Abstract
Objectives
Postoperative atrial fibrillation (POAF) is the most common complication following cardiac surgery. A variety of POAF risk factors has been reported, but study results have been inconsistent or contradictory, particularly in patients with preexisting atrial fibrillation. The incidence of POAF was evaluated in a group of 10,390 cardiac surgery patients among a comprehensive range of risk factors to identify reliable predictors of POAF.
Methods
This 20-year retrospective study examined the relationship between POAF and demographic factors, preoperative health conditions and medications, operative procedures, and postoperative complications. Multivariate logistic regression models were used to evaluate potential predictors of POAF.
Results
Increasing age, mitral valve surgery (OR=1.91), left ventricular aneurysm repair (OR=1.57), aortic valve surgery (OR=1.52), race (Caucasian) (OR=1.51), use of cardioplegia (OR=1.36), use of an intra-aortic balloon pump (OR=1.28), previous congestive heart failure (OR=1.28), and hypertension (OR=1.15) were significantly associated with POAF. The nonlinear relationship between age and POAF revealed the acceleration of POAF risk in patients 55 or older. In patients undergoing coronary artery bypass grafting, increasing age and previous congestive heart failure were the only factors associated with a higher risk of POAF. There was no trend in incidence of POAF over time. No protective factors against POAF were detected, including commonly prescribed categories of medications.
Conclusions
The persistence of the problem of POAF, and the modest predictability using common risk factors, suggest that limited progress has been made in understanding its etiology and treatment.
Introduction
Postoperative atrial fibrillation (POAF) is the most common complication following cardiac surgery. It occurs early in the recovery period after surgery and is associated with an increased incidence of mortality and other morbidities, and contributes significantly to longer hospital stays and higher cost of care.1-4 The reported incidence ranges from 11 to 50%, depending on the patient cohort evaluated.1 As the underlying pathophysiologic mechanisms responsible for POAF remain largely undetermined, the use of statistical models to identify potential predictors of its occurrence is the most practical approach to POAF prevention and evaluation of potential treatments. Various risk factors for POAF have been reported; however, study results have been inconsistent or contradictory, possibly due to small study sample sizes, limited control of confounding factors, and interference from preexisting atrial fibrillation. Only increasing age has been a consistently reported independent risk factor for POAF.2,4,5 Evaluating predictors of POAF across studies has also been difficult because the characteristics of study samples are rarely replicable, and confounding risk factors differ between studies. This may lead to biased or limited generalization to the overall cardiac surgery patient population.
In the 20-year period from 1986 to 2005, 14,960 cardiac surgical procedures were performed at Barnes-Jewish Hospital. This large patient population provided a unique opportunity for a comprehensive study of POAF. The primary objectives of this 20-year retrospective study were to evaluate trends in the incidence of POAF over time and potential predictors of POAF. Various cardiovascular medications are frequently administered to patients as prophylaxis against postoperative arrhythmias. However, the data supporting these practices have been inconsistent. This study also sought to evaluate the effects of various cardiovascular and non-cardiovascular medications on the incidence of POAF when administered preoperatively. An additional objective of the study was to evaluate the association between POAF and postoperative outcomes.
Methods
Study population
From January 1, 1986 to December 30, 2005, 14,960 cardiac surgical procedures were performed at Barnes-Jewish Hospital. From these cases, 10,390 patients without preexisting atrial arrhythmias (atrial fibrillation, atrial flutter, and paroxysmal atrial tachycardia) were selected for inclusion in this study. Appropriate IRB approval was obtained.
Patient ages ranged from 12 to 94 years (median = 64 years), with a mean of 62.3 ±12.9 years. Men comprised 65% of the study population, and over 87% of the patients were Caucasian. Approximately 79% of the procedures were elective, with the remaining being emergent (11%) or urgent (10%) operations. After surgery, all patients received continuous 24-hour telemetry with arrhythmia detection algorithms during their entire hospital stay.
Measures
Patient information, including demographics, preoperative medical history, and peri- and postoperative data were extracted from individual medical records. The onset of POAF was the dichotomous dependent variable in the analyses. POAF was diagnosed as atrial fibrillation/ flutter occurring during the postoperative recovery period before hospital discharge and requiring treatment. This did not include transient, non-sustained arrhythmias or arrhythmias treated only with oxygen or potassium supplementation. Fifty-one independent variables, or potential predictors, were organized into four groups: 1) demographics, 2) preoperative medical history, 3) preoperative medications, and 4) intraoperative procedures (type of surgery performed, and any other procedures or devices employed). A complete list of independent variables is included in Table 1. It should be noted that, although one of the aims of this study was to evaluate the efficacy of various medications as POAF prophylaxis, the reason for administering a particular medication at the time may have been POAF prophylaxis, or a different reason altogether, such as an acute or chronic condition.
Table 1. χ2 and odds ratios for univariate predictors and associates of POAF.
| Variable1 | POAF | Chi-square test | Odds ratio | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| n | (%) | n | (%) | χ2 | p | OR (95% CI) | |
| Demographics | |||||||
| Quartile age group | 766.57 | <.0001 | |||||
| 12 ∼ 54 | 2321 | (86%) | 364 | (14%) | ref | ||
| 55 ∼ 63 | 1888 | (75%) | 624 | (25%) | 2.11 (1.83,2.43) | ||
| 64 ∼ 71 | 1646 | (64%) | 945 | (36%) | 3.66 (3.19,4.19) | ||
| 72 + | 1389 | (53%) | 1213 | (47%) | 5.57 (4.86,6.37) | ||
| Gender | 0.33 | 0.5650 | |||||
| Female | 2541 | (69%) | 1122 | (31%) | |||
| Male | 4703 | (70%) | 2024 | (30%) | |||
| Race | 43.26 | <.0001 | |||||
| African-American | 921 | (78%) | 266 | (22%) | ref | ||
| Caucasian | 6205 | (69%) | 2841 | (31%) | 1.59 (1.37,1.83) | ||
| Other | 107 | (76%) | 33 | (24%) | 1.07 (0.71,1.61) | ||
| Preoperative medical history | |||||||
| Smoking history | 11.60 | 0.0007 | |||||
| No | 2292 | (67%) | 1109 | (33%) | ref | ||
| Yes | 4866 | (71%) | 2019 | (29%) | 0.86 (0.78,0.94) | ||
| Current smoker | 66.53 | <.0001 | |||||
| No | 5201 | (67%) | 2510 | (33%) | ref | ||
| Yes | 1956 | (76%) | 618 | (24%) | 0.65 (0.59,0.73) | ||
| COPD | 29.99 | <.0001 | |||||
| No | 6431 | (71%) | 2676 | (29%) | ref | ||
| Yes | 813 | (63%) | 470 | (37%) | 1.39 (1.23,1.57) | ||
| Hypertension | 35.90 | <.0001 | |||||
| No | 2750 | (73%) | 1001 | (27%) | ref | ||
| Yes | 4494 | (68%) | 2145 | (32%) | 1.31 (1.20,1.43) | ||
| Angina pectoris | 12.30 | 0.0021 | |||||
| None | 2048 | (69%) | 923 | (31%) | ref | ||
| Stable | 2357 | (72%) | 915 | (28%) | 0.86 (0.77,0.96) | ||
| Unstable | 2839 | (68%) | 1308 | (32%) | 1.02 (0.92,1.13) | ||
| Myocardial infarction | 4.29 | 0.0384 | |||||
| No | 3828 | (71%) | 1593 | (29%) | ref | ||
| Yes | 3416 | (69%) | 1553 | (31%) | 1.09 (1.00,1.19) | ||
| CHF | 94.49 | <.0001 | |||||
| No | 5365 | (73%) | 2034 | (27%) | ref | ||
| Yes | 1878 | (63%) | 1111 | (37%) | 1.56 (1.43,1.71) | ||
| NYHA score for CHF | 105.75 | <.0001 | |||||
| 0 | 3304 | (74%) | 1166 | (26%) | ref | ||
| 1 | 20 | (61%) | 13 | (39%) | 1.84 (0.91,3.72) | ||
| 2 | 440 | (62%) | 274 | (38%) | 1.77 (1.50,2.08) | ||
| 3 | 687 | (62%) | 417 | (38%) | 1.72 (1.50,1.98) | ||
| 4 | 730 | (64%) | 407 | (36%) | 1.58 (1.38,1.81) | ||
| Stroke | 5.47 | 0.0194 | |||||
| No | 6648 | (70%) | 2843 | (30%) | ref | ||
| Yes | 596 | (66%) | 303 | (31%) | 1.19 (1.03,1.37) | ||
| Peripheral vascular disease | 19.44 | <.0001 | |||||
| No | 5201 | (67%) | 2510 | (33%) | ref | ||
| Yes | 1956 | (76%) | 618 | (24%) | 1.29 (1.15,1.44) | ||
| Diabetes | 0.93 | 0.3343 | |||||
| No | 5168 | (70%) | 2215 | (30%) | |||
| Yes | 2076 | (69%) | 931 | (31%) | |||
| Renal insufficiency | 9.75 | 0.0018 | |||||
| No | 6653 | (70%) | 2834 | (30%) | ref | ||
| Yes | 576 | (65%) | 309 | (35%) | 1.26 (1.09,1.46) | ||
| Dialysis | 0.16 | 0.6854 | |||||
| No | 7112 | (70%) | 3085 | (30%) | |||
| Yes | 132 | (68%) | 61 | (32%) | |||
| Aortic valve insufficiency | 16.55 | 0.0003 | |||||
| none/mild | 5644 | (69%) | 2420 | (31%) | ref | ||
| moderate | 242 | (62%) | 151 | (38%) | 1.42 (1.15,1.75) | ||
| severe | 263 | (64%) | 151 | (36%) | 1.31 (1.06,1.60) | ||
| Mitral valve insufficiency | 32.84 | <.0001 | |||||
| none/mild | 5435 | (70%) | 2306 | (30%) | ref | ||
| moderate | 548 | (66%) | 281 | (34%) | 1.21 (1.04,1.41) | ||
| severe | 537 | (61%) | 339 | (39%) | 1.49 (1.29,1.72) | ||
| Tricuspid valve insufficiency | 1.73 | 0.4214 | |||||
| none/mild | 5824 | (69%) | 2600 | (31%) | |||
| moderate | 215 | (67%) | 107 | (33%) | |||
| severe | 92 | (73%) | 34 | (27%) | |||
| Aortic valve stenosis | 72.71 | <.0001 | |||||
| No | 6550 | (71%) | 2672 | (29%) | ref | ||
| Yes | 663 | (59%) | 467 | (41%) | 1.73 (1.52,1.96) | ||
| Mitral valve stenosis | 5.78 | 0.0162 | |||||
| No | 7120 | (70%) | 3079 | (30%) | ref | ||
| Yes | 95 | (61%) | 61 | (39%) | 1.48 (1.07,2.05) | ||
| Tricuspid valve stenosis | -- | -- | |||||
| No | 7210 | (70%) | 3141 | (30%) | |||
| Yes | 2 | (100%) | 0 | (0%) | |||
| PCI | 6.38 | 0.0116 | |||||
| No | 6184 | (71%) | 2578 | (29%) | ref | ||
| Yes | 1060 | (65%) | 568 | (35%) | 0.86 (0.76,0.97) | ||
| Previous CABG | 1.47 | 0.2258 | |||||
| No | 6705 | (70%) | 2933 | (30%) | |||
| Yes | 539 | (72%) | 213 | (28%) | |||
| Previous valve procedure | 4.90 | 0.0269 | |||||
| No | 7083 | (70%) | 3097 | (30%) | ref | ||
| Yes | 161 | (77%) | 49 | (23%) | 0.70 (0.50,0.96) | ||
| Previous LVA repair | 0.75 | 0.3859 | |||||
| No | 7232 | (70%) | 3143 | (30%) | |||
| Yes | 12 | (80%) | 3 | (20%) | |||
| Congenital defect repair | 7.34 | 0.0067 | |||||
| No | 7191 | (70%) | 3137 | (30%) | ref | ||
| Yes | 53 | (85%) | 9 | (15%) | 0.39 (0.19,0.79) | ||
| Preoperative medications | |||||||
| Antiarrhythmics | 0.79 | 0.3729 | |||||
| No | 4557 | (69%) | 2001 | (31%) | |||
| Yes | 146 | (67%) | 73 | (33%) | |||
| Beta-blockers | 1.42 | 0.2327 | |||||
| No | 3657 | (69%) | 1631 | (31%) | |||
| Yes | 3556 | (70%) | 1507 | (30%) | |||
| Ca2+-channel blocker | 0.14 | 0.7119 | |||||
| No | 4332 | (69%) | 1906 | (31%) | |||
| Yes | 435 | (70%) | 185 | (30%) | |||
| IV nitrates | 0.21 | 0.6488 | |||||
| No | 6633 | (70%) | 2876 | (30%) | |||
| Yes | 581 | (69%) | 261 | (31%) | |||
| Digitalis | 3.84 | 0.0500 | |||||
| No | 6207 | (70%) | 2624 | (30%) | ref | ||
| Yes | 557 | (67%) | 274 | (33%) | 1.16 (1.00, 1.35) | ||
| Inotropic agents | 0.22 | 0.6381 | |||||
| No | 7039 | (70%) | 3056 | (30%) | |||
| Yes | 175 | (68%) | 81 | (32%) | |||
| ACE-inhibitors | 1.36 | 0.2431 | |||||
| No | 4235 | (69%) | 1868 | (31%) | |||
| Yes | 1639 | (68%) | 768 | (32%) | |||
| Diuretics | 22.27 | < .0001 | |||||
| No | 5203 | (71%) | 2099 | (29%) | ref | ||
| Yes | 1573 | (66%) | 805 | (34%) | 1.27 (1.15, 1.40) | ||
| Aspirin/Ecotrin | 2.28 | 0.1314 | |||||
| No | 2789 | (71%) | 1164 | (29%) | |||
| Yes | 4425 | (69%) | 1974 | (31%) | |||
| Coumadin | 5.32 | 0.0211 | |||||
| No | 3214 | (68%) | 1535 | (32%) | ref | ||
| Yes | 213 | (74%) | 74 | (26%) | 0.7 (0.55, 0.95) | ||
| Anticoagulants (non-Coumadin) | 2.09 | 0.1479 | |||||
| No | 2259 | (68%) | 1058 | (32%) | |||
| Yes | 3293 | (70%) | 1437 | (30%) | |||
| Lipid lowering meds | 1.44 | 0.2301 | |||||
| No | 3238 | (67%) | 1571 | (33%) | |||
| Yes | 575 | (69%) | 253 | (31%) | |||
| Steroids | 2.42 | 0.1196 | |||||
| No | 7009 | (70%) | 3030 | (30%) | |||
| Yes | 205 | (66%) | 107 | (34%) | |||
| Intraoperative procedures | |||||||
| Clinical status | 3.07 | 0.2153 | |||||
| elective | 5695 | (70%) | 2448 | (30%) | |||
| emergent | 797 | (70%) | 343 | (30%) | |||
| urgent | 728 | (67%) | 353 | (33%) | |||
| Valve procedure | 109.28 | <.0001 | |||||
| No | 5853 | (72%) | 2251 | (28%) | ref | ||
| Yes | 1391 | (61%) | 895 | (39%) | 1.67 (1.52,1.84) | ||
| Aortic valve procedure | 80.27 | <.0001 | |||||
| No | 6366 | (71%) | 2555 | (29%) | ref | ||
| Yes | 878 | (60%) | 591 | (40%) | 1.68 (1.50,1.88) | ||
| Mitral valve procedure | 59.07 | <.0001 | |||||
| No | 6699 | (71%) | 2762 | (29%) | ref | ||
| Yes | 545 | (59%) | 384 | (41%) | 1.71 (1.49,1.96) | ||
| Tricuspid valve procedure | 3.70 | 0.0543 | |||||
| No | 7164 | (70%) | 3124 | (30%) | |||
| Yes | 80 | (78%) | 22 | (22%) | |||
| CABG | 9.74 | 0.0018 | |||||
| No | 1607 | (72%) | 612 | (28%) | ref | ||
| Yes | 5637 | (69%) | 2534 | (31%) | 1.18 (1.06,1.31) | ||
| CABG and valve procedure | 204.05 | <.0001 | |||||
| neither | 667 | (81%) | 153 | (19%) | ref | ||
| CABG only | 5186 | (71%) | 2098 | (29%) | 1.76 (1.47,2.12) | ||
| valve only | 940 | (67%) | 459 | (33%) | 2.13 (1.73,2.62) | ||
| both | 451 | (51%) | 436 | (49%) | 4.21 (3.38,5.25) | ||
| LVA repair | 9.13 | 0.0025 | |||||
| No | 7124 | (70%) | 3066 | (30%) | ref | ||
| Yes | 120 | (60%) | 80 | (40%) | 1.55 (1.16,2.06) | ||
| VSD repair | 2.92 | 0.0877 | |||||
| No | 7228 | (70%) | 3133 | (30%) | |||
| Yes | 16 | (55%) | 13 | (45%) | |||
| Ventricular assist device | 0.19 | 0.6665 | |||||
| No | 7167 | (70%) | 3110 | (30%) | |||
| Yes | 76 | (68%) | 36 | (32%) | |||
| Cardioplegia used | 41.86 | <.0001 | |||||
| No | 805 | (79%) | 220 | (21%) | ref | ||
| Yes | 6439 | (69%) | 2926 | (31%) | 1.66 (1.42,1.94) | ||
| IABP used | 34.62 | <.0001 | |||||
| No | 6530 | (71%) | 2712 | (29%) | ref | ||
| Yes | 714 | (62%) | 434 | (38%) | 1.46 (1.29,1.66) | ||
| Cross-clamp time (minutes) | 27.54 | <.0001 | |||||
| 0 | 515 | (75%) | 172 | (25%) | ref | ||
| 3 – 49 | 1733 | (72%) | 679 | (28%) | 1.17 (0.97,1.42) | ||
| 50 – 69 | 1638 | (69%) | 724 | (31%) | 1.32 (1.02,1.61) | ||
| 70 – 97 | 1654 | (69%) | 740 | (31%) | 1.34 (1.10,1.63) | ||
| 98 – 410 | 1575 | (66%) | 800 | (34%) | 1.52 (1.26,1.84) | ||
CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; NYHA = New York Heart Association; PCI = percutaneous cardiac intervention; CABG = coronary artery bypass graft; LVA = left ventricular aneurysm; IABP = intra-aortic balloon pump; VSD = ventricular septal defect.
The year in which the current surgery was performed was not presented in the table due to space constraints, but was included in the multivariate analysis.
Information on postoperative complications such as stroke, length of hospital stay after surgery, and mortality was also collected (listed in Table 2) to look for any association with new onset POAF.
Table 2. Association between new onset POAF and postoperative course (N=10,390).
| Variable | POAF | Chi-square test | Odds ratio | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| n | (%) | n | (%) | χ2 | p | OR (95% CI) | |
| Complication | |||||||
| MI | 8.82 | 0.0030 | |||||
| No | 7201 | (70%) | 3110 | (30%) | ref | ||
| Yes | 43 | (54%) | 895 | (46%) | 1.94 (1.24,3.02) | ||
| Permanent pacemaker | 50.10 | <.0001 | |||||
| No | 7146 | (70%) | 3037 | (30%) | ref | ||
| Yes | 98 | (47%) | 109 | (53%) | 2.62 (1.99,3.45) | ||
| Low cardiac output | 102.79 | <.0001 | |||||
| No | 6839 | (71%) | 2793 | (29%) | ref | ||
| Yes | 405 | (53%) | 353 | (47%) | 2.13 (1.84,2.48) | ||
| Tamponade | 36.58 | <.0001 | |||||
| No | 7132 | (70%) | 3039 | (30%) | ref | ||
| Yes | 112 | (51%) | 107 | (49%) | 2.24 (1.71,2.93) | ||
| Operative re-expl for bleeding | 35.57 | <.0001 | |||||
| No | 6943 | (70%) | 2928 | (30%) | ref | ||
| Yes | 301 | (58%) | 218 | (42%) | 1.72 (1.44,2.06) | ||
| Anticoagulant complications | 36.44 | <.0001 | |||||
| No | 7153 | (70%) | 3053 | (30%) | ref | ||
| Yes | 91 | (49%) | 93 | (51%) | 2.39 (1.79,3.21) | ||
| Transient stroke | 21.24 | <.0001 | |||||
| No | 7167 | (70%) | 3076 | (30%) | ref | ||
| Yes | 77 | (52%) | 70 | (48%) | 2.12 (1.53,2.94) | ||
| Permanent stroke | 56.13 | <.0001 | |||||
| No | 7105 | (70%) | 3004 | (30%) | ref | ||
| Yes | 139 | (49%) | 142 | (51%) | 2.42 (1.91,3.06) | ||
| Deep sternal infection | 20.54 | <.0001 | |||||
| No | 7195 | (70%) | 3095 | (30%) | ref | ||
| Yes | 49 | (49%) | 51 | (51%) | 2.42 (1.63,3.59) | ||
| Superficial sternal infection | 2.82 | 0.0934 | |||||
| No | 7075 | (70%) | 3055 | (30%) | |||
| Yes | 169 | (65%) | 91 | (35%) | |||
| Leg infection | 15.76 | <.0001 | |||||
| No | 7111 | (70%) | 3049 | (30%) | ref | ||
| Yes | 133 | (58%) | 097 | (42%) | 1.70 (1.30,2.22) | ||
| Pneumonia | 247.51 | <.0001 | |||||
| No | 6903 | (72%) | 2721 | (28%) | ref | ||
| Yes | 341 | (46%) | 424 | (55%) | 3.15 (2.72,3.66) | ||
| Acute renal failure | 116.75 | <.0001 | |||||
| No | 6957 | (71%) | 2855 | (29%) | ref | ||
| Yes | 287 | (50%) | 291 | (50%) | 2.47 (2.09,2.93) | ||
| GI complications | 221.27 | <.0001 | |||||
| No | 6869 | (72%) | 2716 | (28%) | ref | ||
| Yes | 375 | (47%) | 430 | (53%) | 2.90 (2.51,3.35) | ||
| Length of stay in hospital (days) | |||||||
| Length of stay in ICU | Median=2 (Q1,Q3: 1, 5) | Median=3 (Q1,Q3: 1, 8) | Z1 = 14.71 | <.0001 | |||
| Length of stay, surg. to disch. | Median=7 (Q1,Q3: 5, 9) | Median=9 (Q1,Q3: 7,15) | Z = 34.64 | <.0001 | |||
| Length of stay, stepdown unit | Median=4 (Q1,Q3: 2, 6) | Median=5 (Q1,Q3: 3, 9) | Z = 16.77 | <.0001 | |||
| Mortality | |||||||
| Mortality | 104.58 | <.0001 | |||||
| No | 6268 | (72%) | 2471 | (28%) | ref | ||
| Yes | 976 | (59%) | 675 | (41%) | 1.75 (1.57,1.96) | ||
| 30-day mortality | 3.57 | 0.0587 | |||||
| No | 6932 | (70%) | 2984 | (30%) | |||
| Yes | 312 | (66%) | 162 | (34%) | |||
| Cause of death: cardiac | 10.34 | 0.0014 | |||||
| No | 6984 | (70%) | 2991 | (30%) | ref | ||
| Yes | 260 | (63%) | 155 | (37%) | 1.39 (1.14,1.71) | ||
| Cause of death: neurologic | 15.02 | 0.0001 | |||||
| No | 7192 | (70%) | 3098 | (30%) | ref | ||
| Yes | 52 | (52%) | 48 | (48%) | 2.14 (1.44,3.18) | ||
| Cause of death: pulmonary | 19.57 | <.0001 | |||||
| No | 7153 | (70%) | 3069 | (30%) | ref | ||
| Yes | 91 | (54%) | 77 | (46%) | 1.97 (1.45,268) | ||
| Cause of death: infection | 5.51 | 0.0189 | |||||
| No | 7194 | (70%) | 3110 | (30%) | ref | ||
| Yes | 50 | (58%) | 36 | (42%) | 1.67 (1.08,2.56) | ||
Mann-Whitney-Wilcoxon test.
Analysis
Descriptive statistics, χ2 tests, Mann-Whitney-Wilcoxon tests, and odds-ratios (OR) were applied to describe the study population and the univariate relationships between individual variables and POAF.
Multivariate logistic regression (MLR) models were used to predict the onset of POAF, with a stepwise selection of significant predictors. Due to the large size of the study population, a stringent selection criterion of p ≤ 0.05 was used for entering and removing independent variables in the model. In a large-scale database with over 50 potential predictors, the presence of missing data is inevitable; its cumulative impact on multivariate analyses due to casewise deletion often overwhelmingly reduces the number of patients available for analysis. To minimize this impact, the MLR incorporated only those independent variables that were significant at the p ≤ 0.10 level after univariate analysis. The onset of POAF was predicted by MLR for all cardiac surgery patients (Model 1), and for patients who only underwent coronary artery bypass grafting (Model 2). Due to casewise deletion caused by missing values, Model 1 had only 5,075 patients available for analysis, which yielded ten significant predictors of POAF. By using only these ten significant predictors in a third MLR model (Model 3), we were able to retain more cases (N = 10,371) while verifying the stability of these ten predictors when reapplied to the entire study population (all cardiac surgery patients).
Information on preoperative warfarin use was missing for 5,354 patients. To avoid casewise deletion of these cases in the comprehensive analysis, this variable was excluded from the MLR models. In a preliminary MLR model, warfarin use was coded as either “yes”, “no”, or “unknown”, to retain missing cases in the analysis. This showed that preoperative warfarin use was not significantly different from no use in terms of predicting POAF. Therefore, it was safe to exclude this variable from the final analyses with minimal risk of losing a predictor of POAF.
Analyses were conducted using SAS software, version 9.1.6 Three statistics were calculated to evaluate the appropriateness of the models: the Hosmer and Lemeshow statistic testing the goodness-of-fit between the observed and predicted occurrence of POAF; the likelihood ratio testing the global significance of the selected predictors of POAF; and the area under the receiver operating characteristic curve (AUC) depicting the adequacy of the model for detecting the onset of POAF.
Results
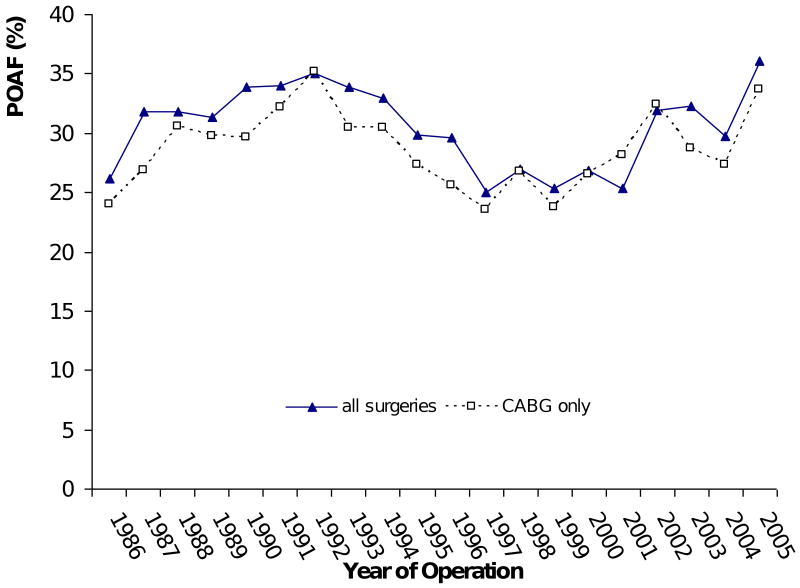
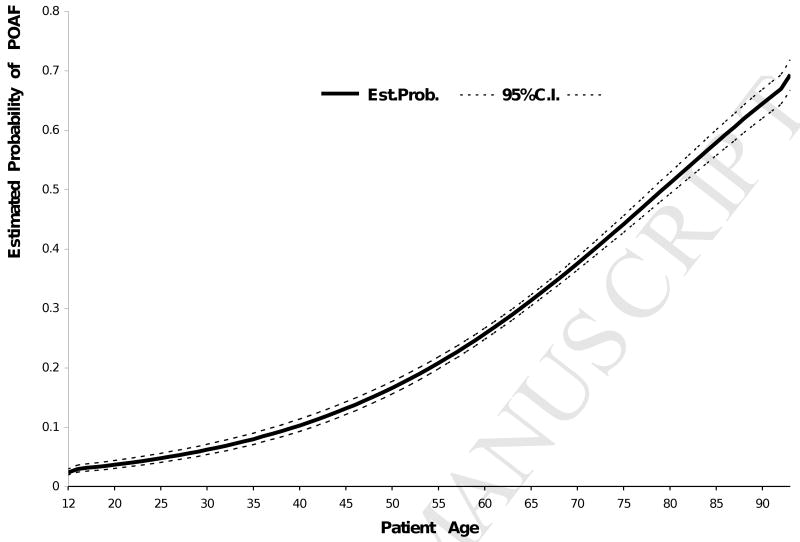
The average incidence of postoperative atrial fibrillation was found to be 30% (range: 25-36%) for the study population over the 20-year period (Figure 1). There was no significant effect of year of surgery on POAF (p = 0.17). The incidence of POAF ranged from 14% to 47% across four different age groups (Table 1). The odds of having POAF in patients 72 years of age or older were 5.6 times (OR = 5.57) those for patients 12-54 years old. While increasing age has consistently been associated with a higher incidence of POAF, the relationship between age and POAF is non-linear. Figure 2 illustrates that beyond age 55, for example, the estimated probability of having POAF rises more steeply, from 20% to 80%, while the probability rises much more slowly (up to 20%) for patients younger than 55.
Figure 1.
Incidence of new onset POAF for all patients and only CABG patients over a 20-year period.
Figure 2.
Estimated probability of POAF by age.
Table 1 presents the results of univariate analysis of the association between individual variables and new onset POAF. Odds ratios and their 95% confidence intervals are presented for those variables that were significantly related to POAF. Caucasian patients had 59% greater odds (OR = 1.59) of having POAF compared with patients of African-American descent. The likelihoods of developing POAF in patients with a preoperative history of congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), hypertension, or aortic valve stenosis were, respectively, about 1.6, 1.4, 1.3, and 1.7 times those of patients without such corresponding histories. Similarly, the incidence of POAF in patients undergoing an aortic or mitral valve procedure was about 1.7 times that in patients undergoing other cardiac procedures.
Twelve of the thirteen types of preoperative medications included in this study did not show a preventive effect against POAF, contrary to what has been reported for some of these medications in either controlled trials7,8 or retrospective studies.1,9 Over the 20-year period, the preoperative use of beta-blockers went from 36% to 59%. Despite this increased use, the incidence of POAF did not decrease. Overall, the incidence was 30% for those patients receiving beta-blockers, versus 31% for those who were not. Likewise, the use of ACE inhibitors went from 5% to 47% over the 20-year period, with no significant reduction in POAF. Although the univariate analysis (Table 1) showed that warfarin reduced the chance of POAF by 30%, this effect was not significant after controlling for other factors in the multivariate analysis. Similarly, patients taking digitalis and/or diuretics had a higher risk of POAF (Table 1), but this effect also did not hold up in the multivariate analysis.
Table 2 shows the co-occurrence of POAF and other postoperative complications, as well as the association between POAF and the length of hospital stay and mortality after cardiac procedures. POAF was positively associated with permanent stroke, the requirement for implantation of a permanent pacemaker, and mortality (OR=2.42, OR=2.62, and OR=1.75, respectively). Among the 14 complications studied other than POAF, 13 had a positive association with POAF (Table 2). The incidence of death from a neurologic cause was more than doubled in POAF patients.
Consistent with the results of other studies,1-3 the occurrence of POAF was associated with a two day longer hospital stay, as evaluated in this study by the median length of stay from surgery to discharge, and a one day longer median length of stay in the intensive care unit and stepdown unit after surgery (see Table 2 for details).
Table 3 presents significant variables as independent predictors of POAF that were detected by the MLR models. The goodness-of-fit, likelihood ratio, and AUC show that the models fit the data adequately.
Table 3. Predictors of new onset POAF and odds ratios (95% CI).
| Predictor | Model 1 All procedures (N = 5,075) | Model 2 CABG-only (N = 3,058) | Model 3 Re-test of Model 1 (N = 10,371) |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Demographics | |||
| Age group | |||
| 12 - 54 | ref | ref | ref |
| 55 - 63 | 1.84 (1.49, 2.27) | 1.77 (1.33, 2.36) | 2.12 (1.84, 2.46) |
| 64 - 71 | 3.39 (2.77, 4.14) | 3.28 (2.50, 4.31) | 3.72 (3.24, 4.29) |
| 72 + | 4.36 (3.59, 5.29) | 4.19 (3.20, 5.49) | 5.34 (4.64, 6.14) |
| Race | |||
| African-American | ref | ref | |
| Caucasian | 1.50 (1.23, 1.83) | 1.51 (1.30, 1.76) | |
| Other | 0.89 (0.46, 1.71) | 1.12 (0.72, 1.73) | |
| Preoperative medical history | |||
| CHF | |||
| No | ref | ref | ref |
| Yes | 1.39 (1.22, 1.58) | 1.46 (1.24, 1.72) | 1.28 (1.16, 1.42) |
| Hypertension | |||
| No | ref | ref | |
| Yes | 1.15 (1.00, 1.32) | 1.15 (1.04, 1.26) | |
| Intraoperative conditions | |||
| Aortic valve procedure | |||
| No | ref | --1 | ref |
| Yes | 1.51 (1.26, 1.81) | 1.52 (1.34, 1.72) | |
| Mitral valve procedure | |||
| No | ref | -- | ref |
| Yes | 1.88 (1.54, 2.30) | 1.91 (1.64, 2.23) | |
| CABG | |||
| No | ref | -- | |
| Yes | 1.23 (1.03, 1.47) | ||
| LVA repair | |||
| No | ref | -- | ref |
| Yes | 1.62 (1.12, 2.34) | 1.57 (1.15, 2.13) | |
| Cardioplegia used | |||
| No | ref | ref | |
| Yes | 1.27 (1.02, 1.47) | 1.36 (1.15, 1.60) | |
| IABP used | |||
| No | ref | ref | |
| Yes | 1.24 (1.04, 1.49) | 1.28 (1.11, 1.46) | |
| Goodness-of-fit: χ2(df); p | 7.44(8); p= .4901 | 1.77(6); p= .9399 | 9.83(8); p= .2770 |
| Likelihood ratio: χ2(df); p | 510.14(13); p<.0001 | 186.55(4); p<.0001 | 1054.41(12); p<.0001 |
| AUC | 0.69 | 0.65 | 0.70 |
CHF = congestive heart failure; CABG = coronary artery bypass graft; LVA = left ventricular aneurysm; IABP = intra-aortic balloon pump.
Not applicable for analysis
For all types of cardiac procedures collectively (Model 1), increasing age was strongly associated with POAF, as expected. The odds of a 72+ year-old developing POAF, for example, are 4.4 times those for a patient younger than 55 years-old. Over the 20-year period, the average patient age ranged from 60 to 64 years. There was no trend in age over the study period. The average age in 1986 was 60±13, and 61±14 in 2005. New onset POAF was 1.5 times as likely to occur among Caucasians as among patients of African-American or other racial/ethnic backgrounds. A history of congestive heart failure was associated with a 40% higher risk of developing POAF, and preexisting hypertension was related to a 15% higher risk of POAF. More importantly, the data seem to indicate that undergoing certain types of cardiac procedures carries a significant risk of POAF: an aortic or mitral valve procedure, coronary artery bypass grafting (CABG), or left ventricular aneurysm (LVA) repair introduces a 51%, 88%, 23%, and 62% higher risk of POAF, respectively. Due to the small number of tricuspid valve procedures and ventricular septal defect (VSD) repairs available for analysis, this study was unable to determine the POAF risk for these procedures. Other studies have reported that the incidence of POAF is greater for combined procedures than for isolated procedures.2 In this database, 8.5% (n=887) of patients had a combined valve and CABG procedure. Although this combined procedure was significant in the univariate analysis (Table 1), it was not a significant predictor in the multivariate analysis.
Increasing the cross-clamp time during a procedure did not significantly increase the risk of POAF. Multivariate analysis also indicated that the use of intraoperative cardioplegia to arrest the heart and intra-aortic balloon pump increased the risk of POAF by 23% and 27%, respectively. However, the POAF risk related to intraoperative cardioplegia may have been affected by the heterogeneous nature of its reference group, which included off-pump CABG (which might have been related to less POAF), type A aortic dissection repairs where circulatory arrest was used, and other surgeries, each of which did not involve cardioplegia but carried varying risks of POAF.
Nearly 80% of the cardiac procedures involved coronary artery bypass grafting, and over 68% of all surgeries were lone CABG procedures. Model 2 analyzed the lone CABG surgeries and revealed that increasing age and history of congestive heart failure were the only two independent risk factors associated with a higher probability of POAF. Patients with a history of congestive heart failure (CHF) were 46% more likely to have POAF. In contrast with the results of Model 1, being of Caucasian descent or having preexisting hypertension did not increase the risk of POAF following lone CABG procedures.
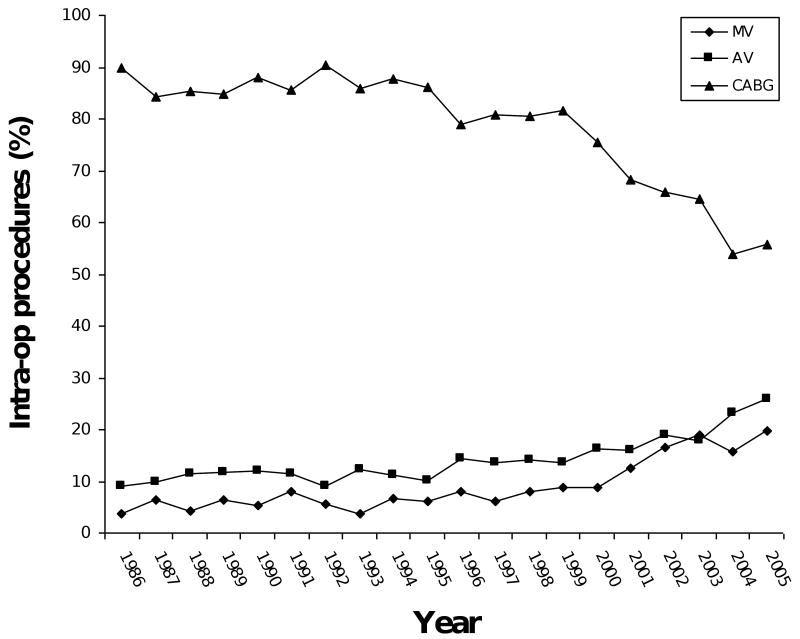
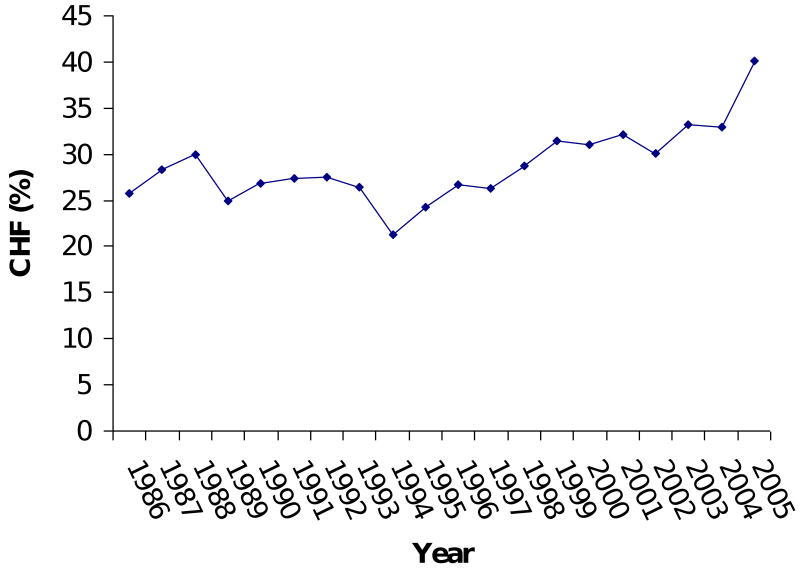
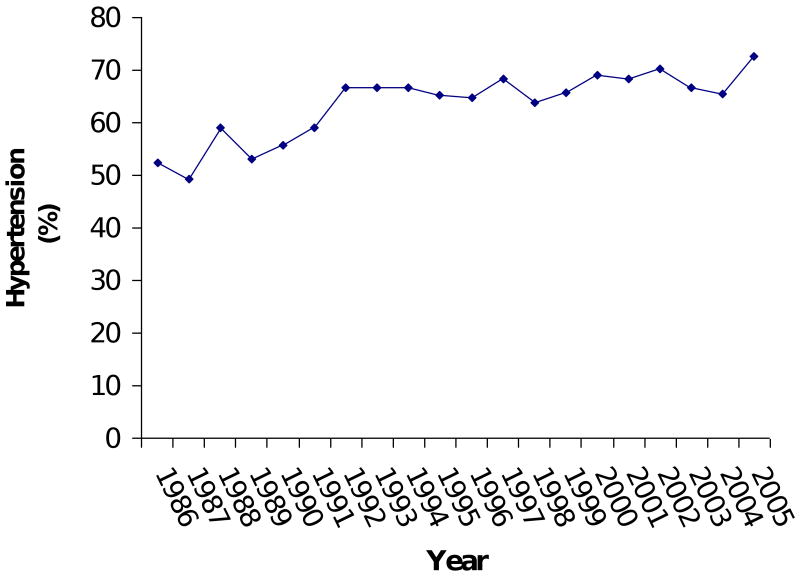
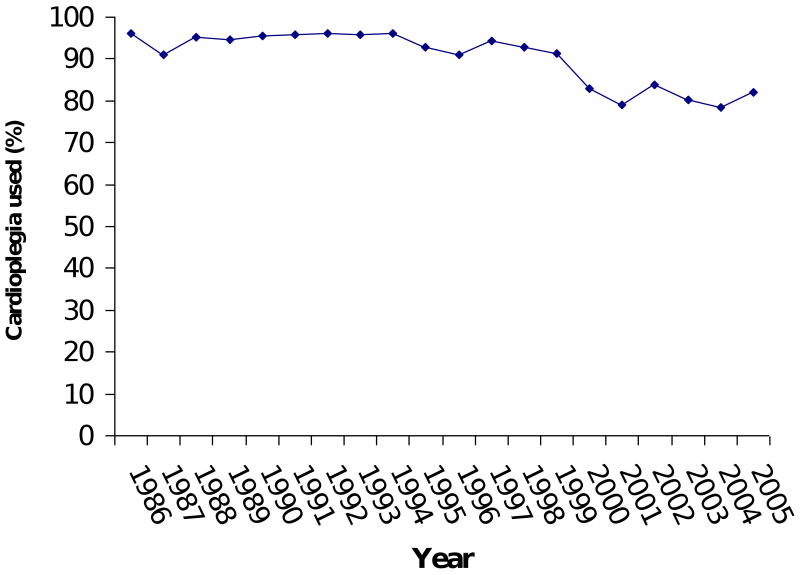
Model 3 imposed the ten variables as predictors on a larger number of patients (N = 10,371) and confirmed the stability of Model 1's results. CABG was the only variable (in Model 1) that turned out to be non-significant in the re-test (Model 3). Over the 20-year period examined, the percentage of patients with CHF increased from 25% to 40% of patients (Figure 3). The percentage of patients with hypertension went from 52% to 72%. Use of cardioplegia went from 96% to 82%. The percentage of patients undergoing CABG procedures went from 90% to 55%, while the percentage of mitral valve and aortic valve procedures went from 4% and 9% to 20% and 26%, respectively.
Figure 3.
Figure 3A: Trends in type of operation. MV: mitral valve procedure. AV: aortic valve procedure. CABG: coronary artery bypass graft procedure.
Figure 3B: Trend in congestive heart failure (CHF).
Figure 3C: Trend in hypertension.
Figure 3D: Trend in the use of cardioplegia.
The Hosmer and Lemeshow test and AUC showed that both Models 1 and 3 were similar in their goodness of fit and adequate discriminative power (AUC=0.70). Even with fewer predictors, Model 3 interpreted the data as well as Model 1. A model with only age as a predictor had an AUC=0.68, illustrating the dominant effect of age on the predictability of POAF.
Discussion
Logistic regression modeling is a useful tool in searching for protective and risk factors for POAF. The results of this method, however, are greatly influenced by the size and characteristics of patient samples and the spectra of factors controlled for in the analysis. The results from this study are comparatively accurate and replicable because they arose from analysis of a comprehensive group of risk factors based on a large population of patients undergoing various cardiac surgical procedures across a 20-year time period. The incidences of POAF from observational studies from the 1980's and contemporary studies are consistent with the findings of the present study.2,10-13 The incidence of POAF oscillated between 25 and 35% over the 20-year period of the current study. Peaks and valleys in the incidence (in Figure 1, for example, the incidence was 36% in 2005 and 24% in 1997) can be explained by the types of operations performed during a particular year, and by patient demographics, according to the MLR results (Table 3). For example, valve procedures have the highest risk of POAF (OR=1.91 for mitral and 1.52 for aortic). Among the patients in 2005, 20% had mitral and 26% had aortic valve procedures, compared with only 6% and 14%, respectively, in 1997. The combination of types of surgery and patient characteristics probably explains the wide range of POAF incidences (11 - 50%) reported by other studies. From 1986 to 2005, the annual percentage of patients with aortic or mitral valve procedures, congestive heart failure, and hypertension increased by 17%, 16%, 14%, and 20%, respectively. Considering that these factors were associated with a significantly higher risk of POAF, while the average incidence of POAF remained around 30%, it is reasonable to conclude that we have made some progress over time in preventing POAF.
Increasing age remains strongly associated with POAF. The incidence of POAF reported in previous studies has ranged from 11 to 50%1, possibly due to variation in the ages of the different study groups used. In the present study, the estimated probability of POAF was less than 20% for patients younger than 55 years, but increased rapidly for patients aged 55 and older, up to a maximum of 80% (Figure 2), indicating a non-linear relationship between age and probability of having POAF. Aging has been associated with remodeling of the atria.14 In particular, one proarrhythmic change is an increase in fibrous tissue between myocytes, which develops with age and is increased by various pathologies. This slows conduction and makes it more heterogeneous, increasing the inducibility of atrial fibrillation.
Among the 51 independent variables studied, 31 were associated with POAF based on univariate analysis (Table 1). However, multivariate analysis identified nine factors that were associated with a higher risk of POAF: increasing age, Caucasian race, a history of CHF, preexisting hypertension, use of intraoperative cardioplegia or an intra-aortic balloon pump, and three types of cardiac surgical procedures (Table 3). Knowing the factors that are associated with a much greater risk of POAF will help direct research and therapeutic efforts toward the patients who need it most. For instance, an estimate based on Model 3 indicates that the chance of developing POAF after an aortic valve procedure for a patient younger than 55 is 43%. The calculated risk would be 76% for a mitral valve procedure involving cardioplegia performed in a 72 year-old Caucasian patient with a history of CHF and hypertension. High risk patients could be candidates for attempted drug prophylaxis or other experimental interventions.
There has also been interest in factors associated with a decreased risk of POAF. For example, smoking has been correlated with a lower risk of POAF2 (the exact reason for this is unknown), patients who have undergone previous cardiac surgeries without experiencing POAF seem less likely to have POAF after subsequent cardiac surgeries (as indicated by the univariate analyses in this study), and pharmacologic prophylaxis with certain drugs is believed to protect against POAF.1,7-9,15-17 The present study did not find evidence, upon multivariate analysis, supporting an association between any of these factors and a decreased risk of POAF.
The preoperative use of drugs, such as beta-blockers, has been inconsistently reported to have either a protective 7,18,19 or no effect against POAF.10,13,20 None of the 13 medications in this study, when taken preoperatively, were found to be related to the onset of POAF. There are several possible explanations for this. First, these drugs are not definitively known to be effective against and were not prescribed to treat atrial fibrillation (patients with preexisting atrial fibrillation were excluded from the study), so it is unsurprising that they were not protective against POAF. Second, the dosages and lengths of time patients were on preoperative medications varied. Third, the fact that a patient was on a specific drug indicated that he or she may have had a comorbid condition requiring treatment with that drug. In such situations, it is difficult to separate the effects of the comorbid condition from those of the drug on the onset of POAF, despite the use of multivariate analysis. Fourth, it was common for patients to be on multiple medications, complicating the association of any single medication's effect with POAF. For these reasons, studies on the effects of pharmacologic prophylaxis for POAF should have stringently controlled experimental designs. It should also be pointed out that postoperative medications were not included in our analyses because we did not have adequate information on when these medications were given postoperatively (e.g. before or after patients developed POAF). It is possible that one or more of the 13 categories of medications studied may be associated with a reduced incidence of POAF, when given preoperatively and continued during the immediate postoperative period.
The discriminative power of the models, as measured by the AUC, is only fair and suggests that the underlying causes and mechanisms of POAF have yet to be determined. Clearly, the strong relationship of age with POAF suggests a need for a greater understanding of the effects of aging on the underlying substrates for POAF. Limitations in data availability did not allow us to test all important factors that have reportedly been associated with POAF: for example, echocardiographic variables quantifying left atrial and ventricular dimensions or function, preoperative use of Amiodarone, on versus off-pump surgery, type of incision and the inflammatory response to surgery (including postoperative pericarditis and markers such as Interleukin-6, C-reactive protein, the neutrophil/lymphocyte ratio, and Plasminogen Activator Inhibitor-110,20-25). These limitations emphasize the need for a more research-friendly centralized patient database with standardization of commonly investigated variables and their definitions.
Conclusion
The incidence of POAF over a 20-year period has not significantly decreased, in this single institution study. Over this study period, the mean incidence of POAF has persisted around 30%. This is consistent with data from other institutions showing that the incidence reported in the 1980's has remained unchanged in recent observational studies, despite the fact that numerous studies have shown a substantial reduction in incidence following various drug therapies. It is unclear why these have not translated to clinical practice. Possibly, there is a loss of efficacy when these therapies are put into a standardized clinical pathway and applied to all patients.13 In this study, antiarrhythmic drugs, when administered preoperatively, were not associated with a significant reduction in POAF. Logistic regression models using traditional preoperative and intraoperative risk factors do not adequately predict POAF. Understanding the underlying pathophysiology of POAF remains elusive, and until there is a better understanding of these mechanisms, it is unlikely that risk factors that do adequately predict POAF will be identified, or that therapies will be developed to treat this condition.
Acknowledgments
This study was supported by NIH Training Grant 2T35HL007815-11A1 and Grants NIH R01 HL 032257, NIH R01 HL085113 and NIH T32 HL007776.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336(20):1429–34. doi: 10.1056/NEJM199705153362006. [DOI] [PubMed] [Google Scholar]
- 2.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 3.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery: current trends and impact on hospital resources. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto K, Ilstrup DM, Schaff HV. Influence of clinical and hemodynamic variables on risk of supraventricular tachycardia after coronary artery bypass. J Thorac Cardiovasc Surg. 1991;101:56–65. [PubMed] [Google Scholar]
- 5.Leitch JW, Thomson D, Baird Dk, Harris PJ. The importance of age as a predictor of artial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100:338–42. [PubMed] [Google Scholar]
- 6.SAS Institute Inc. SAS/STAT User's Guide, Version 8. Cary, NC: SAS Institute Inc.; [Google Scholar]
- 7.Kowey PR, Taylor JE, Rials SJ, Marinchak RA. Meta-analysis of the effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. Am J Cardiol. 1992;69:963–5. doi: 10.1016/0002-9149(92)90802-6. [DOI] [PubMed] [Google Scholar]
- 8.Bradley D, Creswell LL, Hogue CW, Epstein AE, Prystowsky EN, Daoud EG. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:39–47. doi: 10.1378/chest.128.2_suppl.39s. [DOI] [PubMed] [Google Scholar]
- 9.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 10.Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–191. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Saso S, Vecht JA, Rao C, Protopapas A, Ashrafian H, Leff D, et al. Statin therapy may influence the incidence of postoperative atrial fibrillation. Tex Heart Inst J. 2009;36(6):521–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Mauermann WJ, Nuttall GA, Cook DJ, Hanson AC, Schroeder DR, Oliver WC. Hemofiltration during cardiopulmonary bypass does not decrease the incidence of atrial fibrillation after cardiac surgery. Anesth Analg. 2010;110:329–34. doi: 10.1213/ANE.0b013e3181c76bd3. [DOI] [PubMed] [Google Scholar]
- 13.Kim MH, Deeb GM, Morady F, Bruckman D, Hallock LR, Smith KA, et al. Effect of postoperative atrial fibrillation on length of stay after cardiac surgery (The postoperative atrial fibrillation in cardiac surgery study [PACS2]) Am J Cardiol. 2001;87:881–5. doi: 10.1016/s0002-9149(00)01530-7. [DOI] [PubMed] [Google Scholar]
- 14.Pandit SV, Jalife J. Aging and atrial fibrillation research: Where we are and where we should go. Heart Rhythm. 2007;4(2):186–7. doi: 10.1016/j.hrthm.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogue CW, Creswell LL, Gutterman DD, Fleisher LA. Epidemiology, mechanisms, and risks: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:9–16. doi: 10.1378/chest.128.2_suppl.9s. [DOI] [PubMed] [Google Scholar]
- 16.Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of postoperative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27(23):2846–57. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- 17.Mariscalco G, Lorusso R, Klersy C, Ferrarese S, Tozzi M, Vanoli D, et al. Observational study on the beneficial effect of preoperative statins in reducing atrial fibrillation after coronary surgery. Ann Thorac Surg. 2007;84:1158–65. doi: 10.1016/j.athoracsur.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Frost L, Molgaard H, Christiansen EH, Hjortholm K, Paulsen PK, Thomsen PEB. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. Int J Cardiol. 1992;36:253–61. doi: 10.1016/0167-5273(92)90293-c. [DOI] [PubMed] [Google Scholar]
- 19.Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery: a meta-analysis of randomized control trails. Circulation. 1991;84(Suppl III):236–43. [PubMed] [Google Scholar]
- 20.Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ. Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation. 2007;116(Suppl I):1–7. doi: 10.1161/CIRCULATIONAHA.106.677906. [DOI] [PubMed] [Google Scholar]
- 21.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC\AHA\ESC 2006 guidelines for the management of patients with atrial fibrillation - a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:e149–e246. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Daoud EG, Strickberger SA, Man KC, Goyal R, Deeb GM, Bolling SF, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N Engl J Med. 1997;337:1785–91. doi: 10.1056/NEJM199712183372501. [DOI] [PubMed] [Google Scholar]
- 23.Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR. Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial. J Am Coll Cardiol. 1999;34:343–7. doi: 10.1016/s0735-1097(99)00212-0. [DOI] [PubMed] [Google Scholar]
- 24.Ishida K, Kimura F, Imamaki M, Ishida A, Shimura H, Kohno H, et al. Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2006;29(4):501–5. doi: 10.1016/j.ejcts.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Lo B, Fijnheer R, Nierich AP, Bruins P, Kalkman CJ. C-reactive protein is a risk indicator for atrial fibrillation after myocardial revascularization. Ann Thorac Surg. 2005;79:1530–5. doi: 10.1016/j.athoracsur.2004.10.004. [DOI] [PubMed] [Google Scholar]