Abstract
Acute effects of estrogens on mnemonic processes were examined at the behavioral and neurochemical levels. 17β-estradiol and 17α-estradiol influences on memory consolidation were assessed using object placement (OP) and object recognition (OR) tasks. Subjects received treatment immediately after a sample trial (exploring two novel objects), and memory of objects (OR memory) or location of objects (OP memory) was tested 4hr later. Both isomers of estradiol enhanced memory. For spatial memory, 15 and 20μg/kg of 17β-estradiol facilitated OP, while lower and higher doses were ineffective. 17α-estradiol had a similar pattern, but a lower dose was effective. When treatment was delayed until 45min after a sample trial, memory was not enhanced. For non-spatial memory, OR was facilitated at 5μg/kg of 17β-estradiol and at 1 and 2 μg/kg of 17α-estradiol and, similar to OP, lower and higher doses were ineffective. These data demonstrate that beneficial effects of estrogens are dose, time and task dependent, and the dose-response pattern is an inverted-U. Because monoamines are known contributions to memory, brains were removed 30 min after treatment for measurements of dopamine (DA), norepinephrine (NE), serotonin (5-HT), and metabolites. Estrogen elevated 5HT, NE metabolite MHPG, turnover ratio of NE to MHPG, and DA metabolite DOPAC levels in the prefrontal cortex, while NE and MHPG were decreased in the hippocampus. Thus, acute estrogens exert rapid effects on memory consolidation and neural function, which suggests that its mnemonic effects may involve activation of membrane-associated estrogen receptors and subsequent signaling cascades, and that monoamines may contribute to this process.
Keywords: estrogen, 17a-estradiol, 17b-estradiol, spatial memory, object recognition, memory consolidation, HPLC, monoamines, hippocampus, prefrontal cortex
Introduction
The majority of existing research on cognitive effects of estrogen has focused on chronic and/or sub-chronic treatments and has demonstrated that administration of estradiol (E2) to ovariectomized (OVX) animals enhances performance in some, but not all, memory and learning tasks (see Dohanich, 2002 and Luine, 2006, 2008 for review). These studies indicate that estrogen has specific, rather than global, effects on memory systems. Moreover, accumulative evidence has revealed that the beneficial effects of estrogen treatments depend on various factors, including demands of memory tasks (Galea et al., 2001; McLaughlin et al., 2008; Zurkovsky et al., 2007), doses (Holmes et al., 2002; Wide et al., 2004), routes of administration (Garza-Meilandt et al., 2006), duration and timing of treatment (Daniel et al., 2006; Gresack and Frick, 2006a), age (see Frick, 2009 for review), sex (Gibbs, 2008) stress level of subjects (Bowman et al., 2002, 2003, 2009; Luine et al., 2007), environmental enrichment (Gresack et al., 2007a, 2007b) and extent of daily handling (Bohacek and Daniel, 2007)
In contrast, acute effects of estrogen on memory function are less studied, but recent work has shown that acute treatments can influence memory consolidation processes in rats (Luine et al., 2003; Packard et al., 1996; Packard and Teather, 1997a, 1997b; Rhodes and Frye, 2004, 2006) and mice (Fernandez et al., 2008; Gresack and Frick, 2006b; Harburger et al., 2009; Lewis et al., 2008). Some of these studies demonstrated time-limited effects of acute estrogen on memory consolidation using “post-training treatment paradigms”, in which hormone treatment was given after training or the sampling phase of a memory task. This paradigm minimizes possible non-mnemonic or psychological effects of treatments since drug/hormones are not present when animals encounter new information. Packard and Teather (1997a), for example, trained OVX rats in a Morris water maze and immediately gave intraperitoneal injections of 0.1, 0.2 or 0.4mg/kg of 17β-estradiol. The intermediate dose (0.2mg/kg) improved memory for the hidden platform location when tested 24hr later, but the same dose given 2hr after training was ineffective. The results suggested a “critical time and/or dose window” for acute estrogen enhancements of memory consolidation.
Using pre- and post-sampling injection paradigms, we have shown enhanced memory occurs rapidly, within a few hours after acute estrogen treatment (Luine et al., 2003). Interestingly, 17α-estradiol was more potent than 17β-estradiol for hippocampal dependent spatial memory when treatment was given 30min before a sample trial and memory tested 4hr later. Affinity of 17α-estradiol for nuclear estrogen receptors, ERα and ERβ, is much lower compared to 17β-estradiol (Kuiper et al., 1997), but recent studies indicate 17α-estradiol shows greater affinity for some membrane-related estrogen receptors (Toran-Allerand et al., 2002, 2005). Thus, rapid memory enhancement by acute estrogen treatment and the greater potency of 17α-estradiol suggest the responses are, at least in part, mediated via non-genomic pathways. Monoaminergic neurotransmitters are important in rapid membrane associated effects, but few studies have investigated monoamines in relation to rapid effects of estrogen on memory. Non-genomic regulation of monoamine systems following acute estrogen treatment has been reported in some brain areas such as the hypothalamus, an important region for estrogen-regulated sexual behavior, and in the hindbrain, telencephalon, cerebellum (Cornil et al., 2005, 2006) and the nucleus accumbens (Thompson and Moss, 1994). But whether acute estrogen treatments affect monoamines in relation to memory is not known.
The objective of the present study was to investigate acute effects of estrogens on memory consolidation. To achieve this goal, we tested various doses of 17β- and 17α-estradiol given post-sampling in the spatial memory task, object placement, and the non-spatial memory task, object recognition (Ennaceur et al., 1997). Possible contributions of monoamines to estrogen-induced enhancements of memory consolidation were assessed by measuring DA, NE, 5-HT and metabolites in brain areas known important for memory including the prefrontal cortex, the hippocampus, the vertical limb of the diagonal band and the striatum during the period of memory consolidation. The results obtained provide further support for and novel information on estradiol's ability to rapidly enhance memory consolidation in OVX rats.
Materials and Methods
Subjects
Thirty eight female Sprague-Dawley rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN), aged 55-60 days upon arrival and ovariectomized (OVX) by the vender, served as subjects. All rats were double-housed in cages, maintained on a 12/12 hr light-dark cycle (lights on at 7:00AM). They had ad libitum access to low phyto-estrogen food (Chow 2016, 16% protein rodent diet, Harlan Teklad Global Diets, Madison, WI) and water for the entire period of the experiments. Rats were allowed to acclimate to the housing environment for a week and received eight days of habituation sessions prior to behavior experiments (see acclimation/habituation schedule). All experiments were conducted in accordance with the NIH Guide for Care and Use of Animals and the Institutional Animal Care and Use Committee of Hunter College of the City University of New York.
Memory Tasks
Two types of memory tasks, object placement (OP) and object recognition (OR), were adapted from working memory tasks developed by Ennaceur and Aggleton (1987). These tasks measure object place memory (spatial memory) and object recognition memory (non-spatial memory) based on animals spontaneous exploration behavior and their innate tendency of novelty preference. The OR and OP tasks have several advantages to access hormonal effects on memory performance. First, the tasks do not use positive (e.g., food/water rewards) or negative (e.g., electrical shock) reinforcement, which can interact with some factors such as motivation, appetite, thirst and anxiety levels. Second, animals are not required to learn contingency rules for the tasks. Third, stress related confounding variables can be minimized since animals do not encounter stressful circumstances (e.g., forced to swim) during task performance. Finally, because of the nature of OP and OR tasks described above, animals can be used repeatedly for tests (Ennaceur et al., 1997). In the present experiments, the subjects were repeatedly used for the OP and/or OR tests every 10 days.
Object placement (OP)
Spatial memory was tested using the object placement (OP) task as previously described in young and aged rats (Bisagno et al., 2002; Bowman, 2009; Luine et al., 2003; Wallace et al., 2006, 2007). All trials were conducted in a 3 × 3 grid open field made of black Plexiglass (70cm wide × 70cm long × 30cm high), which was placed on a 70cm high table, and external spatial cues (e.g., posters, pictures, a video camera) were available on the walls. Intensity of room light was carefully controlled to illuminate the floor of the chamber evenly. A session consisted of a 3-min sample trial (T1) and a 3-min retention trial (T2) with a 4hr inter-trial interval between T1 and T2. During T1, two identical objects were placed at one end of the open field. The total amount of time rats spent exploring the two objects for 3min was recorded. Exploration behavior was defined when the rats were sniffing at, looking at, or whisking at the objects within 2cm distance. Following an inter-trial delay of 4 hr, a T2 retention trial was given, in which one of the objects was moved to a novel location within the open field. The time spent exploring the objects at the old (familiar location) and the new (novel location) was recorded for 3 min. Objects were candleholders, figurines and statues. The novel location was counterbalanced across treatments. Objects and the floor of the chamber were cleaned with a disinfectant spray after each trial. All trials were videotaped with a SONY camcorder and analyzed later.
Object recognition (OR)
Recognition memory was accessed using the object recognition (OR) task. The basic procedure was the identical to the OP task except that one of the objects used during T1 was replaced with a novel/new object in T2. The exploration time around the old (familiar object) and the new (novel object) were recorded. Objects used for the OR tasks were a variety of cans, containers and bottles. The new object and its position in the open field were counterbalanced across groups.
Acclimation/habituation
Approximately three weeks after ovariectomy and two weeks after arrival, acclimation began and lasted eight days. First, each rat was placed into a 3 × 5 grid open field chamber without objects to explore freely for 6 min. For day 2 through day 5, rats received four habituation sessions to the object recognition task with progressively longer inter-trial intervals (1 min, 15 min, 1 hr, and 2 hr) between T1 and T2. After a 2-day break, three habituation sessions to OP were given with a 10 min, 40 min and 1hr inter-trial intervals.
Estrogen treatments
17α-estradiol and 17β-estradiol were obtained from Sigma-Aldrich Corp (St. Louis, MO). We tested 2-60μg/kg of 17β-estradiol and 0.5-20ug/kg of 17α-estradiol. These doses were selected based on previous studies (see results for details). Hormones were initially dissolved in ethanol stock solutions (10mg /1ml and 1mg /1ml), and diluted with corn oil for injection. The high concentration stock was used to make 10ug/kg or higher injections, and the low concentration stock was used for less than 10ug/kg in order to control ethanol levels (less than 0.006%) in each solution. Control rats received 1ml/kg of corn oil, which contained the corresponding amount of ethanol.
Hormone or vehicle treatment was given using a post-training/sampling treatment paradigm, which was developed based on memory consolidation hypotheses and has been used in a number of memory studies (Gresack and Frick, 2006b; Luine et al., 2003; McGaugh, 1966; Packard, 1998). OVX rats received either a single subcutaneous (sc) injection of estrogen or corn oil at the nape of the neck immediately after a sample trial (immediate post-T1 injection; see Fig. 1A). To test time-dependent effects of estrogen, a delayed post-sampling treatment paradigm was used for one OP session, and rats received hormone treatment 45min after a sample trial (delayed post-T1 injection; see Fig. 1A).
FIG. 1.
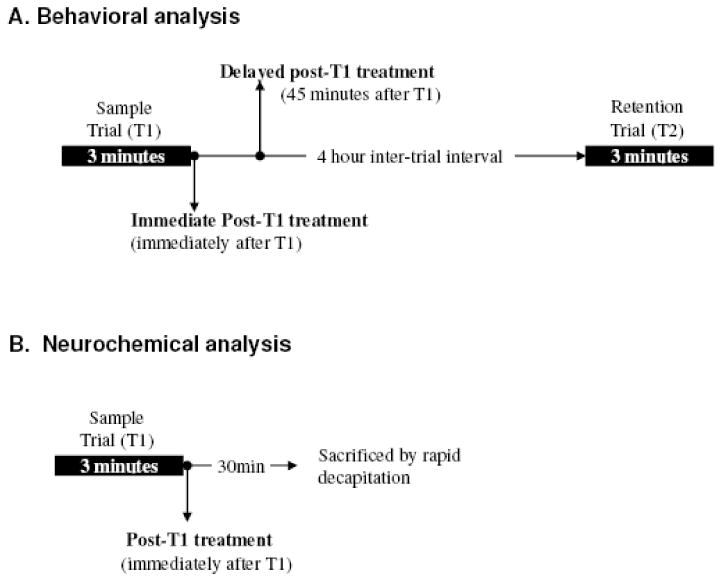
Timeline for hormone treatment. A. OVX rats received a single sc injection of either vehicle or estradiol immediately after T1 (immediate post-T1 treatment) or 45min after T1 (delayed post-T1 treatment). B. For neurochemical analysis, treatment was given immediately after T1. Subjects were sacrificed 30 min after treatment.
To determine the lowest effective doses of acute estradiol, rats were tested approximately every 10 days with doses described above. Treatments (vehicle or estradiol) were counterbalanced in every experiment. For the dose-response relationship study, all doses of estradiol were tested in each experiment using a matched block paradigm (18 or 20 rats per block with equal numbers of rats at each dose). Experiments were repeated every 10 days until each rat received all doses and each dose group contained a sample size of at least eight subjects.
Neurochemical; monoamines, metabolites and activity levels
A week after completion of behavioral study, 12 rats were used for neurochemical analysis. Rats received either 20μg/kg of 17β-estradiol (the most effective dose for OP memory consolidation) or vehicle immediately after a sample trial. 30 min later, they were lightly anesthetized with carbon dioxide and sacrificed by rapid decapitation (Fig.1B). This time interval (30min) was chosen to ensure that we examined neurochemical changes induced by acute estrogen treatment during the time of memory consolidation.
Monoamines and metabolites were measured as described previously (Beck and Luine, 2002; Bisagno et al., 2003; Bowman et al., 2009; Luine et al., 1998; Macbeth et al, 2008). Briefly, following rapid decapitation, the brains were immediately removed, frozen with dry ice and stored at − 70 °C. For sampling, the brains were warmed to approximately freezing and coronally cut into six serial sections with a razor blade, and mounted on a micro slide. Using a 500μm-diameter cannula, tissue samples in the target areas were obtained from the frozen sections under a dissecting microscope with the stage maintained at approximately −11 °C, and placed into a 1.5ml Eppendorf tubes. The number of punches obtained from each brain area was as follows; the prefrontal cortex (6-8 punches); CA1 (4 punches), CA3 (4 punches), and dentate gyrus (4 punches) of the hippocampus; vDB (3 punches) and striatum (2 punches).
The punches were dissolved in 60μl of sodium acetate buffer (pH 5.0), and neurotransmitters obtained through freezing and thawing, which disrupts cellular structures and releases cellular components. α- Methyl-dopamine was added as an internal standard, and samples were centrifuged at 12000 g-force for 12 minutes. The supernatant was removed and the pellet was re-suspended in 200 μl (PFC) or 100μl of 2.0N NaOH (other brain areas) for protein analysis using Bio-Rad reagent (Bio-Rad Laboratories, Hercules, CA, USA). High-performance liquid chromatography (HPLC) with electrochemical detection was used to quantify levels of monoamines and metabolites, including dopamine (DA) and its metabolites, 3-4-dihydroxyphenylalanine (DOPAC) and homovanillic acid (HVA); norepinephrine (NE) and its metabolite 3-Methoxy-4-Hydroxyphenylglycol (MHPG); and serotonin (5-HT) and its metabolite 5-hydroxyindole acetic acid (5-HIAA). The supernatant (40μl) was injected into a Waters Associates chromatographic system (Waters 2690), consisting of an alliance module containing an automated refrigerated, injector pump, Symmetry C18 5μm 4.6 × 150mm reverse-phase column (Novapak, three micron), and an ESA Coulochem III detector (0.48 to 0.50V potential). The mobile phase contained 3% acetonitrile, and peak sharpness was increased by the addition of 100% methanol, approximately 0.5%.
Millennium software (Waters Associates) ran the chromatography system and concentrations of neurotransmitters and metabolites were calculated by reference to standards and the internal standard using peak integration. Monoamine and metabolite concentration were expressed as pg/μg protein. Turnover ratios (metabolite/monoamine) were calculated as a measure of activity.
Data analyses
All data were analyzed using SPSS software (Systat Inc., Chicago, IL., USA). For behavioral analysis, group differences in the total exploration times around two objects during T1 were analyzed using independent t-tests (for two groups) or one-way ANOVAs (for more than two groups). For the retention trial (T2), two-way ANOVAs, group (oil, E2) × object or location (old, new), were used to analyze the time spent exploring the old/familiar objects and the new/novel objects (for OR) or the objects at the old/familiar locations and the new/novel locations (for OP). If a significant interaction (group × exploration times) was found, then independent t-tests were used to test group differences in exploration ratios (T2 NEW/T2 total time). Exploration ratios show % time spent exploring the new objects or the objects in the new location relative to the total time spent exploring the objects in both locations. When more than two groups were tested (dose-response relationship experiments), one-way ANOVAs were used to analyze group differences in exploration ratios with a post-hoc test (Fisher LSD). Data from rats that did not explore the objects during T1 and/or T2 were excluded from statistical analyses. For neurochemical analysis, two-way ANOVAs (group × neurochemical) were used to analyze monoamine and metabolite concentrations and turnover ratios in each brain area. Significant ANOVA results were followed by two-sample t-tests as post-hoc comparisons.
Results
Acute 17β-estradiol; Effects on object placement (OP)
To determine the most effective dose of 17β-estradiol for OP memory consolidation, we first tested 60ug/kg and 30ug/kg in separate sessions because previous studies indicated these doses injected immediately before or after T1 significantly enhanced OP (Luine et al., 2003), or increased spine synapse density in the CA1 hippocampus (MacLusky et al., 2005a, 2005b). During T1, rats spent similar amounts of time exploring the two identical objects for both sessions (12.91-14.13 s). Surprisingly, no significant main effects or interaction were found between 60ug/kg (n=10) and vehicle treated (n=9) group (Fig. 2A) in the retention trial, but a significant interaction was found in 30μg/kg (n=10) vs. vehicle treated (n=9) group (F 1,16=7.97, p<0.012, Fig. 2C). Although the rats in the 30μg/kg group spent more time exploring objects at the new location (12.14 s) than the old location (8.49 s), group differences in exploration ratios between 30μg/kg (ratio=0.59) and vehicle treated (ratio=0.51) were not significant (t 16=1.978, p<0.065; Fig. 2C), suggesting the 30μg/kg dose showed only a trend toward significantly enhancing OP memory.
FIG. 2.
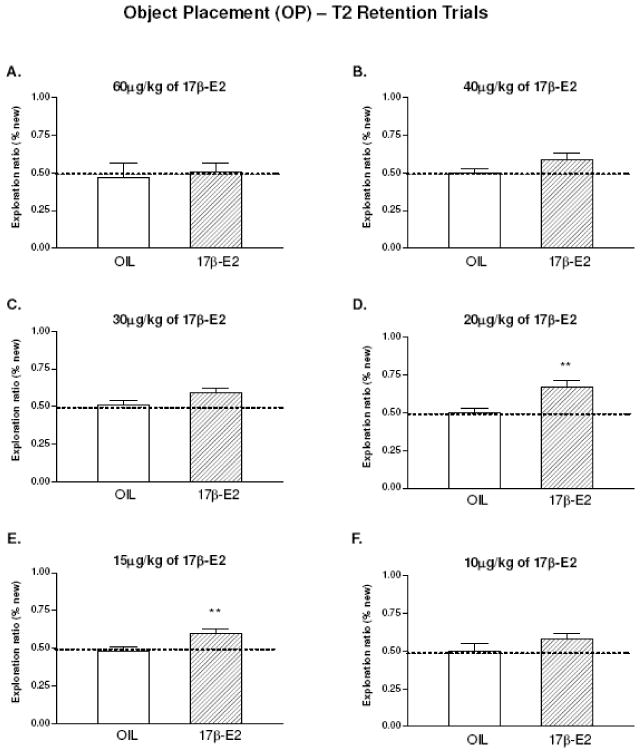
Acute effects of 17β-E2 on object placement (OP). Exploration ratios during retention trials are shown and the dashed line at 0.5 indicates chance level performance (rats spent equal amount of time around objects at old and new locations). n=9-10 in each group. A. OIL vs.60μg/kg of E2. Not significant. B. OIL vs.40μg/kg of E2. Not significant. C. OIL vs.30μg/kg of E2. Significant interaction (F 1, 16=7.97, p<0.012), but no group difference in exploration ratios (t 16=1.978, p<0.065). D. OIL vs.20μg/kg of E2. Significant interaction (F1,18=10.67, p<0.004) and group differences in exploration ratios (t 18=2.943, p<0.009) were found. E. OIL vs.15μg/kg of E2. Significant interaction (F1, 17=8.51, p<0.01) and exploration ratios (t 17=2.988, p<0.008) were found. F. OIL vs. 10μg/kg of E2. Not significant. Data were analyzed by two-way ANOVA (group × location) with two sample t tests. Entries are means ± SEM. *p<0.05; **p<0.01.
Based on the above results, we reasoned that effective doses for memory consolidation might be between 30ug/kg and 60ug/kg, or below 30ug/kg. Thus, we tested doses from 10 to 40ug/kg of 17β-estradiol (n=9-10 in each group). In the T1 sample trials, no significant differences in exploration times (12.05-16.22 s) were found for any session. In the T2 retention trials, ANOVA indicated significant main effects of location (F1,18=9.600, p<0.006) and interactions (F1,18=10.665, p<0.004) for the 20ug/kg, and significant interactions for the 15ug/kg (F1,17= 8.511. p<0.01)dose.. Post-hoc tests revealed that group differences in exploration ratios were also significantly different for 20μg/kg (t 17=2.988, p<0.008) and 15μg/kg (t 18=2.943, p<0.009), indicating that both 20μg/kg (Fig. 2D) and 15μg/kg (Fig. 2E) enhanced OP memory consolidation. In contrast, rats in the 40μg/kg (Fig. 2B) and 10μg/kg (Fig. 2F) groups showed chance level performance. Thus, intermediate doses of 17β-estradiol (15and 20ug/kg) enhance OP memory consolidation, but lower (10ug/kg) and higher (40 and 60ug/kg) doses have no effects.
In order to more precisely determine the dose-response relationship, OP memory performance was tested with doses of 0, 10, 20, 40, and 60ug/kg of 17β-estradiol in the same experiment. During T1, no significant group differences in exploration time (12.77-19.18 s) around the objects were found. Fig. 3A shows the mean exploration ratios of the 0μg (ratio=0.47), 10μg/kg (ratio=0.58), 20μg/kg (ratio=0.67), 40μg/kg (ratio=0.57), and 60μg/kg (ratio=0.51) groups during the retention trial, and there was a significant group difference (F 4, 47=3.191, p<0.021). Post-hoc tests showed that only the 20μg/kg group was significantly different from the control group (p<0.002). Thus, the dose-response relationship, based on percent times spent exploring objects at the new locations, was not a straight line or sigmoidal curve but was an approximately inverted U shape with 20ug/kg of 17β-estradiol exerting the greatest memory enhancing effects.
FIG. 3.
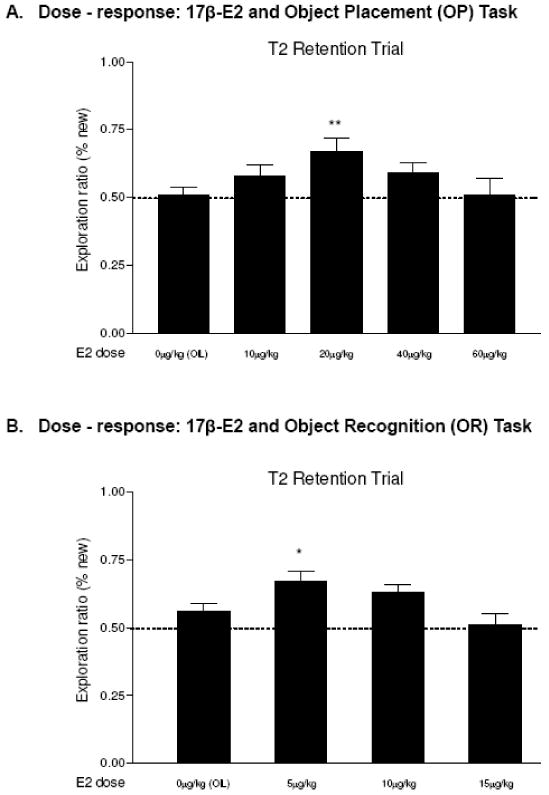
Dose-response for 17β-E2 and memory performance. Data were analyzed by one-way ANOVA with post-hoc test (Fisher LSD). The dashed line at the exploration ratio of 0.5 indicates chance level performance (rats spent equal amount of time around objects at old and new locations). A. Object Placement (OP). ANOVA was significant, F 4, 47=3.191, p<0.021, and 20μg/kg E2 enhanced OP memory (p<0.002). B. Object Recognition (OR). ANOVA was significant, F 3, 52=3.604, p<0.019 and 5μg/kg enhanced memory (p<0.02). Entries are means ± SEMs. *p<0.05; ** p <0.01.
When estrogen treatment was delayed 45 min after T1, memory-enhancing effects of estradiol (Fig. 4A, middle and right panel) were not observed, and both control and 20μg/kg groups spent similar amounts of time exploring the objects around the new and old locations (Fig. 4B, middle panel), and there was no difference in exploration ratios between the two groups (Fig. 4B, right panel). These results indicate that 20μg/kg of estradiol must be present within 45min after T1 to enhance OP memory consolidation.
FIG. 4.
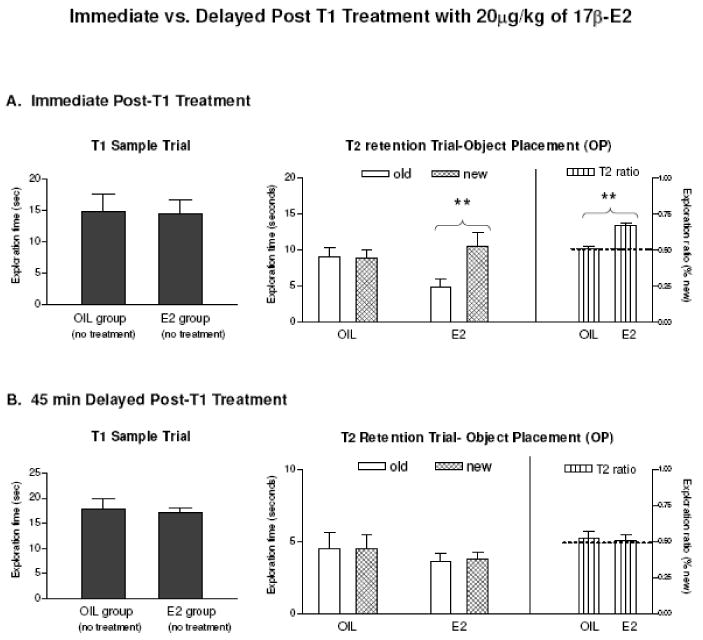
Immediate (A) vs. delayed postT1 treatment (B) with 20μg/kg of 17β-E2 on OP. Left panels: exploration times around objects during T1. Middle panel: time spent exploring objects at old and new locations during T2. Right panel: exploration ratios during T2. Dashed lines at 0.5 indicate chance level performance. Entries are means ± SEM. **p < 0.01.
Acute 17β-estradiol; Effects on object recognition (OR)
For OR memory, we first tested 20μg/kg (n=9) as this dose enhanced OP memory performance, but no significant effect was found. We then examined 5, 10 and 15ug/kg (n=9-10) based on the inverted U seen for OP memory and our previous finding that lower doses of 17β-estradiol enhanced OR compared to OP when given 30min before a sample trial (Luine et al., 2003). During the sampling trials, no significant group differences in exploration times were found (Fig. 5, left panels). In T2, significant main effects of objects were found for 5ug/kg (F1,16=10.49, p<0.005) and 10ug/kg (F 1,17=9.038, p<0.008), and the rats spent more time around the new objects (Fig. 5A and Fig.5B, middle panels), while rats treated with 15μg/kg did not. Post-hoc comparison revealed that only the 5ug/kg group was significantly different from the control group (t 16=2.188, p<0.044, Fig. 5A, right panel), indicating that the lowest tested dose (5μg/kg) enhanced OR memory, but the higher doses (10 and 15ug/kg) were ineffective.
FIG. 5.
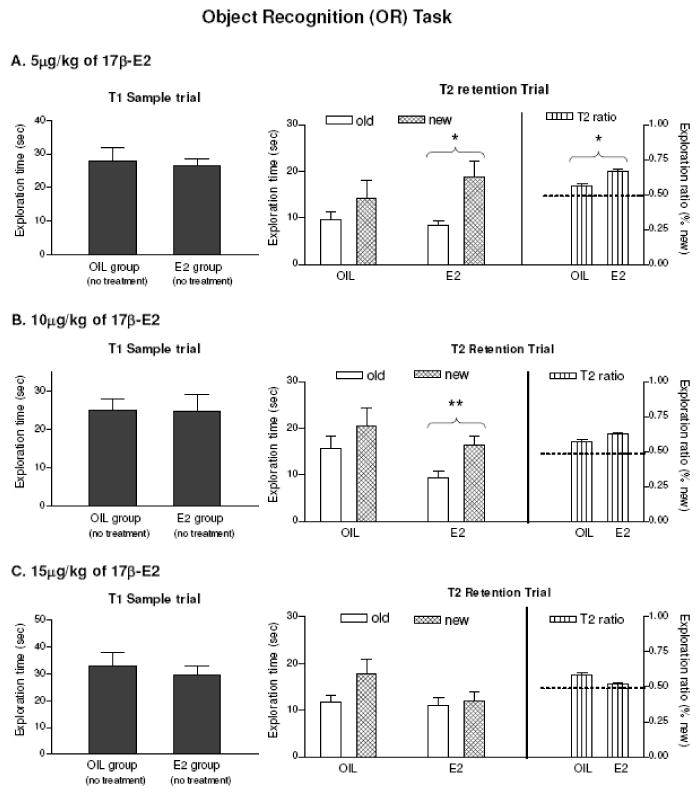
Acute effects of 17β-E2 on object recognition (OR). Time spent around objects during T1 (Left), T2 (Middle) and exploration ratios during T2 (Right). T2 Data were analyzed by two-way ANOVA (group × object) with post-hoc tests. n=9-10. T1 exploration time was not significant for all groups. A. OIL vs. 5ug/kg of E2. Significant interaction (F 1, 16= 10.49, p<0.005) was found and exploration ratios (p<0.04) were different from the control. B. OIL vs. 10ug/kg of E2. Significant interaction (F 1, 17=9.038, p<0.008) was found. C. OIL vs. 15ug/kg of E2. Not significant by ANOVA. Entries are means ± SEM. * P < 0.05, ** p<0.01
The dose-response relationship was examined with 0, 5, 10 and 15ug/kg doses of 17β-estradiol given in the same experiment. During the sampling trials, no significant group differences in exploration times were found. During T2, significant group differences in exploration ratios were found (F 3, 52=3.604, p<0.019) with only 5ug/kg group being different from control (p<0.021). Thus, the dose-response relationship for OR is like OP, an inverted U, and the greatest memory enhancement is at 5μg/kg of 17β-estradiol (Fig. 3B).
Acute 17α-estradiol; Effects on object placement (OP)
Because a number of studies show affinity of 17α-estradiol for membrane associated estrogen receptors (Green et al, 1997; Singh et al, 2000; Wade et al., 2001; Toran-Allerand et al, 2002, 2005; MacLusky et al, 2005a), acute, 17α-estradiol effects on memory consolidation were also investigated. We first tested 20μg/kg of 17α-estradiol since this dose of 17β-estradiol was effective for OP memory enhancement, however, no difference was found. Since OP memory is enhanced at a lower dose of 17α- than 17β-estradiol when treatment is given 30 min before T1 (Luine et al, 2003), we examined dose-response relationship with several lower doses (0, 2, 5, 10μg/kg; n=8-9 in each group). No significant differences in exploration times (12.34-19.11s) were found during T1. Fig. 6A shows the mean exploration ratios of the 0μg (ratio=0.47), 2μg/kg (ratio=0.51), 5μg/kg (ratio=0.68) and 10μg/kg (ratio=0.54) group during the T2 retention trial, and we found a significant difference (F 3, 30=4.06, p<0.015) with the 5μg/kg group being significantly different from the control group (p<0.0019). Thus, the dose-response relationship between 17α-estradiol and OP memory consolidation is an inverted U like 17β-estradiol, but the effective dose of 17α-estradiol is four times lower.
FIG.6.
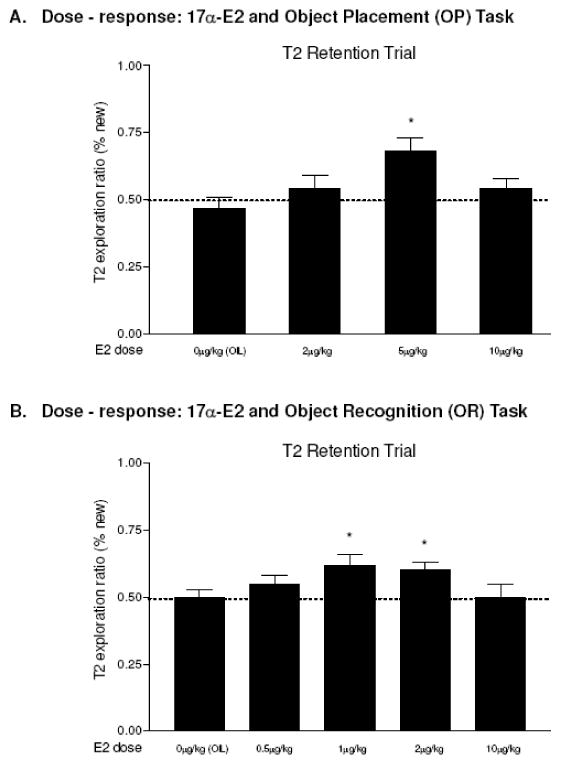
Dose-response for 17α-E2 and memory performance. Data were analyzed by one-way ANOVA with post-hoc test (Fisher LSD). The dashed line at the exploration ratio of 0.5 indicates chance level performance (rats spent equal amount of time around objects at old and new locations). A. Object Placement (OP). ANOVA was significant, F 3, 30=4.06, p<0.015, and 5μg/kg of E2 enhanced OP memory (p<0.0019). B. Object Recognition (OR). ANOVA was significant, F 4, 38=2.763, p<0.041 and both 1μg/kg (p<0.013) and 2ug/kg (p<0.02) of E2 enhanced memory. Entries are means ± SEMs. *p<0.05.
Acute 17α-estradiol; Effects on object recognition (OR)
Finally, we examined the dose-response relationship between 17α-estradiol and OR memory consolidation with 0, 0.5, 1, 2, and 10ug/kg (n=8-9 in each group), and a similar pattern of results to OP was obtained. During T1, rats in all groups spent a similar amount of time (16.43-21.51 s) around the objects. There was a significant group difference in exploration ratios during T2 (Fig. 6B, F 4, 38=2.763, p<0.041) and both 1μg/kg (ratio=0.62, p<0.013) and 2ug/kg (ratio=0.60, p<0.020) groups were significantly different from the control group (ratio=0.50), indicating these doses enhanced OR memory consolidation. Thus, both high and low doses of 17α-estradiol were ineffective, while the intermediate doses facilitated OR memory, revealing a similar inverted U dose-response pattern to 17β-estradiol effects on OR. The most effective doses of both isomers of estradiol for OP and OR memory consolidation are summarized in Table 1.
Table1.
Summary of effective estradiol doses for acute enhancements in memory consolidation
| Memory task | 17β-estradiol | 17α-estradiol |
|---|---|---|
| Object Placement (OP) | 20μg/kg | 5μg/kg |
| Object Recognition (OR) | 5μg/kg | 1, and 2μg/kg |
Monoamines, metabolites and turnover ratios
To determine whether the same dose of 17β-estradiol that enhanced OP memory (20μg/kg) also alters neurochemical levels, monoamines, metabolites, and turnover ratios were measured during the period of memory consolidation. We found significant main effects and/or interactions in the PFC, the CA1 and the DG of the hippocampus, the striatum and the vDB. Data in each brain area and post hoc results are summarized in Table 2.
Table 2.
Entries are average pg/mg protein concentration ± SEM for vehicle-treated control (n=6) and 20μg/kg of 17β-E2 (n=6) treated rats. Values of turnover ratios are multiplied by 10. Data were analyzed by two-way ANOVA (group x neurochemical) in each brain region with two-sample t tests as post hoc comparison. The arrows show significant increase or decrease compared to the control. T p<0.073, *p<0.05, **p<0.01, ***p<0.001. NE, norepinephrine; MHPG, metabolite 3-Methoxy-4-Hydroxyphenylglycol; DA, dopamine; HVA, homovanillic acid; DOPAC, 3-4-dihydroxyphenylalanine; 5-HT, serotonin; 5-HIAA, 5-hydroxyindole acetic acid. Effects of 17β-E2 (20μg/kg) on monoamines, metabolites and turnover ratios in brain areas
| Monoamines Metabolites | Treatment | PFC | CA1 | DG | vDB | Striatum |
|---|---|---|---|---|---|---|
| NE | Control | 3.20 ± 0.19 | 2.04 ± 0.30 | 2.80 ± 0.16 | 2.89 ± 0.23 | 1.67 ± 0.28 |
| E2 | 3.58 ± 0.29 | 1.00 ± 0.15 **↓ | 2.48 ± 0.27 | 2.66 ± 0.20 | 2.12 ± 0.37 | |
| MHPG | Control | 3.11 ± 0.26 | 18.45 ± 2.64 | 3.34 ± 0.35 | 13.18 ± 1.42 | 14.82 ± 1.49 |
| E2 | 6.38 ± 0.41 ***↑ | 12.63 ± 0.69 ⊤↓ | 2.17 ± 0.22 *↓ | 11.35 ± 1.07 | 14.07 ± 0.99 | |
| DA | Control | 0.58 ± 0.09 | 0.46 ± 0.06 | 0.19 ± 0.03 | 20.34 ± 2.82 | 55.26 ± 1.54 |
| E2 | 0.72 ± 0.08 | 0.46 ± 0.07 | 0.20 ± 0.02 | 16.45 ± 2.08 | 69.35 ± 2.37 ***↑ | |
| DOPAC | Control | 0.45 ± 0.05 | 0.15 ± 0.05 | 0.15 ± 0.02 | 1.76 ± 0.25 | 6.10 ± 0.57 |
| E2 | 0.69 ± 0.06 ** ↑ | 0.13 ± 0.03 | 0.15 ± 0.02 | 2.17 ± 0.33 | 7.34 ± 0.63 | |
| HVA | Control | 5.30 ± 0.44 | 0.12 ± 0.02 | 0.09 ± 0.01 | 0.15 ± 0.01 | 0.56 ± 0.07 |
| E2 | 5.83 ± 0.55 | 0.12 ± 0.02 | 0.06 ± 0.01 | 0.24 ± 0.04 | 0.58 ± 0.03 | |
| 5-HT | Control | 2.23 ± 0.23 | 0.71 ± 0.09 | 0.89 ± 0.12 | 2.59 ± 0.20 | 0.76 ± 0.05 |
| E2 | 2.83 ± 0.15 *↑ | 0.79 ± 0.08 | 0.76 ± 0.15 | 2.83 ± 0.40 | 0.72 ± 0.07 | |
| 5-HIAA | Control | 4.09 ± 0.32 | 2.06 ± 0.21 | 2.17 ± 0.22 | 2.28 ± 0.09 | 1.67 ± 0.05 |
| E2 | 4.90 ± 0.29 | 2.20 ± 0.24 | 1.95 ± 0.31 | 2.57 ± 0.29 | 1.67 ± 0.04 | |
| Turnover ratios (× 10) | Treatment | PFC | CA1 | DG | vDB | Striatum |
| MHPG/NE | Control | 9.78 ± 0.76 | 91.47 ± 4.81 | 12.38 ± 1.29 | 46.54 ± 5.15 | 105.55 ± 2.60 |
| E2 | 18.07 ± 1.00 *** ↑ | 105.50 ± 14.43 | 9.10 ± 1.48 | 42.84 ± 2.82 | 78.80 ± 1.61 | |
| HVA/DA | Control | 97.10 ± 10.77 | 3.62 ± 1.06 | 5.02 ± 0.66 | 0.09 ± 0.01 | 0.10 ± 0.00 |
| E2 | 85.18 ± 10.09 | 2.85 ± 0.97 | 3.48 ± 0.36 ⊤↓ | 0.13 ± 0.01 *↑ | 0.09 ± 0.00 | |
| DOPAC/DA | Control | 8.36 ± 1.36 | 5.30 ± 2.50 | 8.98 ± 1.29 | 0.90 ± 0.09 | 1.11 ± 0.01 |
| E2 | 9.90 ± 0.87 | 2.70 ± 0.24 | 8.78 ± 1.24 | 1.32 ± 0.12 *↑ | 1.30 ± 0.03 | |
| 5-HIAA/5-HT | Control | 18.57 ± 0.91 | 31.00 ± 3.87 | 25.73 ± 3.13 | 9.06 ± 0.67 | 22.50 ± 0.17 |
| E2 | 17.50 ± 1.16 | 26.57 ± 1.02 | 27.75 ± 2.71 | 9.81 ± 1.28 | 24.70 ± 0.29 | |
The prefrontal cortex
Significant main effects of group (F 1, 70=31.37, p<0.001), monoamines (F 6, 70=97.24, p<0.001) and interaction (F 6, 70=7.34, p<0.001) were found in the PFC. In general, 17β-estradiol treated OVX rats had substantially higher levels of monoamines and metabolites compared with the vehicle-treated controls. Specifically, 17β-estradiol significantly increased DA metabolite DOPAC concentration (54% higher than control, p<0.01), NE metabolite MHPG concentration (105% higher than control, p<0.001), and 5-HT concentration (27% higher than control, p<0.05). For activity levels, the turnover ratio of NE to MHPG for the estradiol treated group was 85% higher than the vehicle-treated control (p<0.001). Thus, 20μg/kg of 17β-estradiol rapidly and substantially influenced all monoaminergic systems in the PFC, showing significant elevations in either transmitters or metabolites.
The hippocampus
We found significant main effects of group (F 1, 70=5.75, p<0.01), monoamines (F 6, 70=114.13, p<0.001), and interaction (F 6, 70=4.35, p<0.001) in the CA1of the hippocampus, and significant main effects of group (F 1, 70=7.50, p<0.007), monoamines (F 6, 70=89.61, p<0.001), and interaction (F 6, 70=2.70, p<0.02) in the DG of the hippocampus.
There was a general tendency for noradrenergic neurochemicals to be decreased in the hippocampus of the estradiol treated rats. In the CA1 of the hippocampus, for example, NE concentrations of the estrogen treated rats decreased 49% compared to vehicle-treated control (p<0.01). Although it was not statistically significant (p<0.058), NE metabolite MHPG levels also declined, an approximately 32% reduction, in the CA1 of the estradiol treated rats. There were no changes in levels of DA and 5HT neurochemicals in the CA1 after E2.
Similarly in the DG, levels of the NE metabolite, MHPG, were significantly decreased in E2 treated rats, an approximately 35% reduction, compared to controls (p<0.018). HVA/DA turnover ratios also showed a trend toward reduction, (31%; p<0.068). Thus, these data demonstrate that, in contrast to the PFC, 20μg/kg of 17β-estradiol significantly decreased some monoamine concentrations and activity levels in hippocampal areas.
Striatum and vDB
In both areas, acute 17β-estradiol affected only the DA system. In the striatum, significant main effects of group (F 1, 70=18.63, p<0.00), monoamines (F 6, 70=167.05, p<0.001), and interaction (F 6, 70=16.26, p<0.001) were found. DA levels in estradiol treated rats were significantly higher than control rats (26% above control, p<0.001). In the vDB, a significant group x neurochemical interaction (F 6, 69=2.23, p<0.05) was found. E2 significantly increased turnover ratios of HVA/DA (52% above control, p<0.037) and DOPAC/DA (46% above control, p<0.024).
Discussion
The current study examined acute effects of estrogen on memory consolidation and determined dose-response characteristics between hormone treatment and OP (spatial memory) and OR (non-spatial memory) tasks. In addition, we compared levels of monoamines and metabolites in vehicle-treated and estrogen treated OVX rats in brain areas involved in memory function. The present data are consistent with previous findings that post-sampling injection of 17β-estradiol facilitates memory consolidation, and provide novel findings that 17α-estradiol also exerts dose-specific effects on OP and OR memory performance, which supports a recent finding that low doses of 17α-estradiol enhanced contextural fear conditioning, while high doses did not (Barha et al., 2010). Some important relationships between acute estrogen treatment and memory function include : (1) dose-response patterns are an inverted-U for both estrogen isomers in the tasks: (2) the effective time window for the post-sampling treatment is narrower (45min) than previously reported (2hr): (3) non-spatial memory is enhanced at much lower doses of estrogens than spatial memory: (4) 17α-estradiol is more potent than 17β-estradiol in enhancing memory: (5) activity of monoaminergic terminals in areas important for memory, the PFC, hippocampus, vDB and the striatum, are affected by 17β-estradiol at a dose and during the time when enhancement of spatial memory consolidation occurs.
Dose-response patterns
The present study demonstrates that acute post-sampling exposure to 17-β estradiol and/or 17α-estradiol results in rapid memory enhancement when retention is tested 4hr later. This result is compatible with previous findings from our lab (Luine et al, 2003) and others (Rhodes and Frye, 2004), and provides further support for the hypothesis that estrogens have the ability to improve memory rapidly, within a few hours after treatment. Moreover, our data indicate that both isomers of estradiol enhance memory consolidation in a dose-specific manner and that the dose-response patterns are not a classic sigmoid curve, but appear to be an inverted-U. These results are consistent with previous work showing a similar inverted-U dose-response curve using other types of spatial memory tasks, different routes of administration, and longer inter-trial delays in male (Packard et al., 1996) and female rats (Packard and Teather, 1997a, 1997b) and mice (Gresack and Frick, 2006b). Packard and Teather (1997a), for example, utilized a Morris water maze to accesses spatial memory and demonstrated that doses higher and lower than 200μg/kg of 17β-estradiol did not affect retention when tested 24hr after intraperitoneal (ip) injection in a post-training treatment paradigm. This finding was recently replicated by Gresack and Frick (2006b) using the same task, doses and IPinjection in OVX mice. In addition, the current results show that 17α-estradiol exhibits dose-response patterns similar to an inverted-U in both spatial and non-spatial memory tasks. Thus, an inverted U pattern of hormonal effects may be generalized across memory tasks, routes of administration, lengths of inter-trial delays, and types of estrogen isomers.
In fact, this response pattern is not surprising in biological and toxicological studies (Baldi and Bucherelli, 2005; Calabrese and Baldwin, 2001a, 2001b, 2003; Calabrese and Blain, 2005; Zoladz and Diamond, 2008), and inverted-U dose-responses are relatively common characteristics for the post-training injection effects of some hormones, drugs, environmental chemicals and neurotransmitters on performance in memory and learning tasks (Boccia et al., 1998; Clark et al., 1998; Flood et al., 1987; Okuda et al., 2004; Roozendaal, 2000). Packard (1998) argued that an inverted U might represent optimal levels of receptor activation necessary for memory enhancing effects. In this hypothesis, both too little and too much agonist is ineffective because lower doses produce sub-optimal levels of receptor activation, whereas higher doses produce supra-optimal levels which adversely affect receptor activation.
Although mechanisms underlying estrogen effects on memory are not clear, evidence indicates that estrogens exert direct effects on hippocampal synaptic plasticity (see McEwen, 2002; Woolley, 2007; and Spencer et al., 2008 for review) and an association between estrogen-mediated changes in spine density and performance in memory tasks has been reported (McLaughlin et al, 2008; Li et al, 2004; Wallace et al, 2006, 2007). A recent study demonstrated that both isomers of estradiol rapidly altered dendritic spine synapse density and, interestingly, lower doses of 17α-estradiol (15μg/kg) produced greater increases than higher doses (45μg/kg) of the same hormone (MacLusky et al, 2005a), suggesting that dose-response relationships between 17α-estradiol and spine synapse density may also be an inverted U. Thus, their results are consistent with Packard's optimal receptor activation hypothesis, and may account for the inverted U dose-response effects of acute treatment on memory consolidation observed in the current study.
In contrast to the similar dose-response patterns seen in the current and previous studies, the effective doses of 17β-estradiol for spatial memory consolidation were markedly higher in the previous studies, 200μg/kg, (Fernandez et al, 2008; Gresack and Frick, 2006b; Harburger et al., 2009; Lewis et al, 2008; Packard and Teather, 1997a) than in the current study, 20μg/kg. This discrepancy most likely results from differences in length of inter-trial delays, demands of memory tasks, as well as routes of hormone administration. In the current study, retention was tested 4 hr after hormone treatment, while all previous studies tested memory 24and/or 48 hr after treatment. Therefore, it is possible that longer inter-trial delays require more estrogen to enhance memory. Different demands in memory tasks may also influence the dose-response. In this study, spatial memory was measured on the object placement task, which is a relatively stress-free memory task as it uses animal's novelty preference. On the other hand, the Morris water maze is a relatively stressful task as animals are forced to swim to locate a hidden platform. High levels of corticosterone in the limbic system and the hippocampus during the course of the Morris water maze have been reported (Aguilar-Valles et al., 2005). In such environment, higher doses of estrogen might be required to enhance memory consolidation. Alternatively, dose-response relationships for acute post-training/sampling estrogen may not be a simple inverted U, but rather a non-monotonic curve with multiple effective doses (ineffective doses between effective doses) or bimodal dose-responses. Some hormones, environmental chemicals and endocrine disruptors show these non-monotonic dose-response relationships following acute administration in cell culture lines, including time and dose-dependent effects of 17β-estradiol on ERK activation levels (Watson et.al, 2007), and membrane-initiated action of Bisphenol A, an estrogenic environmental chemical, on prolactin release (Wozniak et al., 2005). It is important to note that the lowest tested dose of 17β-estradiol in the previous acute post-training studies (Gresack and Frick, 2006b; Packard and Teather, 1997a) was 100μg/kg, whereas the present study tested the dose range of 10-60μg/kg of 17β-estradiol. Therefore, there is a possibility that both 20 and 200μg/kg are effective but doses in between are ineffective. Since no study has explored this possibility, further research is necessary to investigate possible bimodal dose-responses between acute estrogen and memory consolidation processes.
Time-limited effects of acute estrogen treatment
Previous studies have shown that acute estrogen treatments enhance memory given up to 2hr after a training or sample trial (Luine et al., 2003; Packard et al., 1997a). We evaluated this effect with 20μg/kg of 17β-estradiol given immediately, or 45 min after the sampling trial. Confirming previous findings, OVX rats given estrogen immediately after the sample trial had better spatial memory than the vehicle-treated control (Fig. 4A, right), but OVX rats receiving the treatment 45 min after the sample trial did not, a narrower critical time window than previously reported 2hr (Fig. 4B, right). The differences between this and previous studies are probably due to different inter-trial delays (e.g., 4hr vs. 24hr, respectively) and/or effective estrogen doses (e.g., 20μg/kg vs. 200μg/kg, respectively).
Interestingly, a similar time-dependent effects of acute estrogen treatment was also reported in a neural plasticity study (MacLusky et al., 2005a), which found that increases in dendritic spine synapse density in the hippocampus were greater at 30 min after acute estrogen treatment than that at 4.5 hr regardless of increased circulating estrogen levels over time. MacLusky et al argued that the decline at 4.5 hr and greater potency of the lower dose might reflect down-regulation of the response mechanism. In addition, these biphasic temporal effects of estrogen on hippocampal plasticity suggest that responses may involve different mechanisms; membrane ER mediated responses for the initial rapid increase in spine synapse density at 30 min after treatment, and nuclear mediated responses or integrated actions of genomic and nongenomic pathways for the subsequent decrease at 4.5 hr after treatment.
It should be noted that one possible confound of the current study is that injected hormones might not be fully metabolized and present in the circulating system when animals are tested 4 hr later, and therefore, it is possible that estrogen might have enhanced the retrieval process rather than consolidation and that potential non-mnemonic effects of estrogen might have affected memory performance during retention. But this possibility is unlikely because treatments given immediately after the sample trial enhance memory, while treatments administrated 45 min after the sample trial have no effects.
Optimal doses for spatial vs. non-spatial memory consolidation
The current results indicate that the same dose of estrogen which enhances object memory consolidation, 5μg/kg, is not effective for place memory consolidation. Four times the dose of estradiol (20μg/kg) was necessary to enhance OP memory, indicating that non-spatial memory maybe more sensitive to estradiol than spatial memory. These differences may be due to differential effects of estrogen in distinct brain regions critical for these two types of memories. Although both are hippocampal dependent working memory tasks, object placement is primarily dependent on an intact hippocampus and/or fornix (Ennaceur and Aggleton, 1994), and may also rely on prefrontal cortical input (Ennaceur et al., 1997). On the other hand, object recognition is less dependent on the hippocampus and requires prefrontal cortical activity (Ennaceur et al., 1997). This notion is evidenced by the demonstration that lesioning of the hippocampus impairs memory performance in object placement task, but caused lesser effects in the object recognition task (Broadbent et al., 2004; Mumby et al., 2002). For example, Broadbent et al showed that damage to 30–50% of the dorsal hippocampus caused spatial memory impairment, while lesions of 75–100% were required to impair object recognition memory.
In addition, differences in task demands may also account for different effective doses for object placement and recognition memory, as the cognitive load for spatial memory is greater than non-spatial object recognition memory (Ennaceur et al., 2005). Indeed, objects can be encoded and discriminated through multiple sensory modalities (e.g., vision and tactile) using a variety of cues such as the size, shape, color (wavelength as well as intensity) and texture of objects, while discriminating location of objects involves abstract categorizations and use of “cognitive maps”. Thus, it is consistent with the current result that object memory consolidation is facilitated at lower estrogen doses compared to spatial location memory.
17α-estradiol is more potent than 17β-estradiol
Interestingly, the present results indicate that post-sampling 17α-estradiol was more effective than 17β-estradiol for enhancement of memory consolidation. As shown in Fig. 3 and Fig. 6, about 4-5 fold lower doses of 17α-estradiol, as compared to 17β-estradiol, enhance memory. These results are particularly noteworthy because binding affinity of 17α-estradiol for classic nuclear estrogen receptors, ERα and ERβ, is considerably weaker (42% lower for ERα and 89% lower for ERβ) than 17β-estradiol (Kuiper, et al., 1997), and, therefore, 17α-estradiol is generally considered less active in the brain and does not elicit significant estrogenic responses (Anstead et al., 1997). Thus, our results challenge the general notion of effectiveness of 17α-estradiol and raise a possibility that observed acute estrogen effects on memory consolidation may be mediated through membrane associated estrogen receptor systems. Supporting this hypothesis, several lines of evidence indicate that 17α-estradiol has an equal or even stronger biding affinities to membrane associated ERs (Green et al., 1997; Toran-Allerand et al., 2005; Wade et al., 2001) and can elicit greater estrogenic responses rapidly (MacLusky et al., 2005a; Toran-Allerand et al., 2005). For example, 17α-estradiol has an equal potency to 17β-estradiol to elicit rapid and sustained activation of the MAPK/ERK and phosphatidylinositol 3-kinase-Akt signaling pathways (Singh et al., 2000), and 17α-estradiol is considerably more potent than 17β-estradiol in rapid elevation of hippocampal CA1 dendritic spine synapse density (MacLusky et al., 2005a), which parallels the current behavior data. Interestingly, recent studies have reported that 17-β and 17α-estradiol might be synthesized locally in the brain and affects neural functions rapidly within the seconds to minutes (Hojo et al., 2008; Toran-Allerand et al., 2005; also see Woolley, 2007 for review). In intact rats, Toran-Allerand et al (2005) measured estrogen concentrations in several tissues including the hippocampus, adrenals, ovaries and uterus, and found higher levels of 17α-estradiol than 17β-estradiol in all samples of the brain. In OVX and adrenalectomized mice, 17α-estradiol was also measured in the brain, while 17β-estradiol was not. Although functional effects of elevated 17α-estradiol are not yet known, 17α-estradiol may exert autocrine and/or paracrine effects in the brain (Toran-Allerand et al., 2005), one of which might be on cognitive function.
Acute estrogen altered levels of monoamines
Significant changes in monoamine and metabolite levels, as well as turnover ratios, were found in the PFC, the hippocampus, vDG, and the striatum of OVX rats following acute, 20μg/kg of 17β-estradiol treatment. Thus, our results demonstrate that the same dose of 17β-estratiol that enhanced spatial memory consolidation also altered neurochemical levels, within 30 min after the post-sampling treatment and further suggest that observed rapid changes in monoaminergic activities may contribute to estradiol's enhancement of spatial memory consolidation. Of note is the finding that this dose of 17β-estradiol generally increased neurochemical levels in the PFC, but decreased them in the hippocampus. These results suggest altered neurochemical levels in the PFC may play an important role for spatial memory consolidation. Although spatial memory is considered to be primarily hippocampal rather than PFC dependent, several studies have reported that the PFC-hippocampal projections may be critical for both spatial and non-spatial memory (Floresco et al., 1997; Seamans et al., 1998; Thierry et al., 2000; Wang and Cai, 2006), and suggest possible bidirectional modulation of synaptic strength based on the specific demands of tasks (see Laroche et al., 2000 for review). The current neurochemical and behavior results are consistent with this hypothesis, although it is important to note that in the current behavioral experiments this dose of 17β-estradiol did not enhance OR memory performance.
Several lines of evidence indicate that the PFC is extremely sensitive to changes in the neuromodulatory inputs from NE and DA systems (Arnsten et al., 1997, 2002, 2006; Robbins and Arnsten, 2009). Both DA and NE have beneficial effects on working memory functions in the PFC. However, studies have shown that both excessive and insufficient levels of DA and NE in the PFC induce cognitive impairment, including deficits in spatial working memory in human, rats and monkeys (Murphy et al., 1996a, 1996b; Arnsten 1997, 2007; Zahrt et al., 1997). DA enhances PFC function via D1 receptors, which stimulation increases production of cAMP, while high DA levels decrease it through D2 receptors (Trantham-Davidson et al., 2004). Similarly, NE enhances working memory via α-2 adrenergic receptors and a-1 receptors have opposing effects (Arnsten, 1997). Thus, evidence suggests DA has an inverted U dose-response relationship at D1 receptors (Arnsten and Robbins, 2002; Vijayraghavan et al., 2007), that is, both too little and too much stimulation of D1 receptors impaired working memory in rats (Arnsten, 1997, 2007), and effects may be time-dependent (Hotte et al., 2005). Arnsten (1997) argued that memory impairment occurs with endogenous release of both DA and NE during stress and with the exogenous administration of high and low doses of D1 and α-1 agonists. These observations suggest critical levels of DA and NE are crucial for the optimal PFC functions. In the present study, we observed significantly higher levels of DA and NE metabolites in the PFC following 20μg/kg of estradiol, which suggests more activity in monoaminergic neurons. Thus, increased levels of these neurochemicals in the PFC may contribute to enhancement of spatial memory consolidation, but this change may be excessive for object memory consolidation, and therefore, no beneficial effects were observed in the OR task.
Although mechanisms underlying the function of NE in memory processing are not clear, it has been reported that NE modulates the efficacy of glutamate transmission activating G-protein coupled adrenergic receptors (Scheiderer et al., 2004). Several electrophysiological studies have shown that NE promotes long-term potentiation or LTP in the adult rat hippocampus (Izumi and Zorumski, 2004) and also induces long-term depression or LTD (Scheiderer et al., 2004). Both LTP and LTD are important for certain types of memory formation and considered the cellular model of learning and memory (Kemp and Manahan-Vaughan, 2004: Scheiderer et al., 2004). Specifically, novelty acquisition is associated with induction of hippocampal LTD (Manahan-Vaughan and Braunewell, 1999: Kemp and Manahan-Vaughan, 2007), and performance in a spatial memory task was significantly correlated with the magnitude of LTD in hippocampus (Nakao et al, 2002). Moreover, using a behavior test similar to the OP/OR task, Kemp and Manahan-Vaughan (2004) demonstrated that induction of LTD was correlated with encoding the object location within a spatial context rather than recognition of object themselves, and not triggered by exploration behavior in space. They also showed LTD/LTP induction was regulated by 5-HT4 receptor activation. These findings are particular noteworthy because in our behavior study vehicle-treated OVX rats did not remember the locations of the objects, and showed significantly lower levels of 5-HT in the PFC and higher levels of NE in the CA1 of vehicle-treated rats as compared with 20μg/kg E2 treated rats, while rats that received acute estrogen treatment immediately after the novelty acquisition did remember the locations of objects and had higher levels of 5-HT and lower levels of NE. Therefore, it appears that these neurochemical changes observed in 20μg/kg group might exert “buffering” effects on LTD expression, which in turn resulted in better memory performance in the OP task.
It should be noted that in the current study monoamine and metabolite concentrations were measured with the rats that had been repeatedly used for behavioral tests. Also the rats were given a sample trial 30 min before sacrificed. Therefore it is possible that observed neurochemical changes might be a result of an interaction of behavioral experiences and estrogen treatment. Some studies, for example, have reported that stress levels (Bowman et al., 2002, 2003) and food reward (Beck and Luine, 1999) alters monoamine levels. But this possibility is unlikely since we used extensively habituated subjects to minimize stress and novelty effects, and, as described in Methods section, food reward/training are not required for the memory tasks used in the current study.
In conclusion, the current behavioral and neurochemical data provide new information about acute effects of estrogen on mnemonic function and raise the possibility that monoamines play a role in activating membrane associated neurochemical cascades, which contribute to memory consolidation.
Acknowledgments
The authors thank to G. Mohan, K. Monde, and P. Snyder for assistance in behavior testing, and Dr. Maya Frankfurt for advice and help. This work was part of the dissertation thesis of T. Inagaki. This research was supported by NIH grant GM60654, RR-TT03037 and CUNY Research Grants for Doctoral students.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Valles A, Sanchez E, de Gortari P, Balderas I, Ramirez-Amaya V, Bermudez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82:306–19. doi: 10.1159/000093129. [DOI] [PubMed] [Google Scholar]
- Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids. 1997;62:268–303. doi: 10.1016/s0039-128x(96)00242-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Robbins TW. Neurochemical modulation of prefrontal cortical function in humans and animals. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 51–84. [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–83. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17 1:i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C. The inverted “u-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity Biol Toxicol Med. 2005;3:9–21. doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA. Low Doses of 17alpha-Estradiol and 17beta-Estradiol Facilitate, Whereas Higher Doses of Estrone and 17alpha- and 17beta-Estradiol Impair, Contextual Fear Conditioning in Adult Female Rats. Neuropsychopharmacology. 2010;35:547–59. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: Role of monoamines and amino acids. Brain Research. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–73. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Chronic D-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav. 2003;74:859–67. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Kopf SR, Baratti CM. Effects of a single administration of oxytocin or vasopressin and their interactions with two selective receptor antagonists on memory storage in mice. Neurobiol Learn Mem. 1998;69:136–46. doi: 10.1006/nlme.1997.3817. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav. 2007;52:237–43. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–10. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav. 2009;97:21–9. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–20. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001a;62:330–8. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. U-shaped dose-responses in biology, toxicology, and public health. Annu Rev Public Health. 2001b;22:15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. The hormetic dose-response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246–50. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–73. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–20. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–23. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Dohanich G. Gonadal steroids, learning, and memory. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. Vol. 2. San Diego: Academic Press; 2002. pp. 265–327. [Google Scholar]
- Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–19. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res. 2005;159:247–66. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–7. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Cherkin A, Morley JE. Antagonism of endogenous opioids modulates memory processing. Brain Res. 1987;422:218–34. doi: 10.1016/0006-8993(87)90929-2. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–90. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–26. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol's effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–16. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–83. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW. 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci. 1997;17:511–5. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006a;1115:135–47. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006b;84:112–9. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007a;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Res. 2007b;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–34. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Hotte M, Naudon L, Jay TM. Modulation of recognition and temporal order memory retrieval by dopamine D1 receptor in rats. Neurobiol Learn Mem. 2005;84:85–92. doi: 10.1016/j.nlm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Norepinephrine promotes long-term potentiation in the adult rat hippocampus in vitro. Synapse. 1999;31:196–202. doi: 10.1002/(SICI)1098-2396(19990301)31:3<196::AID-SYN4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–60. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–7. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–8. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–46. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–21. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–90. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN. Neuroendocrinology of Memory and Cognitive Function. In: Lajha Abel, Blaustein Jeffrey D., editors. Handbook of Neurochemistry and Molecular Neurobiology Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. Chap.21. Springer-Verlag; Berlin Heidelberg: 2006. pp. 775–800. [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol. 2007;19:743–51. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–72. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005a;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Environ Health Perspect. Vol. 113. 2005b. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis; pp. 675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–44. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17beta-estradiol effectiveness in altering CA1 spines. Horm Behav. 2008;54:386–95. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996a;93:1325–9. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996b;16:7768–75. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Ikegaya Y, Yamada MK, Nishiyama N, Matsuki N. Hippocampal long-term depression as an index of spatial working memory. Eur J Neurosci. 2002;16:970–4. doi: 10.1046/j.1460-9568.2002.02159.x. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–8. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav Neurosci. 1996;110:626–32. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997a;68:172–88. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997b;8:3009–13. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–39. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav. 2004;78:551–8. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–38. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J Neurophysiol. 2004;91:1071–7. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–21. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–37. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–9. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–50. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–9. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–42. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–82. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V. Impaired recognition memory and decreased prefrontal cortex spine density in aged female rats. Ann N Y Acad Sci. 2007;1097:54–7. doi: 10.1196/annals.1379.026. [DOI] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–36. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007b;72:124–34. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Wozniak AL, Bulayeva NN, Watson CS. Environ Health Perspect. Vol. 113. 2005. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells; pp. 431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–35. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Linear and Non-Linear Dose-Response Functions Reveal a Hormetic Relationship Between Stress and Learning. Dose Response. 2008;7:132–148. doi: 10.2203/dose-response.08-015.Zoladz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


