Abstract
Monogamous species are usually considered to be less likely to exhibit sex differences in behavior or brain structure. Most previous studies examining sex differences in stress hormone responses have used relatively sexually dimorphic species such as rats. We examined the stress hormone responses of monogamous California mice (Peromyscus californicus) to resident-intruder tests. We also tested males and females under different photoperiods, because photoperiod has been shown to affect both aggression and stress hormone responses. Females, but not males showed a significant increase in corticosterone levels immediately following a resident-intruder test. Males but not females showed elevated corticosterone levels under short days. Females tested in aggression tests also showed a significant increase in plasma oxytocin levels, but only when housed in long days. This was consistent with our observation that females but not males had more oxytocin positive cells in the paraventricular nucleus (PVN) when housed under long days. Our data show that sex differences in glucocorticoid responses identified in other rodents are present in a monogamous species.
Keywords: oxytocin, glucocorticoids, paraventricular nucleus, Peromyscus californicus
Introduction
Sex differences in physiological responses to stress are hypothesized to play an important role in behavioral responses to stress. A substantial literature in domestic rats and some mouse strains has documented that females have exaggerated glucocorticoid secretion in response to stress compared to males and that this difference is mediated in part by ovarian steroids (Critchlow et al. 1963; Akinci and Johnston 1993; Jones et al. 1998; Rivier 1999; Weiser and Handa 2009). Intriguingly, two paradigms that are widely used to assess stress responses in humans report exactly the opposite results. Both the Trier Social Stress Test and cold pressor task (Dixon et al. 2004; Helfer and McCubbin 2008) induce larger cortisol responses in males compared to females. Currently the discrepancy between rodent and human studies is not understood. One strategy for resolving these differing results is to investigate the mechanisms regulating stress hormone responses (e.g. Weiser & Handa 2009). A complementary approach is to examine stress hormone responses under different conditions. In general rodent studies examining sex differences in stress responses have been conducted under standard laboratory conditions in species with similar social systems. The environment and other social stimuli can have important effects on the regulation of neuroendocrine systems, including the hypothalamic-pituitary-adrenal (HPA) axis.
Social conflict and aggressive interactions are known to activate the release of glucocorticoids in a wide variety of species (Mendoza et al. 1979; Overli et al. 1999; Abbott et al. 2003), including humans (Gerra et al. 2007). Aggressive behaviors themselves are also tightly regulated, as these behaviors are energetically expensive and risky (Trainor and Marler 2010). In many rodents aggressive behaviors are regulated by photoperiod, which is a reliable predictor of environmental changes such as food availability and temperature (Nelson et al. 1990). In every species that has been examined, both male (Garrett and Campbell 1980; Jasnow et al. 2000; Caldwell and Albers 2004; Trainor et al. 2007; Trainor et al. 2008) and female (Fleming et al. 1988; Scotti et al. 2007; Silva et al. 2010) aggressive behaviors are increased in winter-like short days versus summer-like long days. Based on findings from birds and rodents, it has been hypothesized that adrenal steroids may regulate aggressive behaviors under short days (Soma et al. 2008). For example, adrenalectomy, but not adrenal demedullation blocks melatonin induced increases in aggression of long day housed male Siberian hamsters (Demas et al. 2004). It is less clear whether adrenal hormones could mediate the effect of photoperiod on aggression in females (but see (Gutzler et al. 2009). These data suggest that physiological stress responses to social conflict may be affected by photoperiod.
The majority of studies examining sex differences in stress responses have been conducted on domestic rats, a polygynous species in which males are larger than females. A relevant question then is whether sex differences in stress hormone responses observed in rats generalize to other species with different social systems. Prairie voles are socially monogamous and less sexually dimorphic than rats (Carter et al. 1995). A previous study examined the effect of single housing on corticosterone and oxytocin responses during aggression (Grippo et al. 2007). Single housing potentiated corticosterone secretion in females but not males, whereas single housing increased oxytocin in both males and females. However, these hormone levels were not directly compared to untested animals so it is difficult to determine whether these differences reflect long term or short term changes. Still, these results suggest that sex differences in corticosterone responses may indeed be present in species with monogamous social systems.
We examined hypothalamic, pituitary, and adrenal responses to resident-intruder aggression tests in male and female California mice (Peromyscus californicus). California mice are unique in that males and females form exclusive mating pairs (Ribble 1991). Male-female pairs defend joint territories from both male and female intruders (Ribble and Salvioni 1990), which explains the relatively high aggression levels observed among females relative to other rodent species (Davis and Marler 2003). Males and females were studied under both long day (16L:8D) and short day (8L:16D) photoperiods. Previous studies in California mice show that short days increase aggression in both males (Trainor et al. 2008; Trainor et al. 2009) and females (Silva et al. 2010). We compared plasma corticosterone and oxytocin responses immediately following resident-intruder aggression tests with control animals, and also measured oxytocin immunoreactivity in the paraventricular nucleus (PVN). We examined oxytocin because this hormone is hypothesized to have a buffering effect on glucocorticoids (Carter 1998; Neumann et al. 2000). Oxytocin is released peripherally during stress, although this response appears to be more context specific than glucocorticoid release (Gibbs 1984; Carter and Lightman 1987; Sanders et al. 1990), and there is growing evidence the effects of oxytocin may be stronger females versus males (Insel and Hulihan 1995; Young et al. 2001; Bales and Carter 2003; Goodson et al. 2009). Finally we used immunohistochemistry to measure phosphorylation of extracellular signal-regulated kinase (ERK) in the PVN as a marker of brain activity. Phosphorylation of ERK occurs rapidly, making it an ideal marker for changes in brain activity associated with rapid changes hormone secretion (Kwon et al. 2006). Using this integrative approach, we examined sex differences in hormonal and hypothalamic responses to social conflict.
Materials and Methods
Adult male and female California mice were bred in our lab colony and randomly assigned to be housed in long days (16L:8D) or short days (8L:16D) for 8 weeks. All testing procedures were approved by the UC Davis Institutional Animal Care and Use Committee. Animals were maintained in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The aggression tests and behavioral results have been described elsewhere (Trainor et al. 2009; Silva et al. submitted) and will be briefly described here.
Behavior Testing and Tissue Processing
Each mouse was individually housed for 3 days and then tested in either a resident-intruder test, aggression test or a control test in which the lid of the cage was removed and replaced to simulate an aggression test. All females were lavaged on the day of testing and were only used if in diestrus. All tests were conducted under dim red light 30 to 90 min after lights out (1400 Pacific Standard Time). Tests were 7 min for males and 10 min for females (due to lower aggression levels in females). All residents were tested with same-sex intruders that were unfamiliar and group-housed. Immediately after the test, each mouse was anesthetized with isoflurane and rapidly decapitated. The brain was rapidly removed and placed in 5% acrolein in phosphate saline buffer (PBS). Brains were cryoprotected in 25% sucrose and frozen at −40° C. Trunk blood was collected in heparinized tubes and centrifuged to collect plasma.
To determine whether slightly shorter aggression tests (7 min) for males versus females (10 min) could have affected our results, we conducted examined corticosterone levels in males tested in 10 min aggression tests. Six males were individually housed for 3 days under long day photoperiods. Each male was tested in a 10 min aggression test with a group-housed male intruder. Immediately after the aggression test each resident was anesthetized with isoflurane and a retroorbital blood sample was collected. Plasma was isolated and frozen for corticosterone assays.
Radioimmunoassay and Enzyme Immunoassay
Corticosterone was assayed using an I125 labeled radioimmunoassay kit (MP Biomedicals, Solon, OH) that has been used previously with California mice (Glasper and DeVries 2004). California mice have very high baseline corticosterone levels, so samples were diluted 1:2000. The sensitivity of this assay is 25 ng/mL. The intra-assay coefficient of variation was 2.99%. Plasma oxytocin was assayed using an enzyme immunoassay kit (Assay Designs, Ann Arbor, MI). When assay concentrations for serial dilutions of a California mouse plasma pool were compared with standards, computed regression lines did not differ in slope (P > 0.25). The sensitivity of this assay is 15.6 pg/mL. Plasma was diluted 1:2 for analysis and the inter- and intra-assay coefficients of variation were 3.5% and 5.32% respectively.
Immunohistochemistry
Brains were sectioned at 40 μm for immunohistochemistry. Alternate sections of the posterior PVN were stained with either pERK or OT corresponding to −0.82 and −0.94 in the mouse brain atlas (Paxinos and Franklin 2002). This location has been described as the lateral magnocellular PVN in California mice, with the caveat this region contains both parvocellular and magnocellular neurons (de Jong et al. 2009). Staining for pERK was conducted using the ABC method as previously described (Trainor et al. 2009). Sections were incubated in 1% sodium borohydride in PBS and then blocked in 10% normal goat serum and 0.3% hydrogen peroxide. Sections were incubated in rabbit anti-pERK (1:250, Cell Signaling, Danvers, MA) antibody dissolved in 2% normal goat serum and 0.5% triton X (TX) in PBS. Sections were washed in PBS and incubated in biotinylated goat anti-rabbit antibody (1:500, Vector Laboratories, Burlingame, CA). Sections were then washed in PBS and incubated in avidin-biotin complex (Vector Laboratories) and then washed again in PBS. Sections were developed in nickel-enhanced diaminobenzidine for 2 min (Vector Laboratories). Sections were mounted on slides, dehydrated and coverslipped with Permount (Fisher, Pittsburgh, PA).
Oxytocin in the PVN was visualized using immunofluorescence. Sections were incubated in 1% sodium borohydride and then blocked in 10% normal donkey serum. Sections were then incubated in mouse anti-OT (1:3000, Millipore, Billerica, MA) in 2% normal donkey serum and 0.5 % TX in PBS overnight at 4° C. Sections were washed in PBS and then incubated in DyLight 549 conjugated donkey anti-mouse antibody (1:500, Jackson Immunolabs, West Grove, PA) in PBS TX for 2 h. Sections were washed in PBS, mounted, and coverslipped with Vectashield (Vector Laboratories). For each mouse a control section was run in which the primary antibody was omitted. None of these sections showed positive staining. In a control experiment, sections of the PVN that were preincubated with oxytocin peptide (Sigma, St. Louis, MO) showed no positive staining.
Image Analysis
Slides were analyzed using a Zeiss Axioimager equipped with an Axiocam MRC camera. For all cell counts 2 images of the PVN were taken at approximately Bregma −0.82 and −0.94 and saved as a tiff file. For pERK stained slides images of the posterior PVN were taken under brightfield conditions. For OT stained slides a Zeiss 43HE filter (A:550 E:605) was used to visualize fluorescently stained OT slides. The number of immunopositive cells in each brain area was then counted in a box (0.18 × 0.23 mm) using Image J (NIH, Bethesda, MD) by an observer unaware of treatment assignments. A constant threshold of staining was set to determine positively stained cells, and the number of positive cells was counted using the “analyze particles” function of Image J. For each mouse, the number of cells was average across the 2 sections for statistical analyses. In the SON, there were few OT positive cells, so cell counts were conducted by eye.
Statistical Analyses
For variables in which males and females were run together in the same assay (corticosterone RIA, OT ELISA and immunohistochemistry), data were analyzed with three way ANOVA testing for sex, photoperiod, and behavior testing. For pERK immunostaining data, sections from males and females were run in separate assays, so these data were not analyzed in a single statistical analysis. For pERK cell counts, males and females were analyzed separately using two-way ANOVA testing for photoperiod and behavior testing. Planned comparisons were used to compare control and aggression tested animals within each sex and photoperiod combination. Spearman rank correlations were used to correlate behavior with hormone and cell count data. Corticosterone values were log transformed for analysis.
Results
Plasma Corticosterone and Oxytocin Levels
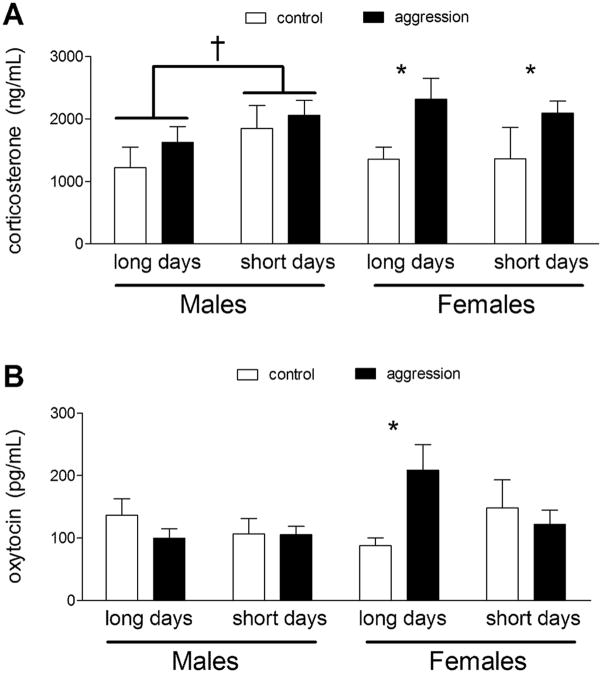
Analyses of corticosterone in males and females showed an overall effect of aggression testing (F1,50 = 8.15, p < 0.01) and a photoperiod × sex interaction (F1,50 = 4.49, p < 0.05). A clear pattern emerged when males and females were analyzed separately using two-way ANOVA including photoperiod and aggression testing as factors. In males corticosterone was increased in short days (Fig. 1A; F1,29 = 4.63, p < 0.05) whereas aggression testing had no effect. In contrast, females had increased corticosterone when tested in aggression tests (Fig. 1A; F1,21 = 6.51, p < 0.05) whereas photoperiod had no effect. It is possible that the slightly shorter tests used for males could have prevented us from detecting an increase in corticosterone. To test this possibility we examined corticosterone levels in males tested in 10 min aggression tests and compared these values to males tested in 7 min aggression tests and controls. There was no significant difference in corticosterone levels between control males, males tested in 7 min aggression tests, or males tested in 10 min aggression tests (Table 2, F1,18 = 0.79, p = 0.47). There was no significant difference in the total amount of aggression observed between 7 min and 10 min aggression tests nor was there a difference in the relative rate of aggression in each test (Table 2, all p’s > 0.1). These data indicate that the lack of a corticosterone response in males can not be explained by the use of 7 min aggression tests.
Figure 1.
Plasma corticosterone (A) and oxytocin (B) levels measured by radioimmunoassay and enzyme immunoassay respectively. For males, corticosterone levels were increased in short days whereas in females aggression testing increased corticosterone levels. In females oxytocin levels were increased by aggression testing under long days but not short days. * p < 0.05 planned comparison between control and aggression, † p< 0.05 effect of photoperiod
Table 2.
Corticosterone levels in males tested in 7 min and 10 min aggression test
| n | Corticosterone (ng/mL) | Total Bites | Attack latency (s) | Bites (freq/per min) | Relative Attack Latency | |
|---|---|---|---|---|---|---|
| control | 5 | 1,218±331 | n/a | n/a | n/a | n/a |
| 7 min | 10 | 1,627±250 | 4.9±0.9 | 177±45 | 0.7±0.13 | 0.42±0.11 |
| 10 min | 6 | 1,162±353 | 3.8±1.3 | 221±91 | 0.38±0.13 | .47±0.400 |
Analyses of peripheral OT levels detected a photoperiod × sex × behavior interaction (F1,59 = 4.75, p < 0.03). Planned comparisons showed that in long days, females tested in aggression tests had significantly higher plasma oxytocin levels than controls (Fig. 1B). There was no difference between control and aggression-tested females under short days, and in males there were no significant differences between control and aggression-tested individuals.
Corticosterone and OT levels were not correlated with aggressive behaviors in males or females. Males were more aggressive than females, and males were significantly more aggressive in short days (Table 1). Although females showed higher aggression levels in short days this difference was not statistically significant.
Table 1.
Aggressive behavior in male (7 min test) and female (10 min test) California mice from Trainor et al. (2010) and Silva et al. (2010)
| Bites (freq) | Attack Latency (s) | |||
|---|---|---|---|---|
| Long days | Short days | Long days | Short days | |
| Male | 4.9±0.8 | 13.8±2.9* | 177±46 | 90±45* |
| Female | 1.2±0.6 | 2.4±0.9 | 433±70 | 360±82 |
Oxytocin and pERK Immunoreactivity in PVN
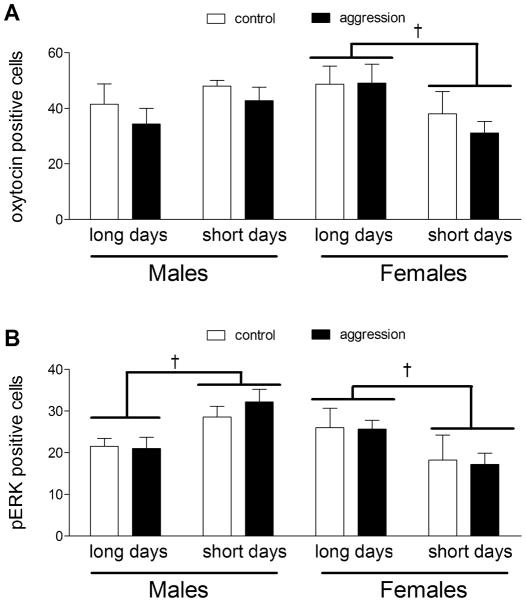
In the PVN, there was a significant photoperiod × sex interaction for the number of OT immunopositive cells (Fig. 2F, 3A; F1,48 = 6.70, p = 0.01). When males and females were analyzed in separate ANOVAs, a clear pattern emerged. Females housed in short days had significantly fewer OT positive neurons versus females in long days (Fig. 3A, F1,24 = 5.0, p < 0.05) whereas photoperiod did not have a significant effect on the number of OT positive neurons in males (Fig. 3A; F1,24 = 1.8, p > 0.18). There were no significant differences in the number of OT positive neurons in the SON (data not shown, all p’s > 0.2).
Figure 2.
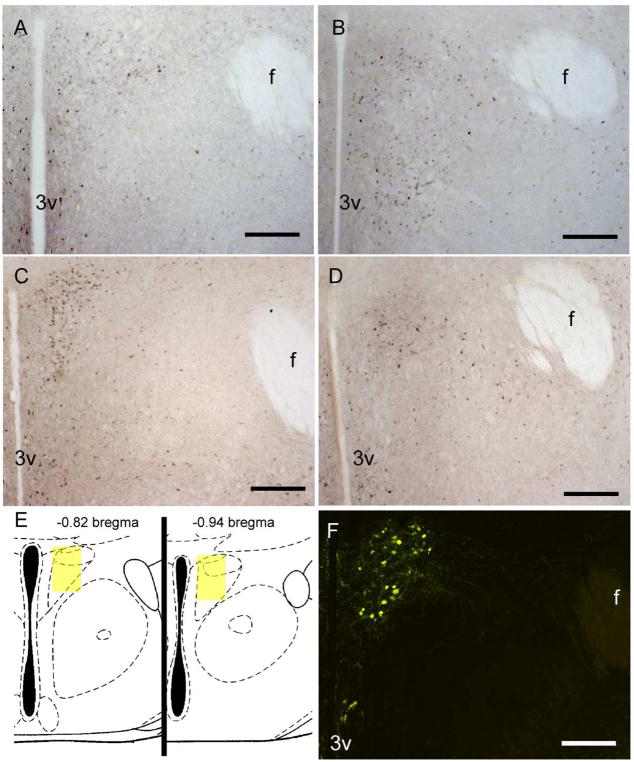
Photomicrographs of pERK and oxytocin in California mice. Male California mice had more pERK positive cells in short days (B) compared to long days (A). In contrast, females had more pERK positive cells in long days (C) than short days (D). Representation of the quantification areas used for microscopic analyses (E, Reproduced from Paxinos & Franklin 2002 with permission from Academic Press). Oxytocin positive cells were counted in the PVN (F). Scale bars = 200 μm.
Figure 3.
Cell counts of oxytocin (A) and pERK (B) immunopositive cells in the PVN. Females had more oxytocin immunoreactive cells under long days compared to short days. No differences in the number of oxytocin positive cells were detected in the parvocellular region or in males. Males had more pERK positive cells when housed under short days whereas females had more pERK positive cells when housed under long days. † p< 0.05 effect of photoperiod
In the PVN, males had significantly more pERK positive cells when housed in short days versus long days (Figs. 2A, 2B, 3B; F1,24 = 10.3, p < 0.01). In contrast, females had more pERK positive cells when housed in long days compared to short days (Figs. 2C, 2D, 3B; F1,24 = 4.48, p < 0.05). There were no effects of behavior testing or interaction on pERK staining (all p’s > 0.1). There were no significant correlations between the number of pERK cells with plasma corticosterone or oxytocin levels.
Discussion
Sex differences in neuroendocrine mechanisms are typically studied in species with relatively large sex differences in size, morphology, and parental care (Ball and McCarthy 2008). It is often assumed that monogamous species will have fewer sex differences in neurobiological mechanisms of behavior. However in the monogamous California mouse, we observed substantial sex differences in hormonal responses to social conflict and in how the hypothalamus responds to photoperiod. In males, short days increased the number of pERK cells in the PVN and also increased corticosterone levels. In contrast, females had more pERK positive cells in the PVN under long days, as well as more oxytocin neurons. The increased number of oxytocin neurons in the PVN was consistent with the surge in plasma oxytocin seen only in long day females following aggression tests. In addition, female residents responded to aggressive encounters with an increase in corticosterone whereas male residents did not, similar to previous reports showing exaggerated corticosterone responses to stress in female rodents (Critchlow et al. 1963; Weiser and Handa 2009). Short days increases aggression in male and female California mice, the neurobiological and hormonal mechanisms associated with these behaviors show important sex differences.
Sex Differences in Corticosterone Responses to Photoperiod and Social Conflict
Female California mice had increased corticosterone levels when tested in an aggression test, regardless of the photoperiod. In contrast, Davis & Marler (2003) observed no difference in corticosterone between control and aggression-tested female California mice. An important difference between the two studies is that we collected samples immediately after testing whereas Davis & Marler collected samples 60 min after testing. Together, these data suggest that female California mice have an increase in corticosterone during aggressive interactions that returns to baseline within 1 hr. A previous study in prairie voles also reported that females, but not males, increased corticosterone secretion 10 min after resident-intruder tests (Grippo et al. 2007). Similarly, social instability tests (in which cage mates were rotated, increasing aggressive interactions) increased corticosterone in females but not males (Haller et al. 1998). These rodent studies are complemented by field studies on African wild dogs and dwarf mongooses that demonstrated that dominant females had higher cortisol levels than subordinates, whereas dominant and subordinate males had similar cortisol levels (Creel et al. 1996). Overall, these studies in non-human animals suggest that the HPA axis in females may be more sensitive to social conflict than males. In this study we did not examine the hormonal or neuronal responses of intruders to aggressive encounters. An additional consideration for our study is that all females tested were in diestrus. Estrogens are known to regulate CRH transcription in the brain (Vamvakopoulos and Chrousos 1993; Grino et al. 1995), so it is possible that hormonal responses to aggression tests may differ during other stages of the estrous cycle. Ongoing experiments are addressing these questions.
A second important sex difference in glucocorticoid secretion was seen in the effect of photoperiod. As observed in other species of rodents, male California mice had increased corticosterone levels in short days versus long days (Nelson et al. 1996; Ronchi et al. 1998; Bilbo and Nelson 2003; Prendergast et al. 2007). In female California mice there was no effect of photoperiod on corticosterone levels, in contrast to previous results in hamsters which showed increased female basal and stress-induced cortisol levels in short days (Bilbo and Nelson 2003). Previous studies examining the effect of photoperiod on glucocorticoid levels in hamsters collected blood samples during the light (inactive) phase whereas our samples were collected in the dark (active) phase. Glucocorticoid levels generally increase towards the end of the inactive phase (Guillemin et al. 1959; Kalsbeek et al. 1996), so this could be an important difference. However, in another study we observed that female corticosterone levels are actually decreased in short days when samples are collected during the light phase (Steinman, Crean & Trainor submitted). This suggests that circadian rhythms in corticosterone can not explain the absence of an effect of photoperiod in females. An alterative possibility is that the unique breeding system of California mice may influence corticosterone secretion in females. Siberian hamsters breed under long day summer conditions. In contrast, in at least one population of California mice peak breeding activity occurs during the rainy season from November to May (Ribble 1992). One possible reason why short days do not increase corticosterone levels in female California mice is that elevated corticosterone levels could interfere with reproduction, as exposure to high glucocorticoid levels is known to limit placental and fetal growth (Seckl and Holmes 2007).
Sex Differences in Oxytocin Responses to Photoperiod and Social Conflict
In females, plasma OT levels were increased following social conflict under long days but not short days. This finding is consistent with previous studies showing that OT plays an important role in regulating social interactions in female rodents (Veenema and Neumann 2008). We also observed that females housed in long days had more OT positive neurons in the PVN under long days versus short days, and aggression testing did not affect the number of cells. These results suggest that increased production of OT in magnocellular neurons under long days may contribute to a greater ability to release oxytocin via the posterior pituitary during aggression tests. Oxytocin is released peripherally under a number of stressful contexts including forced swim (Wotjak et al. 1998) and restraint (Lang et al. 1983). Interestingly, in rats maternal aggression tests induce peripheral OT release in the intruders but not the lactating resident (Neumann et al. 2001). We observed somewhat similar results in that short day female California mice did not show an increase in plasma OT levels. This raises the question as to whether differences in oxytocin release, either centrally or peripherally may mediate the effect of photoperiod on aggressive behavior in females.
Although neuropeptides can be released peripherally, neuropeptides can be released within the brain either from dendritic or axonal terminals (Ludwig and Pittman 2003). Thus, it is possible that the increased number of OT neurons in long day females might affect the activation of OT receptors within the brain. In rats, female intruders exposed to aggressive residents showed increased release of OT within the PVN but not the lateral septum or central nucleus of the amygdala (Bosch et al. 2004). Peripheral and central release of OT can be either coordinated or independent depending on the context (Neumann et al. 1993). Thus, it can not be assumed that our measurements of oxytocin in plasma accurately reflect central release of oxytocin. Further study will be needed to determine whether the increased number of OT positive cells in the PVN in long days affects OT function in the brain.
In males we did not observe any effect of photoperiod or behavior testing on plasma oxytocin levels or the number of oxytocin positive cells in the PVN. This could indicate that OT does not play an important role in regulating male aggression. Alternatively, OT could have more subtle effects on aggression that could not be detected with our experimental design. There is growing evidence that oxytocin has important effects on social recognition (Insel and Fernald 2004). For example, male OT knockout mice showed a deficit in remembering previously encountered males (Ferguson et al. 2000). This could have important effect on aggressive behavior in a more naturalistic setting, as aggressive encounters between individuals with adjacent territories are usually less intense than encounters with unfamiliar individuals (Wilson 1975).
Sex Differences in Effects of Photoperiod on pERK Immunoreactivity
In the PVN, photoperiod affected the number of pERK positive cells, but this effect differed between males and females. In males there were more pERK positive cells under short days whereas for females there were more pERK positive cells under long days. These patterns of expression did not correspond with sex differences in corticosterone or oxytocin secretion. However, it is possible that double labeling of pERK and either oxytocin or corticotropin releasing hormone could identify separate populations of cells that correspond more closely to patterns of hormone secretion.
In previous studies we observed different patterns of aggression induced pERK expression in males (Trainor et al. 2010) and females (Silva et al. 2010). These studies also revealed important sex differences in patterns of pERK expression following resident-intruder aggression tests. In the bed nucleus of the stria terminalis, immunostaining and western blotting experiments showed that pERK (but not total ERK) expression is elevated by aggression testing but only under short days. A similar pattern was observed in the medial amygdala of males, although western blotting showed that pERK expression was elevated in both control and aggression tested short day males. Females showed a much different pattern. In the BNST and MEA, pERK expression was elevated by aggression testing, regardless of photoperiod. For males then, there is a consistent upregulation of pERK expression in short days across several brain areas, most notably the MEA and PVN. This pattern is generally consistent with elevated corticosterone in short day males. In contrast pERK in the BNST and MEA is aggression induced in females, which is generally consistent with elevated corticosterone in aggression tested females. Future studies will be needed to determine whether these sex differences in pERK expression have an effect on corticosterone expression or whether corticosterone in turn alters pERK expression.
Conclusions
Most previous studies examining sex differences in glucocorticoid responses to stress in rodents have utilized sexually dimorphic species, raising the question of whether these differences persist in species with different social systems. We observed in the monogamous California mouse that females show elevated glucocorticoid responses during aggression tests whereas males do not. Females also did not show an increase in corticosterone in short days, unlike other photoperiod sensitive rodents that have been studied. A major question for future study is whether these differences in glucocorticoid responses have long term consequences for behavior. For example glucocorticoid responses could have important consequences in determining whether an individual wins or loses aggressive encounters in the future (Earley and Hsu 2008). Our findings suggest that there could be important differences in the form and function of how males and females respond behaviorally to winning experiences.
Acknowledgments
The authors thank Michael Steinman and Julie VanWesterhuzyzen for technical help and Karen Bales for helpful discussions. This work supported by NIH R01 MH85069 and a grant from the UC Davis Academic Senate (B.C.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Akinci MK, Johnston GAR. Sex differences in the effects of acute swim stress on binding to GABAA receptors in mouse brain. J Neurochem. 1993;60:2212–2216. doi: 10.1111/j.1471-4159.1993.tb03507.x. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Ball GF, McCarthy MM. The neuroendocrine control of sex specific behavior in vertebrates: lessons from mammals and birds. Curr Top Dev Biol. 2008;83:213–248. doi: 10.1016/S0070-2153(08)00407-9. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Sex differences in photoperiodic and stress-induced enhancement of immune function in Siberian hamsters. Brain, Behavior, and Immunity. 2003;17:462–472. doi: 10.1016/s0889-1591(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Bunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Effects of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004;46:444–449. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter DA, Lightman SL. Oxytocin responses to stress in lactating and hyperprolactinaemic rats. Neuroendocrinology. 1987;46:532–537. doi: 10.1159/000124876. [DOI] [PubMed] [Google Scholar]
- Creel S, Creel NM, Monfort SL. Social stress and dominance. Nature. 1996;379:212–212. [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm Behav. 2003;44:189–198. doi: 10.1016/s0018-506x(03)00128-4. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Demas GE, Polacek KM, Durazzo A, Jasnow AM. Adrenal hormones mediate melatonin-induced increases in aggression in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2004;46:582–591. doi: 10.1016/j.yhbeh.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Earley RL, Hsu YY. Reciprocity between endocrine state and contest behavior in the killifish, Kryptolebias marmoratus. Horm Behav. 2008;53:442–451. doi: 10.1016/j.yhbeh.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Phillips A, Rydall A, Levesque L. Effects of Photoperiod, the Pineal Gland and the Gonads on Agonistic Behavior in Female Golden Hamsters (Mesocricetus auratus) Physiology & Behavior. 1988;44:227–234. doi: 10.1016/0031-9384(88)90143-6. [DOI] [PubMed] [Google Scholar]
- Garrett JW, Campbell CS. Changes in social behavior of the male golden hamster accompanying photoperiodic changes in reproduction. Horm Behav. 1980;14:303–318. doi: 10.1016/0018-506x(80)90020-3. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Raggi MA, Moi G, Branchi B, Moroni M, Brambilla F. Experimentally induced aggressiveness in heroin-dependent patients treated with buprenorphine: comparison of patients receiving methadone and healthy subjects. Psychiatry Res. 2007;149:201–213. doi: 10.1016/j.psychres.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sciences. 1984;35:487–497. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Glasper ER, DeVries AC. Social structure influences effects of pair-housing on wound healing. Brain, Behavior, and Immunity. 2004;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grino M, Hery M, Paulmyer-Lacroix O, Anglade G. Estrogens decrease expression of the corticotropin-releasing factor gene in the hypothalamic paraventricular nucleus and of the proopiomelanocortin gene in the anterior pituitary of ovariectomized rats. Endocrine. 1995;3:395–398. doi: 10.1007/BF02935643. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Sue Carter C. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R, Dear WE, Liebelt RA. Nyctohemeral variations in plasma free corticosteroid levels in the rat. Proc Soc Exp Biol Med. 1959;101:394–395. doi: 10.3181/00379727-101-24955. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Photoperiodic regulation of adrenal hormone secretion and aggression in female Syrian hamsters. Horm Behav. 2009;56:481–489. doi: 10.1016/j.yhbeh.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Fuchs E, Halasz J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bulletin. 1998;50:33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Helfer SG, McCubbin JA. Does gender affect the relation between blood pressure and pain sensitivity? Int J Behav Medicine. 2008;8:220–229. [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL, Bartness TJ, Demas GE. Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus) Horm Behav. 2000;38:102–110. doi: 10.1006/hbeh.2000.1604. [DOI] [PubMed] [Google Scholar]
- Jones BC, Sarrieau A, Reed C, Azar MR, Mormède P. Contribution of sex and genetics to neuroendocrine adaptation to stress in mice. Psychoneuroendocrinology. 1998;23:505–517. doi: 10.1016/s0306-4530(98)00014-6. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamic-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16:5555–5565. doi: 10.1523/JNEUROSCI.16-17-05555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience. 2006;142:1281–1292. doi: 10.1016/j.neuroscience.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Lang RE, Heil JWE, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends in Neurosciences. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Mendoza S, Coe C, Lowe D, Levine S. The physiological response to group formation in adult male squirrel monkeys. Psychoneuroendocrinology. 1979;3:221–230. doi: 10.1016/0306-4530(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Badura LL, Goldman BD. Mechanisms of seasonal cycles of behavior. Annu Rev Psychol. 1990;41:81–108. doi: 10.1146/annurev.ps.41.020190.000501. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Fine JB, Demas GE, Moffatt CA. Photoperiod and population density interact to affect reproductive and immune function in male prairie voles. Am J Physiol Rg Integr Comp Physiol. 1996;270:R571–577. doi: 10.1152/ajpregu.1996.270.3.R571. [DOI] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypotalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Toschi N, Ohl F, Torner L, Kromer SA. Maternal defence as an emotional stressor in female rats: correlation of neuroendocrin and behavioural parameters and inolvement of brain oxytocin. European Journal of Neuroscience. 2001;13:1016–1024. doi: 10.1046/j.0953-816x.2001.01460.x. [DOI] [PubMed] [Google Scholar]
- Overli O, Harris CA, Winberg S. Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol. 1999;54:263–275. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. New York: Academic Press; 2002. [Google Scholar]
- Prendergast BJ, Kampf-Lassin A, Yee JR, Galang J, McMaster N, Kay LM. Winter day lengths enhance T lymphocyte phenotypes, inhibit cytokine responses, and attenuate behavioral symptoms of infection in laboratory rats. Brain, Behavior, and Immunity. 2007;21:1096–1108. doi: 10.1016/j.bbi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble DO, Salvioni M. Social organization and nest coocupancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- Ribble DO. Lifetime reproductive success and its correlates in the monogamous rodent, Peromyscus californicus. J Animal Ecology. 1992;61:457–468. [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Ronchi E, Spencer RL, Krey LC, McEwen BS. Effects of photoperiod on brain corticosteroid receptors and the stress response in the golden hamster (Mesocricetus auratus) Brain Research. 1998;780:348–351. doi: 10.1016/s0006-8993(97)01303-6. [DOI] [PubMed] [Google Scholar]
- Sanders G, Freilicher J, Lightman SL. Psychological stress of exposure to uncontrollable noise increases plasma oxytocin in high emotionality women. Psychoneuroendocrinology. 1990;15:47–58. doi: 10.1016/0306-4530(90)90046-c. [DOI] [PubMed] [Google Scholar]
- Scotti MAL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Hormones and Behavior. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Silva AL, Fry WHD, Knoblauch N, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behavioural Brain Research. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AL, Fry WHD, Knoblauch N, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behavioural Brain Research. doi: 10.1016/j.bbr.2009.12.038. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Scotti MAL, Newman AEM, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Frontiers in Neuroendocrinology. 2008;29:476–489. doi: 10.1016/j.yfrne.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Rowland MR, Nelson RJ. Photoperiod affects estrogen receptor alpha, estrogen receptor beta, and aggressive behavior. Eur J Neurosci. 2007;26:207–218. doi: 10.1111/j.1460-9568.2007.05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53:192–199. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Crean KK, Fry WHD, Sweeney C. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.10.050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Crean KK, Fry WHD, Sweeney C. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2010;165:325–336. doi: 10.1016/j.neuroscience.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Aggression and territoriality. In: Breed MD, Moore J, editors. Encyclopedia of Animal Behavior. Oxford: Academic Press; 2010. [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. J Clinical Investigation. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Progress in Brain Research. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EO. Sociobiology: the new synthesis. Cambridge, Mass: Harvard University Press; 1975. [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of petidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]