbit pup, the increase of ghrelin precedes the onset of AA in nursed pups in present results and in our previous publication (Morgado et al., 2008). (3) Ghrelin increases and AA persists in fasted subjects. A ghrelin increase during AA is present in fasted rats under a schedule of food restriction (Drazen et al., 2006). In addition, in humans, rhythmic secretion of ghrelin persists in subjects fasted at the expected time of the previous meal (Avram et al., 2005; Natalucci et al., 2005), similar to our present results in fasted rabbit pups. In all cases the stomach is empty. This increase in response to fasting has been interpreted as a learned response (Drazen et al., 2006), but also could be an evidence of a putative FEO (LeSauter et al., 2009). Collectively, our results and those of the literature indicate that ghrelin also plays a role in the appetitive component of feeding by increasing the AA activity that precedes mealtime, besides its well known role in food intake. However, this role must be modulatory as in mice with targeted mutations of the ghrelin receptor gene (Blum et al., 2009) or deficient of preproghrelin (Szentirmai et al., 2010), FAA is still present, though significantly attenuated, at the same time as their wild-type littermates.
The differences we found in behavior and physiological parameters when pups are fed during the day (Caba et al., 2008; Morgado et al., 2008) or during the night (present results) are in agreement with studies in other species. In rats, food-restricted during the day (11:00 h), absolute and relative adrenal weight significantly increased in comparison to subjects food-restricted during the night (19:00 h); moreover the normal Cort pattern in night-fed subjects is maintained, whereas in subjects fed during the light phase, this pattern is completely disrupted (Belda et al., 2005). Ghrelin was not measured in this latter study, but in humans food intake during the night results in a phase advance, instead of a phase delay, in the rhythm of ghrelin (Goel et al., 2009). In the rat, timing of maternal care and deprivation during the first postnatal week, either during the day or during the night, affects the circadian rhythms of Cort and locomotor behavior after weaning (Yoshihara et al., 2005). In rabbits, we previously demonstrated that behavioral activity of pups is different either nursed during day or night, as mentioned above (Caba et al., 2008). This evidence, together with those in other species, let us to suggest that disruption of the natural time of nursing affects behavior and physiology of pups.
One possible mechanism for the differential expression of ghrelin in fasted pups according to the timing of nursing would be through the mother's milk between day and night. It is clear that rabbit pups cannot differentiate directly between subjective day and night; nonetheless, they are indeed exposed to different day/night cues through composition of mother's milk. For instance, in the rat, milk contains melatonin during the night, but not during the day (Rowe and Kennaway, 2002) and this hormone can be transferred from milk to plasma and tissues of suckling rats, including their brain (Reppert and Klein, 1978). Moreover, in humans, 7% of the genes of the epithelial cells that produce milk-fat globules and synthesize lipids, proteins and carbohydrates for milk from the mammay gland are expressed with circadian periodicity (Maningat et al., 2009). In the rabbit, there are to our knowledge no studies of circadian variations in milk quality, but there is evidence that mothers behave differently when nursing their pup during the day or during the night. Mothers forced to nurse during the day, which is their inactive phase, show a much greater locomotor activity both at nursing time and during the entire light/dark cycle compared to does nursing during the night, which is in their natural active period (Meza et al., 2008). Further studies are warranted to explore possible circadian changes in the composition of rabbit's milk to understand the differences in physiology and behavior of rabbit pups nursed either during the mother's day (Caba et al., 2008) or during the night as in the present study.
In addition to ghrelin, other parameters, such as Cort and FFA also indicated the synchronizing role of nursing in very young pups, with some persistence. Regarding Cort, note that while Cort concentrations were much higher at 48 and 52 h in fasted pups in comparison to the equivalent time points in nursed pups, values did not remain high, but were still under circadian control, as reported in fasted adult rats (Dallman et al., 1999). The present result, in conjunction with our previous publications (Morgado et al., 2008; Rovirosa et al., 2005) also show that in addition to the elevation of Cort at the time of nursing there is another transient elevation at 18:00 h in the afternoon. Similarly in rats under a restricted feeding schedule, Cort peaks at the time of food presentation as well as in the evening with an attenuated peak (Honma et al., 1992; Moberg et al., 1975). As mentioned earlier adult rabbits are nocturnal and Cort shows an elevation in late afternoon, reaching a peak at 18:00 h (Szeto et al., 2004). It is therefore possible that this elevation at 18:00 h is related to the development of the endogenous circadian rhythm of Cort that will be established in the adult and to which pups were exposed in utero. Future experiments should explore in detail this possibility.
In the present study, FFA was rhythmic with maximum values four hours after nursing or the expected time of nursing in fasted subjects, and then decreased steadily. This differs from the pattern seen in adult rats fed ad libitum, where FFA are not rhythmic, but where a daily rhythm emerges when subjects are exposed to a restricted feeding schedule and which persists during fasting conditions (Martinez-Merlos et al., 2004). This evidence, together with present and previous results in rabbits (Morgado et al., 2008) suggests that FFA is driven by an endogenous time-keeping system. More specifically, it was proposed recently that FFA is probably part of the synchronizing signal for the FEO (Escobar et al., 2009). Thus nursing may be a potent exteroceptive synchronizer in rabbit pups as early as 7 days of age.
Other parameters did not show persistence during fasting including stomach weight and liver glycogen. During fasting, glycogen values are almost negligible, similar to those in adult rats (Escobar et al., 1998) and in neonatal rabbits feeding during daytime (Escobar et al., 2000). The cyclic pattern of liver glycogen indicates a similar homeostatic process in both the rabbit pup and the adult rat and as a consequence of the limited availability of food during a short period every day. At this age, daily nursing seems to induce a catabolic state in the pups, as had been established in adult rats under a restricted food paradigm (Báez-Ruiz et al., 2005; Escobar et al., 1998; Escobar et al., 2009; Mistlberger, 2009). Around the third week of age, food must become less important as an entraining signal, since pups start to eat solid food, their eyelids open and light is established as the prevailing entraining signal, just as in other adult mammals.
The pattern of circulating leptin was not as expected: it did not rise after feeding in nursed pups and showed a rise independent of any persistence pattern in fasted pups. Leptin is a key regulator of energy homeostasis that produces an inhibition of feeding and stimulates energy expenditure. Leptin administration decreases food intake and leptin values decrease during fasting or during restriction of food intake in adult rodents (reviewed in Ahima and Flier, 2000). Leptin concentrations in rabbit pups remain stable in nursed subjects, although this does not rule out an increase after feeding that probably was not detected due to the timing of sampling, as suggested by the small increase four hours after nursing. In contrast, values in fasted subjects steadily increased up to three fold at 72 h after last nursing. This result was surprising as we expected to detect low leptin values during fasting, as reported in adult mice after 12 h of food restriction (Ahima et al., 1998). However, in the same study lowering of leptin values were not found in fasted 8-day-old pups (Ahima et al., 1998), suggesting that leptin secretion in response to fasting differs between neonatal and adult mice. In fact, the intracerebroventricular and i.p. administration of leptin to 7-10 days old mice did not affect milk intake, contrary to what is observed at 28 days of age (Mistry et al., 1999). Moreover, leptin values are low during the first days of life, but around the beginning of the second week of age a surge of leptin has been reported both in mice and rats (Ahima et al., 1998; Devaskar et al., 1997; Proulx et al., 2001; Rayner et al., 1997). This increase is transient, e.g. in mice values start to rise at PD4, increase up to 10 fold at PD10, then sharply decrease at P16 (Ahima et al., 1998). Based on the above evidence, we consider that the increasing concentration of leptin in our rabbits at P9 is related probably to a developmental process rather than to fasting. It had been proposed that during development, increased leptin may play a role as a neurotrophic factor fundamental for organization of hypothalamic circuitry involved in the control of food intake and energy homeostasis in adults (reviewed in Bouret and Simerly, 2004). On this basis, we propose that the apparent contradictory plasma concentration in fasted 8-9 day-old subjects could indicate that the surge of this hormone is essential for neural development in the rabbit, a hypothesis that should be explored in the future. Besides this hypothesis of a developmental effect, there is another possibility related to the method of sampling which may explain our results for leptin. As sampling proceeds, less pups remains in the nest. It is well known that littermates are very important for thermoregulation of pups, which is challenged during sampling. But the number of siblings present in the nest probably did not affected significantly the data in the present study, because thermoregulation is only critical for growth and survival of pups from birth to postnatal day 5, but not for those of P6-P10, as in our study, even when they are raised alone from birth at 25°C (Bautista et al., 2003). In addition, the rise of leptin at the end of fasting is not due to acute hypoglycemia as already mentioned. We conclude therefore that leptin in the rabbit pups is not related to feeding as reported in adults, but could be related to some developmental process.
Although milk ingestion has been proposed as the main zeitgeber (Jilge et al., 2000), it is clear that pups are exposed to tactile, acoustic and pheromonal stimuli from the mother during nursing. Regarding olfactory cues, several studies had explored the role of the mammary pheromone (MP), known as 2-methyl-but-2-enal (Schaal et al., 2003). This pheromone is released from the mother's nipples together with milk (Moncomble et al., 2005), and it plays a conditioning role in the nipple-search behavior of pups during the first week of age (Hudson, 1985; Kindermann et al., 1994). Moreover, rabbit pups show prandial and circadian variation to MP (Montigny et al., 2006). In this regard it is necessary to explore in detail the role of the MP as a zeitgeber to determine its contribution to the entraining of pups from relative to that of milk ingestion, which produces an important gut distention, as well as other stimuli from the mother. Thus, the rabbit offers the opportunity to explore the impact of maternal deprivation without exposing pups to artificial long periods of maternal separation, as pups remain most of their time alone in the nest, and only need to be fed once every 24 h.
In summary, the results of this and our previous studies (Morgado et al., 2008; Rovirosa et al., 2005) suggest that Cort, stomach weight, glucose, liver glycogen and FFA are affected by nursing in a similar way, whether nursing occurs during day or nighttime, whereas ghrelin appears to be affected only when nursing occurs during nighttime. Finally and in contrast with other physiological parameters, leptin seems more related to a neural developmental process than to food intake and energy homeostasis at the age of pups used in this study.
Acknowledgments
This work was supported by National Institutes of Health/Fogarty Grants R01TW006636 (M.Caba), and TW/HD-00668 (P. Michael Conn, to partially support E. Morgado at ONPRC, Beaverton, OR), and a Grant by CONACYT, Ref. 82764 (M. Caba). We gratefully acknowledge Biol. Mercedes Acosta for her invaluable help maintaining and caring for the rabbit colony. Additionally we are grateful to SSA-SESVER, particularly to former director Jon Rementería Sempé and Dr. Oscar I. Barroso Aragón and also to Dr. Pascal Poindron for helpful comments on this manuscript and to Dr. Armando Martínez Chacón for his advice with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Neural basis of timing and anticipatory behaviors. Eur J Neurosci. 2009;30:1643–1649. doi: 10.1111/j.1460-9568.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endoc Metab. 2005;90:2982–2987. doi: 10.1210/jc.2004-1785. [DOI] [PubMed] [Google Scholar]
- Báez-Ruiz A, Escobar C, Aguilar-Roblero R, Vázquez-Martínez O, Díaz-Muñoz M. Metabolic adaptations of liver mitochondria during restricted feeding schedules. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1015–G1023. doi: 10.1152/ajpgi.00488.2004. [DOI] [PubMed] [Google Scholar]
- Bautista A, Drummond H, Martínez-Gómez M, Hudson R. Thermal benefit of sibling presence in the newborn rabbit. Dev Psychobiol. 2003;43:208–215. doi: 10.1002/dev.10134. [DOI] [PubMed] [Google Scholar]
- Belda X, Ons S, Carrasco J, Armario A. The effects of chronic food restriction on hypothalamic-pituitary-adrenal activity depend on morning versus evening availability of food. Pharmacol Biochem Behav. 2005;81(1):41–46. doi: 10.1016/j.pbb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodosi B, Gardi J, Hadju I, Szentirmai E, Obal F, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1971–1979. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinol. 2004;145:2621–2626. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- Brecchia G, Bonanno A, Galeati G, Federici C, Maranesi M, Gobbetti A, Zerani M, Boiti C. Hormonal and metabolic adaptation to fasting: Effects on the hypothalamic–pituitary–ovarian axis and reproductive performance of rabbit does. Domestic Animal Endocrinology. 2006;31:105–122. doi: 10.1016/j.domaniend.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Broekhuizen S, Mulder JL. Differences and similarities in nursing behaviour of hares and rabbits. Acta Zool Fenn. 1983;174:61–63. [Google Scholar]
- Caba M, González-Mariscal G. The Rabbit pup, a natural model of nursing-anticipatory activity. Eur J Neurosci. 2009;30:1697–1706. doi: 10.1111/j.1460-9568.2009.06964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Caba M, Tovar A, Silver R, Morgado E, Meza E, Zavaleta Y, Juárez C. Nature's food anticipatory experiment: entrainment of locomotor behavior, suprachiasmatic nucleus and dorsomedial hypothalamic nucleus by suckling in rabbit pups. Eur J Neurosci. 2008;27:432–443. doi: 10.1111/j.1460-9568.2008.06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformation as a bridge between parametric and nonparametric statistics. Am Statistician. 1981;35:132–133. [Google Scholar]
- Corino C, Mourot J, Magni S, Pastorelli G, Rosi F. Influence of dietary conjugated linoleic acid on growth, meat quality, lipogenesis, plasma leptin and physiological variables of lipid metabolism in rabbits. J Anim Sci. 2002;80:1020–1028. doi: 10.2527/2002.8041020x. [DOI] [PubMed] [Google Scholar]
- Cummins DE, Purnell JQ, Frayo RS, Schmidova K, Wiesse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Devaskar S, Ollesch C, Rajakumar R, Rajakumar P. Developmental changes in ob gene expression and circulating leptin peptide concentration. Biochem Biophys Res Commun. 1997;238:44–47. doi: 10.1006/bbrc.1997.7237. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz M, Vázquez-Martínez O, Aguilar-Roblero R, Escobar C. Anticipatory changes in liver metabolismo and entrainment of insulina, glucagon, and corticosterone in food-restricted rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2048–R2056. doi: 10.1152/ajpregu.2000.279.6.R2048. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D'Alessio DA, Seeley R, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Escobar C, Díaz-Muñoz M, Encinas F, Aguilar-Roblero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1309–R1316. doi: 10.1152/ajpregu.1998.274.5.R1309. [DOI] [PubMed] [Google Scholar]
- Escobar C, Hudson R, Martínez-Gómez M, Aguilar-Roblero R. Metabolic correlates of the circadian pattern of suckling-associated arousal in young rabbits. J Comp Physiol. [A] 2000;186:33–38. doi: 10.1007/s003590050004. [DOI] [PubMed] [Google Scholar]
- Escobar C, Cailloto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: the driving force for food-anticipatory activity. Eur J Neurosci. 2009;30:1665–1675. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- Goel N, Stunkard AJ, Rogers NL, Van Dongen HPA, Allison KC, O'Reardon JP, Ahima RX, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24(1):85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mariscal G, Lemus AC, Aguilar-Roblero R. The number of suckling pups modulates circadian periodicity of nursing rabbits. Dev Psychobiol. 2009;57:586. [Google Scholar]
- Gonzalez-Mariscal G, Díaz-Sánchez V, Melo AI, Beyer C, Rosenblatt JS. Maternal behavior in New Zealand White rabbits: quantification of somatic events, motor patterns and steroid plasma levels. Physiol Behav. 1994;55:1081–1089. doi: 10.1016/0031-9384(94)90391-3. [DOI] [PubMed] [Google Scholar]
- Hassid WZ, Abraham S. Chemical procedures for analysis of polysaccharides. In: Collowick SP, Kaplan C, editors. Methods in enzymology. III. New York: Academic; 1957. pp. 34–50. [Google Scholar]
- Honma KI, Honma S, Hiroshige T. Feeding-associated corticosterone Peak in rats under various feeding cycles. Am J Physiol Reg Integr Comp Physiol. 1984;246:R721–R726. doi: 10.1152/ajpregu.1984.246.5.R721. [DOI] [PubMed] [Google Scholar]
- Honma KI, Noe Y, Honma S, Katsuno Y, Hiroshige T. Roles of paraventricular catecholamines in feeding-associate corticosterone rhythm in rats. Am J Physiol Endocrinol Metab. 1992;25:E948–E955. doi: 10.1152/ajpendo.1992.262.6.E948. [DOI] [PubMed] [Google Scholar]
- Hudson R. Do newborn rabbits learn the odor stimuli releasing nipple-search behavior? Dev Psychobiol. 1985;18:575–585. doi: 10.1002/dev.420180612. [DOI] [PubMed] [Google Scholar]
- Jászberényi M, Bujdosó E, Bagosi Z, Telegdy G. Mediation of the behavioral, endocrine and thermoregulatory actions of ghrelin. Horm Behav. 2006;50:266–273. doi: 10.1016/j.yhbeh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jilge B. The rabbit: a diurnal or a nocturnal animal? J Exp Anim Sci. 1991;34:170–183. [PubMed] [Google Scholar]
- Jilge B. The ontogeny of circadian rhythms in the rabbit. J Biol Rhythms. 1993;8:247–260. doi: 10.1177/074873049300800307. [DOI] [PubMed] [Google Scholar]
- Jilge B. Ontogeny of the rabbit's circadian rhythms without an external zeitgeber. Physiol Behav. 1995;58:131–140. doi: 10.1016/0031-9384(95)00006-5. [DOI] [PubMed] [Google Scholar]
- Jilge B, Kuhnt B, Landers W, Rest S. Circadian thermoregulation in suckling rabbit pups. J Biol Rhythms. 2000;15:329–335. doi: 10.1177/074873000129001431. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Kindermann U, Hudson R, Distel H. Learning of suckling odors by newborn rabbits declines with age and suckling experience. Dev Psychobiol. 1994;27:111–122. doi: 10.1002/dev.420270205. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. PNAS. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maningat PD, Sen P, Rijnkels M, Sunehag AL, Hadsell DL, Bray M, Haymond MW. Gene expression in the human mammary epithelium during lactation: the milk fat globule transcriptome. Physiol Genomics. 2009;37:12–33. doi: 10.1152/physiolgenomics.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Merlos MT, Ángeles-Castellanos M, Díaz-Muñoz M, Aguilar-Roblero R, Mendoza J, Escobar C. Dissociation between adipose tissue signals, behavior and the food-entrained oscillator. J Neuroendocrinol. 2004;181:53–63. doi: 10.1677/joe.0.1810053. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Moar KM, Rayner DV, Trayhurn P, Hoggard N. Regulation of leptin receptor and NPY gene expression in hypothalamus of leptin-treated obese (ob/ob) and cold-exposed lean mice. FEBS Lett. 1997;402(2-3):185–188. doi: 10.1016/s0014-5793(96)01525-6. [DOI] [PubMed] [Google Scholar]
- Meza E, Juárez C, Morgado E, Zavaleta Y, Caba M. Brief daily suckling shifts locomotor behavior and induces PER1 protein in paraventricular and supraoptic nuclei, but not in the suprachiasmatic nucleus, of Rabbit does. Eur J Neurosci. 2008;28:1394–1403. doi: 10.1111/j.1460-9568.2008.06408.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosc Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci. 2009;30:1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol Regul Integr Comp Phiysol. 1999;277:R742–R747. doi: 10.1152/ajpregu.1999.277.3.R742. [DOI] [PubMed] [Google Scholar]
- Moberg GP, Bellinger LL, Mendel VE. Effect of meal feeding on daily rhythms of plasma corticosterone and growth hormone in the rat. Neuroendocrinology. 1975;19:160–169. doi: 10.1159/000122436. [DOI] [PubMed] [Google Scholar]
- Moncomble AS, Coureaud G, Quennedey B, Langlois D, Perrier G, Schaal B. The mammary pheromone of the rabbit: from where does it come? Anim Behav. 2005;69:29–38. [Google Scholar]
- Montigny D, Coureaud G, Schaal B. Rabbit pup response to the mammary pheromone: from automatism to prandial control. Physiol Behav. 2006;89:742–749. doi: 10.1016/j.physbeh.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Morgado E, Gordon MK, Miñana-Solís MC, Meza E, Levine S, Escobar C, Caba M. Hormonal and metabolic rhythms associated with the daily scheduled nursing in Rabbit pups. Am J Physiol Regul Integr Comp Physiol. 2008;295:R690–R695. doi: 10.1152/ajpregu.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Hayashida T, Kuroiwa T, Ankara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- Novák M. Colorimetric ultramicro method for the determination of free fatty acids. J Lipid Res. 1965;6:431–433. [PubMed] [Google Scholar]
- Proulx K, Clavel S, Nault G, Richard D, Walker DD. High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology. 2001;142:4607–4616. doi: 10.1210/endo.142.11.8512. [DOI] [PubMed] [Google Scholar]
- Rayner DV, Dalgliesh GD, Duncan JS, Hardie IJ, Hoggard N, Trayhurn P. Postnatal development of the ob gene system: elevated leptin levels in suckling fa/fa rats. Am J Physiol Regul Integr Comp Physiol. 1997;273:R446–R450. doi: 10.1152/ajpregu.1997.273.1.R446. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Klein DC. Transport of maternal [3H] melatonin to suckling rats and the fate of [3H] melatonin in the neonatal rat. Endocrinology. 1978;102:582–588. doi: 10.1210/endo-102-2-582. [DOI] [PubMed] [Google Scholar]
- Rovirosa MJ, Levine S, Gordon MK, Caba M. Circadian rhythm of corticosterone secretion in the neonatal rabbit. Dev Brain Res. 2005;158:92–96. doi: 10.1016/j.devbrainres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Rowe SA, Kennaway DJ. Melatonin in rat milk and the likehood of its role in postnatal maternal entrainment of rhythms. Am J Physiol Regul Integr Comp Physiol. 2002;282:R797–R804. doi: 10.1152/ajpregu.00228.2001. [DOI] [PubMed] [Google Scholar]
- Schaal B, Coureaud G, Langlois D, Giniés, Sémon E, Perrier G. Chemical and behavioural characterization of the rabbit MP. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Suckow MA, Douglas FA. The laboratory rabbit. Second CRC Press; 1997. [Google Scholar]
- Sugino T, Yamaura J, Yamagishi M, Ogura A, Hayashi R, Kurosa Y, Kojima M, Kangawa K, Hasegawa Y, Teraschima Y. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem Biophys Res Commun. 2002;298:785–788. doi: 10.1016/s0006-291x(02)02572-x. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–141. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. Am J Physiol Regul Integ Comp Physiol. 2010;298:R467–R77. doi: 10.1152/ajpregu.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Gonzales JA, Spitzer SB, Levine JE, Zaias PG, Saab N, Schmeiderman N, McCabe PM. Circulating levels of glucocorticoid hormones in WHHL and NZW rabbits: circadian cycle and response to repeated social encounter. Psychoneuroendocrinol. 2004;29:861–866. doi: 10.1016/S0306-4530(03)00153-7. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Otsuki Y, Yamazaki A, Honma S, Yamasaki Y, Honma KI. Maternal deprivation in neonatal rats alters the expression of circadian system under light-dark cycles and restricted daily feeding in adulthood. Physiol Behav. 2005;85:646–654. doi: 10.1016/j.physbeh.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Denenberg BH, Anderson CA. Frequency of suckling in the pup. Science. 1965;150:1835–1836. doi: 10.1126/science.150.3705.1835. [DOI] [PubMed] [Google Scholar]