Abstract
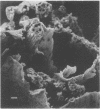
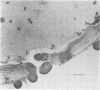
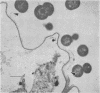
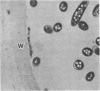
Fiber degradation in Bermuda grass and orchard grass was evaluated gravimetrically and by scanning and transmission electron microscopy after incubation with pure cultures of rumen bacteria. Lachnospira multiparus D-32 was unable to degrade plant cell wall components. Butyrivibrio fibrisolvens 49 degraded 6 and 14.9% of the fiber components in Bermuda grass and orchard grass, respectively, and Ruminococcus albus 7 degraded 11.4% orchard grass fiber but none in Bermuda grass. Both B. fibrisolvens and R. albus lacked capsules, did not adhere to fiber, and degraded only portions of the more easily available plant cell walls. R. flavefaciens FD-1 was the most active fiber digester, degrading 8.2 and 55.3% of Bermuda and orchard grass fiber, respectively. The microbe had a distinct capsule and adhered to fiber, especially that which is slowly degraded, but was able to cause erosion and disorganization of the more easily digested cell walls, apparently by extracellular enzymes. Results indicated that more digestible cell walls could be partially degraded by enzymes disassociated from cellulolytic and noncellulolytic bacteria, and data were consistent with the hypothesis that the more slowly degraded plant walls required attachment. Microbial species as well as the cell wall architecture influenced the physical association with and digestion of plant fiber.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Gordon G. L., Hogan J. P. Rumen bacterial and fungal degradation of Digitaria pentzii grown with or without sulfur. Appl Environ Microbiol. 1983 Sep;46(3):738–748. doi: 10.1128/aem.46.3.738-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of Butyrivibrio fibrisolvens: a gram-positive bacterium. J Bacteriol. 1977 Mar;129(3):1506–1512. doi: 10.1128/jb.129.3.1506-1512.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen J. A., Dehority B. A. Degradation and utilization of hemicellulose from intact forages by pure cultures of rumen bacteria. Appl Microbiol. 1970 Sep;20(3):362–368. doi: 10.1128/am.20.3.362-368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradel C. M., Dehority B. A. Fermentation of isolated pectin and pectin from intact forages by pure cultures of rumen bacteria. Appl Microbiol. 1972 Feb;23(2):332–340. doi: 10.1128/am.23.2.332-340.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Stack R. J., Hungate R. E. Muralytic Activities of Ruminococcus albus 8. Appl Environ Microbiol. 1984 May;47(5):1141–1145. doi: 10.1128/aem.47.5.1141-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Partial characterization of the extracellular carboxymethylcellulase activity produced by the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1983 May;29(5):504–517. doi: 10.1139/m83-080. [DOI] [PubMed] [Google Scholar]
- Lamed R., Setter E., Bayer E. A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983 Nov;156(2):828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Ruminococcus flavefaciens Cell Coat and Adhesion to Cotton Cellulose and to Cell Walls in Leaves of Perennial Ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jan;35(1):156–165. doi: 10.1128/aem.35.1.156-165.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherwood J. M. Cellulose degradation by Ruminococcus. Fed Proc. 1973 Jul;32(7):1814–1818. [PubMed] [Google Scholar]
- Miura H., Horiguchi M., Ogimoto K., Matsumoto T. Nutritional interdependence among rumen bacteria during cellulose digestion in vitro. Appl Environ Microbiol. 1983 Feb;45(2):726–729. doi: 10.1128/aem.45.2.726-729.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E. Effect of 3-Phenylpropanoic Acid on Capsule and Cellulases of Ruminococcus albus 8. Appl Environ Microbiol. 1984 Jul;48(1):218–223. doi: 10.1128/aem.48.1.218-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham W. R., Akin D. E. Rumen fungi and forage fiber degradation. Appl Environ Microbiol. 1984 Sep;48(3):473–476. doi: 10.1128/aem.48.3.473-476.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowicz M., Heinrichova K., Ziołecki A. An exopectate lyase of Butyrivibrio fibrisolvens from the bovine rumen. J Gen Microbiol. 1982 Nov;128(11):2661–2665. doi: 10.1099/00221287-128-11-2661. [DOI] [PubMed] [Google Scholar]
- Wood T. M., Wilson C. A., Stewart C. S. Preparation of the cellulase from the cellulolytic anaerobic rumen bacterium Ruminococcus albus and its release from the bacterial cell wall. Biochem J. 1982 Jul 1;205(1):129–137. doi: 10.1042/bj2050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gylswyk N. O., Labuschagne J. P. Relative efficiency of pure cultures of different species of cellulolytic rumen bacteria in solubilizing cellulose in vitro. J Gen Microbiol. 1971 Apr;66(1):109–113. doi: 10.1099/00221287-66-1-109. [DOI] [PubMed] [Google Scholar]