Abstract
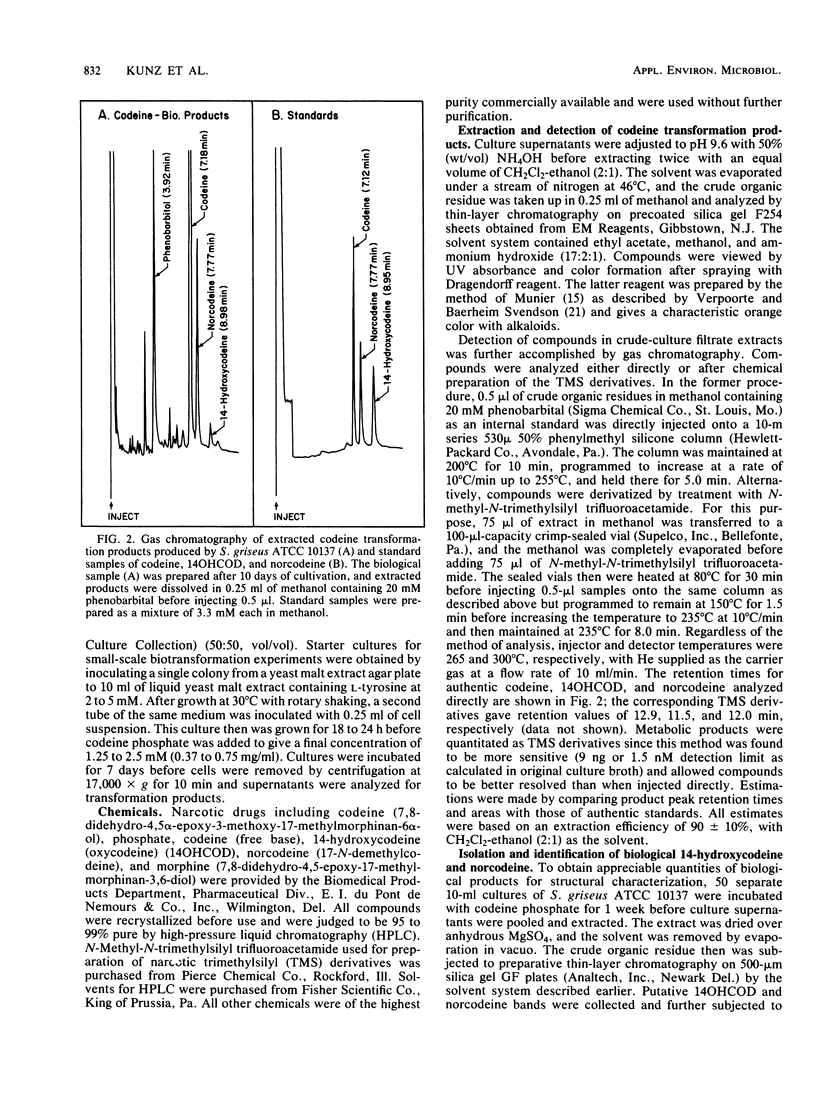
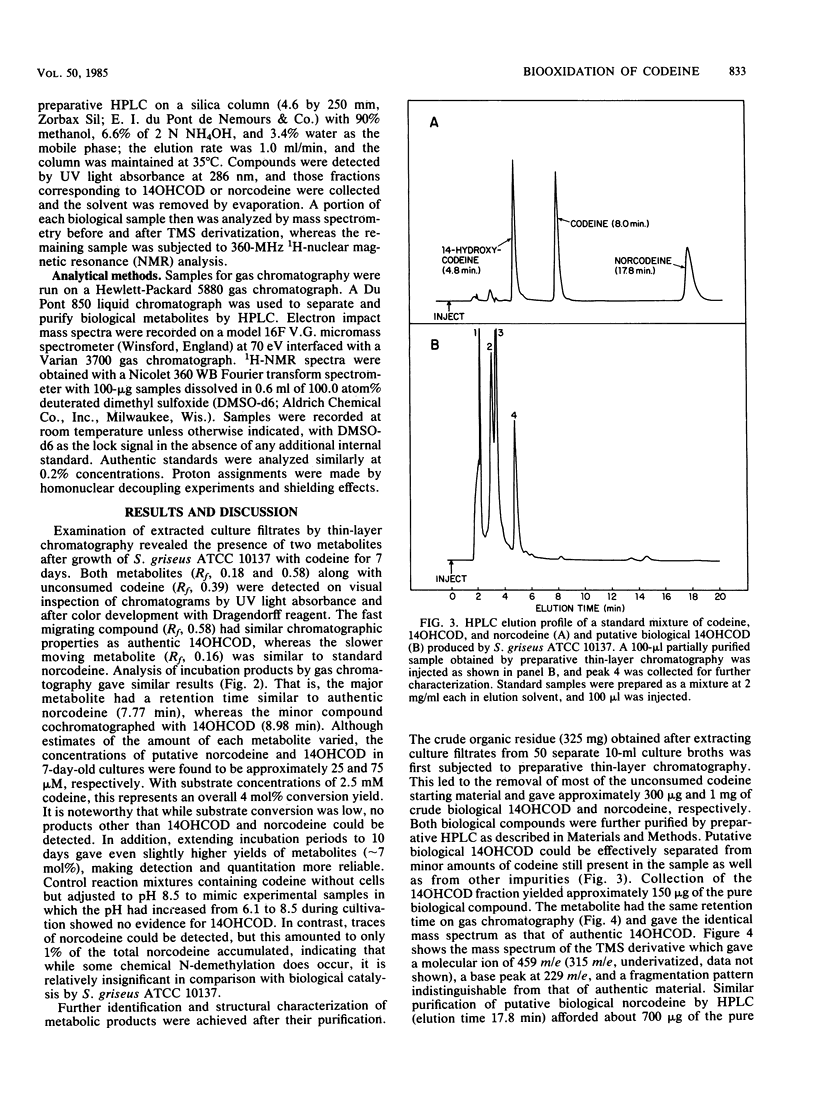
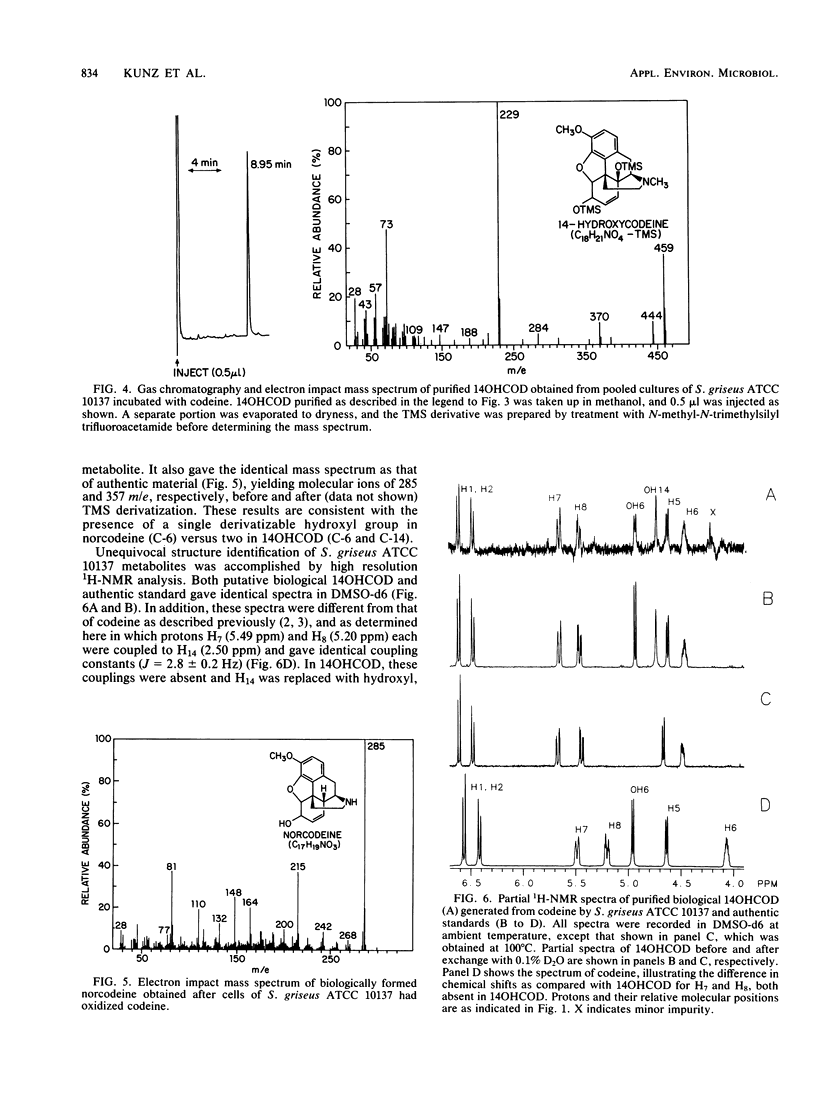
14-Hydroxycodeine and norcodeine were rigorously identified as products arising from codeine oxidation by Streptomyces griseus ATCC 10137. Both products were routinely detected in extracted culture filtrates after growth of cells in the presence of codeine for 1 week. Under these conditions, about 4 mol% of the codeine starting material was consumed, with norcodeine and 14-hydroxycodeine representing the only identifiable transformation products (molar ratio, 4:1, respectively). Extraction of a series of culture filtrates and purification of the pooled metabolites by thin-layer and high-pressure liquid chromatography led to the isolation of both biological products, the structures of which were verified by high-resolution mass spectrometry and proton nuclear magnetic resonance spectroscopy. The identities of both biological products were further confirmed by comparison of their spectral properties with those of authentic standards. This is the first report providing structural evidence for the biological formation of 14-hydroxycodeine from codeine and of codeine oxidation by S. griseus.
Full text
PDF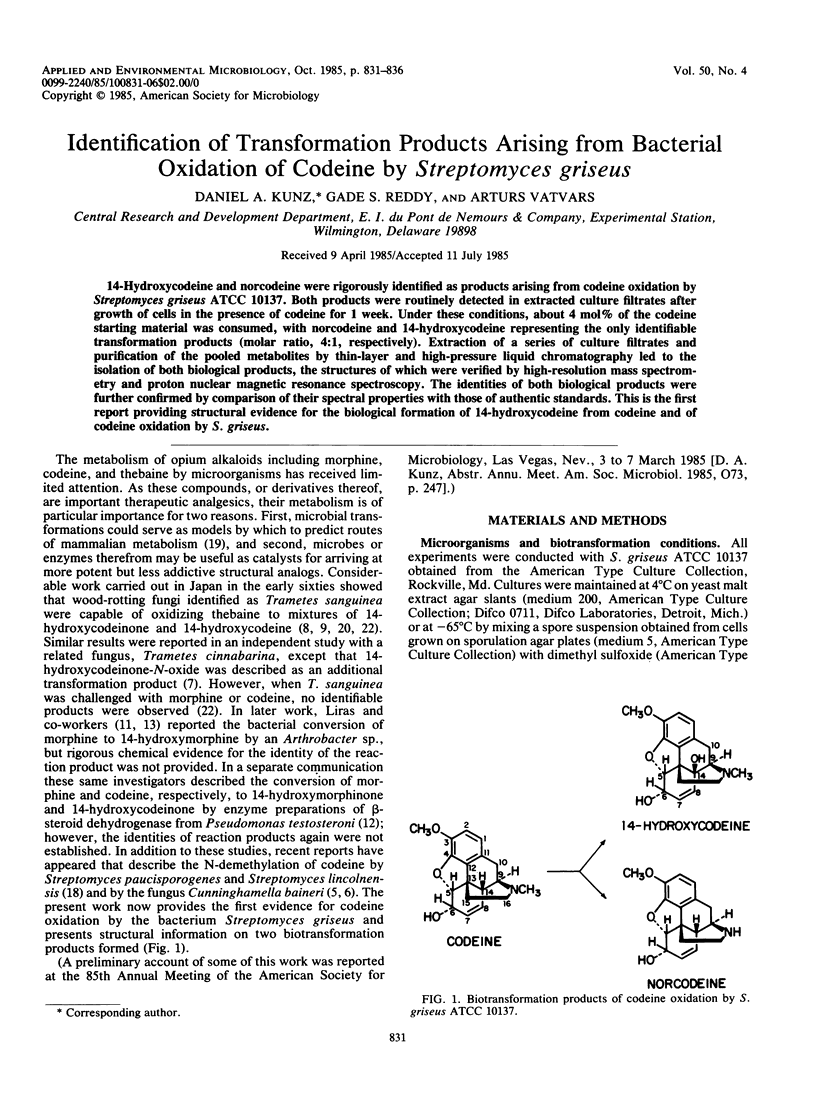

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida K., Uchida K., Iizuka K., Okuda S., Tsuda K., Uemura T. Incorporation of molecular oxygen into a morphine alkaloid, 14-hydroxycodeinone by Trametes sanguinea. Biochem Biophys Res Commun. 1966 Jan 4;22(1):13–16. doi: 10.1016/0006-291x(66)90594-8. [DOI] [PubMed] [Google Scholar]
- Gröger D., Schmauder H. P. Mikrobielle Umwandlung von Thebain. Experientia. 1969 Jan 15;25(1):95–96. doi: 10.1007/BF01903920. [DOI] [PubMed] [Google Scholar]
- IIZUKA K., YAMADA M., SUZUKI J., SEKI I., AIDA K., OKUDA S., ASAI T., TSUDA K. [Studies in the field of microbiological decomposition. XVI. Decomposition of alkaloids of the morphine group by Trametes sanguinea]. Chem Pharm Bull (Tokyo) 1962 Jan;10:67–70. doi: 10.1248/cpb.10.67. [DOI] [PubMed] [Google Scholar]
- Liras P., Kasparian S. S., Umbreit W. W. Enzymatic transformation of morphine by hydroxysteroid dehydrogenase from Pseudomonas testosteroni. Appl Microbiol. 1975 Oct;30(4):650–656. doi: 10.1128/am.30.4.650-656.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liras P., Umbreit W. W. Transformation of morphine by resting cells and cell-free systems of Arthrobacter sp. Appl Microbiol. 1975 Aug;30(2):262–266. doi: 10.1128/am.30.2.262-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNIER R. Séparation des alcaloïdes de leurs N-oxydes par microchromatographie sur papier. Bull Soc Chim Biol (Paris) 1953;35(10):1225–1231. [PubMed] [Google Scholar]
- YAMADA M., IIZUKA K., OKUDA S., ASAI T., TSUDA K. [Studies in the field of microbiological decomposition. XVII. Transformation of alkaloids in the morphine series by Trametes sanguinea. (2)]. Chem Pharm Bull (Tokyo) 1962 Oct;10:981–984. [PubMed] [Google Scholar]


