Abstract
Childhood cancer survivors who have had pelvic or central nervous system surgery or have received alkylator-containing chemotherapy or pelvic radiotherapy as part of their cancer therapy may experience urinary bladder late effects. This article reviews the medical literature on long-term bladder complications in survivors of childhood cancer and outlines the Children’s Oncology Group Long Term Followup (COG LTFU) Guidelines related to bladder function. An overview of the treatment of bladder late effects and recommended counseling for survivors with these complications are presented.
Keywords: urinary tract, urinary bladder, late effect, child, neoplasm
Over the last several decades, improvements in treatment and supportive care have led to long-term survival rates for childhood cancer that exceed 80%.1 This success has produced a growing population of childhood cancer survivors who are at risk for a variety of medical complications and functional impairments related to their cancer or its treatment. Among the long-term effects described in childhood cancer survivors are abnormalities of bladder function. Severe impairment of bladder function due to pelvic or central nervous system surgery, pelvic radiotherapy, or alkylating agent chemotherapy may lead to hydronephrosis and/or renal dysfunction, sometimes necessitating reconstructive surgery to prevent renal function deterioration. Less severe bladder functional impairment may produce bothersome symptoms such as urinary frequency, urgency or incontinence that diminish quality of life.
This paper will review how normal bladder function may be altered by pediatric cancer treatment. Methods of identifying patients at risk for bladder dysfunction will be discussed, with particular focus on the risk-based screening guidelines outlined by the Children’s Oncology Group (COG).2 Finally, the diagnostic approach for evaluating bladder dysfunction will be outlined, and prevention and/or management of conditions causing bladder dysfunction will be reviewed.
Normal Bladder Function
The bladder has two primary functions: storage of urine and micturition (voiding). Urine storage requires two basic elements. First, maintenance of a low, stable pressure during bladder filling must be achieved. This occurs as a function of the viscoelastic properties of the detrusor muscle and normal neural innervation. Second, continence must be maintained by closure of the bladder base during bladder filling.
There are a number of mechanisms whereby normal bladder function can be altered by cancer treatment. Fibrosis or direct injury to the bladder can affect the viscoelastic properties of the bladder, resulting in decreased compliance and elevated bladder pressure during filling. Sustained high intravesical pressures impede ureteral peristalsis, leading to hydronephrosis and or reflux.3 Nerve damage either centrally or peripherally due to pelvic or central nervous system surgery can also have a deleterious effect on bladder compliance and storage of urine. Control of the external sphincter can also be affected by nerve damage.
Bladder Late Effects Following Treatment for Childhood Cancer
Hemorrhagic Cystitis
Hemorrhagic cystitis (HC) is a condition in which irritation of the lining of the bladder leads to exposure of the submucosal blood vessels and bleeding.11 Chemotherapy including the oxazophorine alkylating agents cyclophosphamide and ifosfamide and radiation therapy to the bladder have been implicated in the development of HC.4-6 Patients who receive both treatment modalities are at the highest risk.5 The mediator of bladder injury in HC following chemotherapy is acrolein,7, 8 which is an hepatic breakdown product of ifosfamide9 and cyclophosphamide10 that is excreted in the urine. Most HC occurs acutely during cancer therapy; however, it may also become a chronic recurring problem with a timeframe as long as 20 years after completion of therapy.4 The incidence of hemorrhagic cystitis after pediatric cancer therapy is difficult to measure with certainty, but has been reported to be 15% in Ewing sarcoma patients treated with cyclophosphamide.5
Radiation-induced HC may be either acute or chronic, the latter due to endarteritis that leads to tissue hypoxia, ischemia and necrosis.12 Radiation doses >30 Gy to the whole bladder or >60 Gy to a portion of the bladder are risk factors for development of HC [Table I]. Radiomimetic chemotherapeutic agents such as the anti-tumor antibiotics dactinomycin and doxorubicin in combination with radiation may contribute additional risk .4,6,13
Table I.
COG LTFU Screening Guidelines for Bladder Late Effects
| Therapeutic Exposure |
Potential Late Effect |
Risk Factors | Highest Risk Factors | Recommendations | Guideline Score |
Health Counseling/ Further Considerations |
|---|---|---|---|---|---|---|
| Section 11: Cyclophosphamide Ifosfamide |
Hemorrhagic cystitis Bladder fibrosis Dysfunctional voiding Vesicoureteral reflux Hydronephrosis |
Higher cumulative doses (decreased incidence with use of Mesna) Combined with pelvic radiation Alcohol use Smoking |
Cyclophosphamide dose ≥ 3 gm/m2 Pelvic radiation dose ≥ 30 Gy |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream Annual urinalysis |
1 | Counseling: Counsel to promptly report dysuria or gross hematuria. Further Considerations: Urine culture, spot urine calcium/creatinine ratio, and ultrasound of kidney and bladder for patients with microscopic hematuria (defined as ≥5 RBC/HFP on at least 2 occasions). Nephrology or urology referral for patients with culture- negative microscopic hematuria AND abnormal ultrasound and/or abnormal calcium/creatinine ratio. Urology referral for patients with culture negative macroscopic hematuria |
| Section 12: Cyclophosphamide |
Bladder malignancy |
Combined with pelvic radiation Alcohol use Smoking |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream Annual urinalysis |
2A | Same as Section 11 | |
|
| ||||||
| Section 80: Radiotherapy ≥ 30 Gy to whole abdomen, pelvis, sacral spine |
Hemorrhagic cystitis |
Higher radiation dose (≥ 30 Gy to entire bladder; ≥ 60 Gy to portion of bladder) |
Combined with cyclophosphamide and/or ifosfamide |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream |
2A | Same as Section 11 |
| Section 81: Radiotherapy ≥ 30 Gy to whole abdomen, pelvis, sacral spine |
Bladder fibrosis Dysfunctional voiding Vesicoureteral reflux Hydronephrosis |
Higher radiation dose (≥ 45 Gy) Radiation to entire bladder Combined with: - Cyclophosphamide - Ifosfamide - Vincristine |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream Annual urinalysis |
1 | Urologic consultation for patients with incontinence or dysfunctional voiding |
|
|
| ||||||
| Section 82: Radiotherapy to whole abdomen, pelvis, sacral spine |
Bladder malignancy |
Combined with cyclophosphamide or ifosfamide Alcohol use Smoking |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream Annual urinalysis |
2A | Same as Section 11 | |
|
| ||||||
| Section 109: Cystectomy |
Cystectomy- related complication: Chronic urinary tract infection Renal dysfunction Vesicoureteral reflux Hydronephrosis Reservoir calculi Spontaneous neobladder perforation Vitamin B12/ folate/ carotene deficiency |
Annual urology evaluation | 1 except reservoir calculi = 2A and Vitamin B12/ folate/ carotene deficiency = 2B |
None | ||
|
| ||||||
| Section 119: Neurosurgery – spinal cord |
Neurogenic bladder Urinary incontinence |
Tumor adjacent to or compressing spinal cord or cauda equina Radiation dose ≥ 45 Gy to lumbar and/or sacral spine and/or cauda equina |
Injury above the level of the sacrum Radiation ≥50 Gy to lumbar and/or sacral spine and/or cauda equina |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream |
1 | Counseling: Counsel regarding adequate fluid intake, regular voiding, seeking medical attention for symptoms of voiding dysfunction or urinary tract infection, compliance with recommended bladder catheterization regimen. Further Considerations: Urologic consultation for patients with dysfunctional voiding or recurrent urinary tract infections |
| Section 126: Pelvic surgery |
Urinary incontinence Urinary tract obstruction |
Tumor adjacent to or compressing spinal cord or cauda equina Retroperitoneal node dissection Extensive pelvic dissection Radiation to the bladder, pelvis, and/or lumbar-sacral spine and/or lumbar/sacral spine |
Annual history, including hematuria, urinary urgency/frequency, urinary incontinence/retention, dysuria, nocturia, abnormal urinary stream |
1 | Same as section 119 | |
Guideline scores for the COG Long Term Follow-up Guidelines
1. There is uniform consensus of the panel that: (1) there is high-level evidence linking the late efect with the therapeutic exposure and (2) the screening recommendation is appropriate based on the collective clinical experience of panel members
2A. There is uniform consensus of the panel that: (1) there is lower-level evidence linking the late effect with the therapeutic exposure and (2) the screening recommendation is appropriate based on the collective clinical experience of panel members
2B. There is non-uniform consensus of the panel that: (1) there is lower-level evidence linking the late effect with the therapeutic exposure and (2) the screening recommendation is appropriate based on the collective clinical expeirence of panel members
3. There is major disagreement that the recommendation is appropriate
Hemorrhagic cystitis is usually painless, although patients may report urinary urgency, frequency, dysuria, suprapbic pain and occasional bladder spasms. The amount of blood loss is variable, but life-threatening hemorrhage can occur. Infection with adenovirus or BK virus may contribute to the risk of HC.14
A number of treatments for prevention of treatment-inducedHC have been tried. Vigorous hydration, catheter drainage and continuous bladder irrigation may decrease symptoms during therapy. Two drugs that bind to acrolein and limit its damage are N-acetylcysteine (Mucomyst) and 2-mercaptoethane sodium sulfonate (Mesna).7,8 The incidence of HC is decreased in patients whom receive mesna during cyclophosphamide therapy.15 Mesna administration is now the standard of care for most pediatric cancer protocols utilizing high dose cyclophosphamide.
Bladder Fibrosis and Direct Bladder Injury
Long-term bladder fibrosis and contracture has been reported in patients previously treated for HC related to oxazaphosphorine-alkylating agents. Jerkins et al reported bladder contracture in 3 of 23 patients presenting with HC following treatment of childhood cancer. All had received cyclophosphamide and 18 had also undergone radiation treatment.16
The effects of radiation therapy on bladder function can be acute and/or chronic. A number of investigators have examined the late effects of radiation therapy on bladder function utilizing an animal model.17-22 Changes in bladder function with diminished bladder compliance are noted between days 170 and 245 after irradiation and correlates with radiation dosage.17 These late changes are attributed to fibrosis as a consequence of collagen deposition in the bladder wall. Unlike acute injury, these changes are not reversible. Cumulative radiation dose >45 Gy to the whole bladder poses the highest risk for bladder toxicity [Table I].23-24
Yeung et al evaluated 11 children with pelvic rhabdomyosarcoma, of whom 7 had involvement of the bladder or prostate.25 After mean follow-up of 6.6 years, all 7 of the irradiated children had enuresis, an abnormal bladder capacity, and/or an abnormal voiding pattern. In four children, urodynamic studies demonstrated a reduced functional bladder capacity.
Cancer surgery involving the lower genitourinary tract has the potential to impair normal function of the bladder and normal voiding mechanisms. Myogenic/neurologic impairment may occur due to transection of bladder muscle and nerves during surgery to remove the bladder or adjacent pelvic tumors.26-28 Partial cystectomy can affect bladder function by reducing the bladder volume.24, 29 The greater the amount of bladder resected, the more likely bladder volume and function will be compromised. Hays et al. reviewed the outcomes of 40 patients treated for primary bladder rhabdomyosarcoma.24 Partial cystectomy was performed in 33 of the 40 patients. The extent of partial cystectomy ranged from 15% to 80% of the bladder mucosal surface. All 40 patients received chemotherapy and many received cyclophosphamide in conjunction with radiation therapy. Information regarding bladder function was obtained by patient survey. Nine of the 19 irradiated patients had symptoms suggesting a small or contracted bladder. Further analysis of this group suggested that patients receiving greater than 40 Gy radiation had a higher incidence of bladder symptoms. All three patients receiving greater than 50 Gy had bladder complications.
Neurogenic Bladder
Any cancer therapy that results in injury to the innervation of the bladder can have deleterious effects on bladder function resulting in impaired bladder storage, inability to void and/or incontinence. Neural innervation to the bladder can be affected at several levels: brain, spinal cord, and peripheral nerves. These areas can be affected by tumor growth alone, or surgery and radiation used to treat the tumor. The effect on bladder function is dependent on the level of injury.
Brain tumors and their treatment can produce centrally-mediated bladder dysfunction. This is usually manifested by urinary incontinence. There is loss of inhibition of the bladder and the patient is unable to prevent voiding. This may be associated with detrusor overactivity or uninhibited bladder contractions.30 Lesions above the pons do not affect the voiding reflex. This results in less impact on the upper urinary tract than is commonly seen with spinal cord problems.
Spinal cord injury (SCI) may occur from direct compression of the cord by tumor.31 Resection of tumor and radiation may also injure the cord. SCI can be complete or incomplete. In complete disruption of the spinal cord, the bladder and external sphincter are disconnected from central control and inhibition.32 This decentralization of the bladder results in detrusor overactivity and poor storage of urine. Acutely, there may be a period of spinal shock, during which there is detrusor underactivity or areflexia and impairment of bladder emptying. Once bladder contractility returns, there is discoordination between the bladder and the external sphincter leading to high voiding pressures. If untreated, the elevated bladder pressures lead to deterioration of renal function.3
Peripheral nerve damage from extensive pelvic surgery27, 28 can cause impaired bladder contractility. Bladder storage will be affected if there is complete denervation, much like that noted with SCI. Damage to the nerves can occur during resection of pelvic tumors, and even as a consequence of lesser, non-extirpative surgery such as ureteral reimplantation.33 High doses of radiotherapy (>50 Gy) to the lumbar or sacral spine or to the cauda equina may also cause nerve damage that results in neurogenic bladder.34
Secondary Malignancy of the Bladder
Another late effect of cancer therapy is the development of secondary malignancies. Prior exposure to cyclophosphamide for both benign and malignant conditions35-39 has been reported to be associated with bladder carcinoma. Cyclophosphamide has also been implicated in the development of leiomyosarcoma of the bladder.40-41 The risk of bladder cancer after cyclophosphamide is dose dependent. Travis et al found an excess risk of bladder cancer following high dose cyclophosphamide exposure in older patients with non-Hodgkin lymphoma.39 However, a dose relationship of cyclophosphamide and bladder cancer in pediatric cancer survivors has not been established. A recent report from the Childhood Cancer Survivor Study found only 5 cases of secondary bladder cancer among 13,136 patients.42 These occurred following treatment of non-Hodgkin lymphoma (1 patient), leukemia (2 patients) and soft tissue sarcoma (2 patients). There is one reported case of transitional cell carcinoma of the bladder developing after treatment of Hodgkin lymphoma with nitrogen mustard and pelvic radiotherapy.43 Mutations in p53 have been reported in 50% of bladder cancer,44 but the mutations have a different spectrum when induced by cyclophosphamide.45 Khan et al reported that the mutation spectrum matches the phorphoramide mustard adduction sequences detected by a repetitive primer extension assay.45
Gross hematuria is the most common presentation of bladder carcinoma but some tumors are detected incidentally on imaging evaluation for other problems. Most of these tumors are of low grade and stage and can be managed endoscopically. The risk of recurrence is higher in patients whose bladder carcinoma develops after cyclophosphamide.35
The COG LTFU Guidelines for Bladder Complications
The COG LTFU guidelines are risk-based, exposure-related recommendations for the identification and management of late effects due to therapies utilized in the treatment of childhood cancer, and are designed for asymptomatic survivors presenting for routine medical follow-up two or more years after completion of cancer therapy. More extensive evaluation is warranted for survivors with symptoms suggesting illness or organ dysfunction. Patient education materials called “Health Links” accompany the guidelines; both the guidelines and Health Links can be downloaded from http://www.survivorshipguidelines.org. The methods used to develop the guidelines is explained in more detail in the Supplemental Appendix and Supplemental Table.46
The COG LTFU guidelines related to bladder dysfunction are outlined in Table I. The guidelines specify the particular bladder complications that may result from childhood cancer therapy, along with the particular risk factors that have been linked to each condition. The screening recommendations for bladder late effects are limited to an annual history and urinalysis in survivors with potentially bladder-toxic therapeutic exposures.
Evaluation of Bladder Function
Evaluation of bladder function begins with a careful history with an emphasis on voiding. The examiner should elicit information regarding voiding pattern (number of voids per day), character of the urinary stream (caliber and force), evidence of straining to void or intermittency of the stream, and degree of incontinence. For young survivors, parents may not be able to provide specific information regarding their child’s voiding history.47 The best means to obtain these data is for the family to complete a voiding diary and questionnaire at home over several days [Table II].48 The family can prospectively record the time and amount of each void, frequency of incontinent episodes and the relationship to time of voiding, and evidence of urgency or dysuria. Gross hematuria is generally well documented by family members, as this usually results in prompt referral for evaluation. The COG Guidelines recommend that survivors treated with alkylating agents undergo annual urinalysis to screen for microscopic hematuria. This test is readily available and can screen for occult bladder malignancy and renal disease. The finding of >5 RBC/HPF on at least two occasions should prompt a urine culture, spot urine calcium/creatinine ratio and ultrasound of the kidney and bladder [Table I]. Patients with culture-negative microscopic hematuria and macroscopic hematuria and/or abnormal calcium/creatinine ratio should be referred to a nephrologist or urologist.
Table II.
Dysfunctional Voiding Scoring System
| Over the last month | Almost never | Less than half the time |
About half the time |
Almost every time |
Not available |
|---|---|---|---|---|---|
| 1. I have had wet clothes or wet underwear during the day |
0 | 1 | 2 | 3 | NA |
| 2. When I wet myself, my underwear is soaked. |
0 | 1 | 2 | 3 | NA |
| 3. I miss having a bowel movement every day. |
0 | 1 | 2 | 3 | NA |
| 4. I have to push for my bowel movements to come out. |
0 | 1 | 2 | 3 | NA |
| 5. I only go to the bathroom one or two times each day. |
0 | 1 | 2 | 3 | NA |
| 6. I can hold onto my pee by crossing my legs, squatting or doing the “pee dance”. |
0 | 1 | 2 | 3 | NA |
| 7. When I have to pee, I cannot wait. | 0 | 1 | 2 | 3 | NA |
| 8. I have to push to pee. | 0 | 1 | 2 | 3 | NA |
| 9. When I pee it hurts. | 0 | 1 | 2 | 3 | NA |
| 10. Parents to answer. Has your child experienced something stressful e.g. new baby, new school, School problems, Abuse (sexual/physical), Home problems (divorce/death) |
No (0) | Yes (3) | |||
| TOTAL | |||||
Abnormal values: >9 (males), >6 (females)
Reprinted from 38 with permission.
In patients with a positive history, the physical examination should begin with palpation of the abdomen to detect a distended bladder. Examination of the genitalia includes direct visualization of the urethral orifice. Rectal examination is also done to exclude a pelvic mass and to assess anal sphincter tone. A limited neurologic examination can assess innervation of the genital and perineal area. It may be useful to observe the patient’s urinary stream during voiding. If there is any suggestion of decreased force of the urine stream (stranguria), a urinary flow rate and ultrasound measurement of residual urine is performed. The urinary flow rate is calculated by measuring volume voided divided by the number of seconds required to empty the bladder, and can be compared to established normal values.
Patients with bladder dysfunction should be referred to an urologist. A renal and bladder ultrasound can assess bladder thickness, capacity, and adequacy of emptying, as well as identify hydronephrosis and renal abnormalities. Voiding cystourethrography may be indicated in patients with a history of infection and or hematuria. This study can visualize the urethra in males with stranguria and exclude vesicoureteral reflux, which is more common in patients with a decreased functional bladder capacity. More formal urodynamic evaluation of bladder function is undertaken in patients with incontinence. Cystometry can measure the bladder capacity and determine bladder compliance. If the urinalysis shows hematuria, with or without elevated protein, cystoscopy may be indicated.
Treatment of Bladder Late Effects
Hemorrhagic Cystitis
When HC occurs as a late effect of treatment, the degree of hematuria determines the intervention. Most cases of that occur following therapy are mild and can be managed with increased hydration. For persistent or recurrent bleeding, alternative therapeutic considerations include prostaglandins,49 hyperbaric oxygen to promote wound healing,50 conjugated estrogens that may decrease capillary fragility,51 or pentosan polysulfate to serve as an anti-fibrinolytic and anti-coagulant.52 Recently, use of anti-Factor VII a has had success in survivors following radiation-induced hemorrhagic cystitis for pelvic malignancies.53, 54 If the bleeding fails to improve with these measures or results in urinary retention, cystoscopy and clot extraction with instillation of astringents such as alum or silver nitrate is the next step. If these measures fail or there is life threatening hemorrhage, formalin may be instilled. It can be effective in stopping life threatening hemorrhage but poses a risk of bladder fibrosis and contracture.55 Other measures that can be utilized for severe hemorrhage include embolization of the vesical arteries, or urinary diversion.56 If symptoms of a urinary tract infection are present (i.e., fever, flank or back pain, urinary urgency, hesitancy, and/or frequency), a urinalysis, urine culture, complete blood count, and determination of BUN and creatinine may be indicated.
Non-Surgical Treatment of Bladder Dysfunction
The bladder capacity may be physically reduced due to partial cystectomy. Capacity may be functionally reduced due to fibrosis or nerve damage. The consequences of a reduced bladder capacity vary. In some survivors, the only symptom may be urinary frequency and/or urgency. Other patients will present with urge incontinence; either the result of a significant reduction in bladder capacity or inability to inhibit the detrusor contraction.
Although most patients seek treatment for the symptoms of a reduced capacity, reduced bladder compliance may have a greater impact on the kidneys. The ability of the bladder to store urine at low intravesical pressures is essential to prevent upper urinary tract damage.3 A bladder storing urine at elevated pressures may impede ureteral peristalsis and result in hydronephrosis and renal damage over time. These changes can occur silently if the patient does not manifest voiding complaints.
Decreased bladder compliance is usually initially managed with antimuscarinic medication (e.g. oxybutinin, tolterodine) to ensure frequent complete emptying of the bladder. A therapeutic effect should be observed within a few weeks of starting these medications. The patient should be observed to have decreased urinary frequency or decreased incontinent episodes after initiation of therapy. However, repeat urodynamic evaluation to assess improvement in bladder compliance is needed.
Surgical Treatment of Bladder Dysfunction
Surgery may be necessary for management of bladder complications of cancer treatment. Abnormalities of bladder storage due to either reduced capacity or poor bladder compliance with elevated pressures require bladder enlargement or augmentation. Augmentation is most often performed by incorporating a bowel segment into the native bladder in order to increase its capacity and lower storage pressures. The intestinal segment is sutured to the native bladder after it is reconfigured to create a spherical structure.
Complete removal of the bladder, cystectomy, mandates reconstruction of a new urinary reservoir. In some patients, a passive conduit can be constructed to drain the urine to an abdominal stoma that drains continuously to a bag. Ileal conduit diversion or the similar colonic conduits utilize an intestinal segment to create an incontinent abdominal stoma. While one end of the segment is brought to the skin, the other is closed off and the ureters are then implanted into the bowel segment. This type of diversion is used less commonly today, but may be a temporary measure until a continent reconstruction can be performed at the completion of therapy or when the patient is older. Continent reconstructions using intestinal segments to replace the bladder are most commonly performed today. The two major subtypes are orthotopic neobladders and continent diversions. The orthotopic neobladder is anastomosed to the native urethra, with preservation of the sphincteric mechanisms [Figure 1]. In a continent diversion, a cutaneous channel is brought out the anterior abdominal wall and is used as a route for catheterization [Figure 2].57 Central to all of these techniques is the principle of clean intermittent catheterization which allows for safe and efficient emptying of reconstructed bladders that cannot be emptied volitionally.
Figure 1.
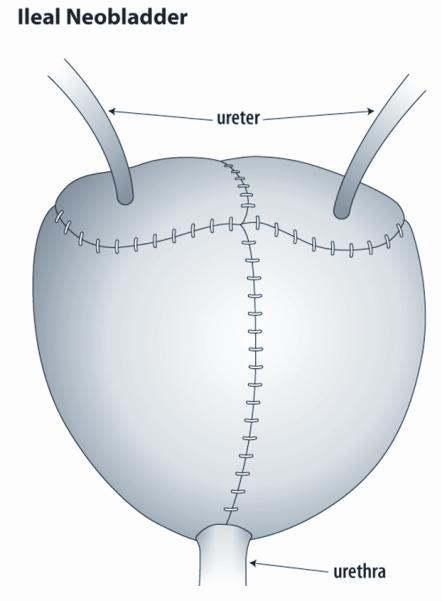
Ileal neobladder. This figure shows a bladder constructed of ileum to replace the native bladder removed at time of cystectomy. The small intestine is opened and reconfigured into a spherical shape. The bowel segment is sewn to the native urethra above the pelvic floor. The ureters are reimplanted into the new reservoir. The patient may be able to void spontaneosly but some patients require intermittent catheterization to empty the neobladder.
Figure 2.
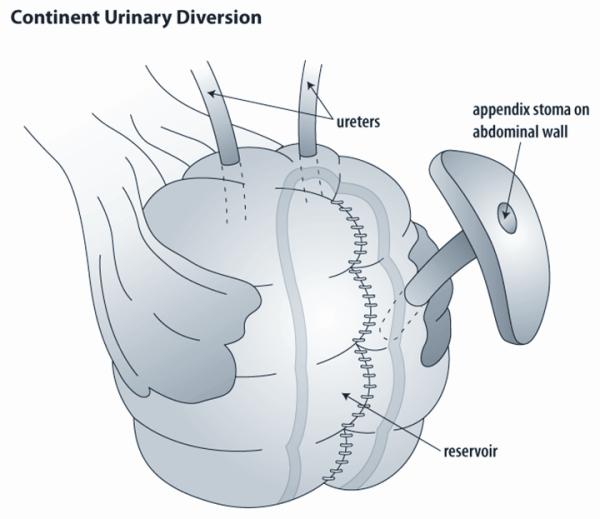
Continent urinary reservoir. This figure shows a continent diversion made to replace the bladder following cystectomy. These reservoirs can be constructed from the either colon alone or a combination of colon and ileum. A catheterizable channel is constructed from either the native appendix or a small piece of ileum tapered into a narrow channel. This is implanted into the reservoir and then brought to the skin. The reservoir is emptied intermittently by catheterization. The ureters are reimplanted into the new reservoir.
Counseling the Patient with Bladder Complications
Survivors who have received potentially bladder-toxic therapy should be counseled about the symptoms and signs of urinary tract complications and the risk of renal impairment posed by inadequate treatment of bladder dysfunction. The COG Health Link “Bladder Health After Childhood Cancer” is a useful patient education tool that provides a lay summary of long-term bladder complications and guidelines for seeking medical intervention (available at www.survivorshipguidelines.org).
Patients with HC should contact their physician whenever the HC fails to resolve promptly, or if symptoms of a urinary tract infection or unexplained complication are present. Survivors with neurologic impairment of the bladder need frequent evaluation of bladder function by the urologist. Urodynamic studies of the bladder can monitor bladder pressures to ensure that filling pressures remain low. Frequent emptying of the bladder is necessary to avoid high sustained bladder pressures that can lead to hydronephosis or vesicoureteral reflux. This will also decrease risk for urinary tract infections. Patients who have undergone bladder reconstructive procedures with intestinal segments also require careful monitoring and annual evaluation by the urologist. Bladder reconstruction with intestinal segments does increase the risk of urinary infections, urinary calculi, electrolyte disturbances and bladder perforation. The latter can present as acute peritonitis and may be fatal if not recognized promptly.58 Although rare, a more ominous late complication of such surgery is secondary tumor formation with an estimated risk of 1-3%.59 The development of malignancy after enterocystoplasty has been reported with a latency period of 5-10 years. These patients may benefit from yearly endoscopy of the bladder to detect any premalignant changes.
Conclusions
The COG LTFU guidelines offer a comprehensive summary of the risk factors associated with long-term bladder complications after treatment for childhood cancer, based on evidence in the medical literature and expert panel review. Further studies will be required to confirm the validity of the screening recommendations and to better define more precisely the risk factors associated with certain late effects.
Survivors who have been exposed to therapies that are potentially toxic to the bladder deserve careful ongoing follow-up in order to detect bladder complications so that intervention can be accomplished in a timely fashion. Urologists, internists, pediatricians, nurses, and psychologists, as well as other medical specialists, may be necessary to provide appropriate interventions for the patient at risk for bladder complications following treatment of cancer in childhood.
Supplementary Material
Contributor Information
Michael Ritchey, Mayo Clinic College of Medicine Scottsdale, AZ.
Fernando Ferrer, Connecticut Children’s Medical Center Hartford, CT.
Patricia Shearer, Cancer Survivor Program University of Florida Shands Cancer Center Gainesville, FL.
Sheri L. Spunt, Department of Oncology St. Jude Children’s Research Hospital, Memphis, TN; Department of Pediatrics University of Tennessee, Memphis, TN.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. CA Cancer J Clin. 2008 Mar-Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004 Dec 15;22(24):4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 3.McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981 Aug;126(2):205–209. doi: 10.1016/s0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 4.Heyn R, Raney RB, Jr, Hays DM, et al. Late effects of therapy in patients with paratesticular rhabdomyosarcoma. Intergroup Rhabdomyosarcoma Study Committee. J Clin Oncol. 1992 Apr;10(4):614–623. doi: 10.1200/JCO.1992.10.4.614. [DOI] [PubMed] [Google Scholar]
- 5.Stillwell TJ, Benson RC, Jr, Burgert EO., Jr. Cyclophosphamide-induced hemorrhagic cystitis in Ewing’s sarcoma. J Clin Oncol Jan. 1988;6(1):76–82. doi: 10.1200/JCO.1988.6.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Hale GA, Marina NM, Jones-Wallace D, et al. Late effects of treatment for germ cell tumors during childhood and adolescence. J Pediatr Hematol Oncol. 1999 Mar-Apr;21(2):115–122. doi: 10.1097/00043426-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Brock N. The development of mesna for the inhibition of urotoxic side effects of cyclophosphamide, ifosfamide, and other oxazaphosphorine cytostatics. Recent Results Cancer Res. 1980;74:270–278. doi: 10.1007/978-3-642-81488-4_32. [DOI] [PubMed] [Google Scholar]
- 8.Brock N, Pohl J, Stekar J. Detoxification of urotoxic oxazaphosphorines by sulfhydryl compounds. J Cancer Res Clin Oncol. 1981;100(3):311–320. doi: 10.1007/BF00410691. [DOI] [PubMed] [Google Scholar]
- 9.Sarosy G. Ifosfamide--pharmacologic overview. Semin Oncol. 1989 Feb;16(1 Suppl 3):2–8. [PubMed] [Google Scholar]
- 10.Levine LA, Richie JP. Urological complications of cyclophosphamide. J Urol. 1989 May;141(5):1063–1069. doi: 10.1016/s0022-5347(17)41173-6. [DOI] [PubMed] [Google Scholar]
- 11.Sklar CA, LaQuaglia MP. The long-term complications of chemotherapy in childhood genitourinary tumors. Urol Clin North Am. 2000 Aug;27(3):563–568. doi: 10.1016/s0094-0143(05)70103-8. [DOI] [PubMed] [Google Scholar]
- 12.Williams J, Clarke D. Pelvic radiation necrosis and radiation cystitis. In: Kindwall E, editor. Hyperbaric Medicine Practice. Best Publishing; Arizona: 1994. p. 505. [Google Scholar]
- 13.Tefft M, Lattin PB, Jereb B, et al. Acute and late effects on normal tissues following combined chemo- and radiotherapy for childhood rhabdomyosarcoma and Ewing’s sarcoma. Cancer. 1976 Feb;37(2 Suppl):1201–1217. doi: 10.1002/1097-0142(197602)37:2+<1201::aid-cncr2820370831>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Gorcynska E, Turkiewicz D, Rybka K, et al. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005 Oct;11(10):797–804. doi: 10.1016/j.bbmt.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Luce JK, Simons JA. Efficacy of mesna in preventing further cyclophosphamide-induced hemorrhagic cystitis. Med Pediatr Oncol. 1988;16(6):372–374. doi: 10.1002/mpo.2950160603. [DOI] [PubMed] [Google Scholar]
- 16.Jerkins GR, Noe HN, Hill D. Treatment of complications of cyclophosphamide cystitis. J Urol. 1988 May;139(5):923–925. doi: 10.1016/s0022-5347(17)42718-2. [DOI] [PubMed] [Google Scholar]
- 17.Dorr W, Beck-Bornholdt HP. Radiation-induced impairment of urinary bladder function in mice: fine structure of the acute response and consequences on late effects. Radiat Res. 1999 Apr;151(4):461–467. [PubMed] [Google Scholar]
- 18.Kallfass E, Kramling HJ, Schultz-Hector S. Early inflammatory reaction of the rabbit coeliac artery wall after combined intraoperative (IORT) and external (ERT) irradiation. Radiother Oncol. 1996 May;39(2):167–178. doi: 10.1016/0167-8140(96)01708-2. [DOI] [PubMed] [Google Scholar]
- 19.Kraft M, Oussoren Y, Stewart FA, Dorr W, Schultz-Hector S. Radiation-induced changes in transforming growth factor beta and collagen expression in the murine bladder wall and its correlation with bladder function. Radiat Res. 1996 Dec;146(6):619–627. [PubMed] [Google Scholar]
- 20.Lundbeck F, Oussoren Y, Stewart FA. Early and late damage in the mouse bladder after radiation combined with cyclophosphamide or cisplatinum, evaluated by two different functional assays. Acta Oncol. 1993;32(6):679–687. doi: 10.3109/02841869309092452. [DOI] [PubMed] [Google Scholar]
- 21.Woloschak GE, Chang-Liu CM, Jones PS, Jones CA. Modulation of gene expression in Syrian hamster embryo cells following ionizing radiation. Cancer Res. 1990 Jan 15;50(2):339–344. [PubMed] [Google Scholar]
- 22.Yue TL, Wang XK, Olson B, Feuerstein G. Interleukin-1 beta (IL-1 beta) induces transforming growth factor-beta, (TGF-beta 1) production by rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1994 Nov 15;204(3):1186–1192. doi: 10.1006/bbrc.1994.2588. [DOI] [PubMed] [Google Scholar]
- 23.Mangar SA, Foo K, Norman A, et al. Evaluating the effect of reducing the high-dose volume on the toxicity of radiotherapy in the treatment of bladder cancer. Clin Oncol (R Coll Radiol) 2006 Aug;18(6):466–473. doi: 10.1016/j.clon.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Hays DM, Raney RB, Wharam MD, et al. Children with vesical rhabdomyosarcoma (RMS) treated by partial cystectomy with neoadjuvant chemotherapy, with or without radiotherapy. A report from the Intergroup Rhabdomyosarcoma Study (IRS) Committee. J Pediatr Hematol Oncol. 1995 Feb;17(1):46–52. doi: 10.1097/00043426-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Yeung CK, Ward HC, Ransley PG, Duffy PG, Pritchard J. Bladder and kidney function after cure of pelvic rhabdomyosarcoma in childhood. Br J Cancer. 1994 Nov;70(5):1000–1003. doi: 10.1038/bjc.1994.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozkan KU, Bauer SB, Khoshbin S, Borer JG. Neurogenic bladder dysfunction after sacrococcygeal teratoma resection. J Urol. 2006 Jan;175(1):292–296. doi: 10.1016/S0022-5347(05)00012-1. [DOI] [PubMed] [Google Scholar]
- 27.Mosiello G, Gatti C, De Gennaro M, et al. Neurovesical dysfunction in children after treating pelvic neoplasms. BJU Int. 2003 Aug;92(3):289–292. doi: 10.1046/j.1464-410x.2003.04326.x. [DOI] [PubMed] [Google Scholar]
- 28.Cruccetti A, Kiely EM, Spitz L, Drake DP, Pritchard J, Pierro A. Pelvic neuroblastoma: Low mortality and high morbidity. J Pediatr Surg. 2000 May;35(5):724–728. doi: 10.1053/jpsu.2000.6076. [DOI] [PubMed] [Google Scholar]
- 29.Raney B, Anderson J, Jenney M, et al. Late effects in 164 patients with rhabdomyosarcoma of the bladder/prostate region: a report the International workshop. J Urol. 2006 Nov;176(5):2190–2194. doi: 10.1016/j.juro.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 30.Blaivas JG. The neurophysiology of micturition: a clinical study of 550 patients. J Urol. 1982 May;127(5):958–963. doi: 10.1016/s0022-5347(17)54147-6. [DOI] [PubMed] [Google Scholar]
- 31.Hoover M, Bowman LC, Crawford SE, et al. Long-term outcome of patients with intraspinal neuroblastoma. Med Pediatr Oncol. 1999 May;32(5):353–359. doi: 10.1002/(sici)1096-911x(199905)32:5<353::aid-mpo8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Decter RM, Bauer SB. Urologic management of spinal cord injury in children. Urol Clin North Am. 1993 Aug;20(3):475–483. [PubMed] [Google Scholar]
- 33.Barrieras D, Lapointe S, Reddy PP, et al. Urinary retention after bilateral extravesical ureteral reimplantation: does dissection distal to the ureteral orifice have a role? J Urol. 1999 Sep;162(3 Pt 2):1197–1200. doi: 10.1016/S0022-5347(01)68130-8. [DOI] [PubMed] [Google Scholar]
- 34.Pieters RS, Niemierko A, Fullerton BC, Nunzenrider JE. Cauda equina tolerance to high-dose fractionated irradiation. Int J Radiat Oncol Biol Phys. 2006 Jan 1;64(1):251–257. doi: 10.1016/j.ijrobp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Hoenig DM, McRae S, Chen SC, Diamond DA, Rabinowitz R, Caldamone AA. Transitional cell carcinoma of the bladder in the pediatric patient. J Urol. 1996 Jul;156(1):203–205. [PubMed] [Google Scholar]
- 36.Agarwala S, Hemal AK, Seth A, Gupta AK, Bhatnagar V, Mitra DK. Transitional cell carcinoma of the urinary bladder following exposure to cyclophosphamide in childhood. Eur J Pediatr Surg. 2001 Jun;11(3):207–210. doi: 10.1055/s-2001-15486. [DOI] [PubMed] [Google Scholar]
- 37.Kersun LS, Wimmer RS, Hoot AC, Meadows AT. Secondary malignant neoplasms of the bladder after cyclophosphamide treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2004 Mar;42(3):289–291. doi: 10.1002/pbc.10451. [DOI] [PubMed] [Google Scholar]
- 38.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001 Apr 18;93(8):618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 39.Travis LB, Curtis RE, Glimelius B, et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1995 Apr 5;87(7):524–530. doi: 10.1093/jnci/87.7.524. [DOI] [PubMed] [Google Scholar]
- 40.Parekh DJ, Jung C, O’Conner J, Dutta S, Smith ER., Jr. Leiomyosarcoma in urinary bladder after cyclophosphamide therapy for retinoblastoma and review of bladder sarcomas. Urology. 2002 Jul;60(1):164. doi: 10.1016/s0090-4295(02)01701-6. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen-Bjergaard J, Jonsson V, Pedersen M, Hou-Jensen K. Leiomyosarcoma of the urinary bladder after cyclophosphamide. J Clin Oncol. 1995 Feb;13(2):532–533. doi: 10.1200/JCO.1995.13.2.532. [DOI] [PubMed] [Google Scholar]
- 42.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006 Jan 20;24(3):476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 43.DeLair SM, White RW, Kurzrock EA. Secondary transitional cell carcinoma and nitrogen mustard treatment. Urology. 2005 Jun;65(6):1226–1227. doi: 10.1016/j.urology.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 44.Reznikoff CA, Belair CD, Yeager TR, et al. A molecular genetic model of human bladder cancer pathogenesis. Semin Oncol. 1996 Oct;23(5):571–584. [PubMed] [Google Scholar]
- 45.Khan MA, Travis LB, Lynch CF, et al. p53 mutations in cyclophosphamide-associated bladder cancer. Cancer Epidemiol Biomarkers Prev. 1998 May;7(5):397–403. [PubMed] [Google Scholar]
- 46.Winn RJ, Botnick WZ. The NCCN guideline program: a conceptual framework. Oncology. 1997 Nov;11(11A):25–32. [PubMed] [Google Scholar]
- 47.Bloom DA, Seeley WW, Ritchey ML, McGuire EJ. Toilet habits and continence in children: an opportunity sampling in search of normal parameters. J Urol. 1993 May;149(5):1087–1090. doi: 10.1016/s0022-5347(17)36304-8. [DOI] [PubMed] [Google Scholar]
- 48.Farhat W, Bagli DJ, Capolicchio G, et al. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol. 2000 Sep;164(3 Pt 2):1011–1015. doi: 10.1097/00005392-200009020-00023. [DOI] [PubMed] [Google Scholar]
- 49.Ippoliti C, Przepiorka D, Mehra R, et al. Intravesicular carboprost for the treatment of hemorrhagic cystitis after marrow transplantation. Urology. 1995 Dec;46(6):811–815. doi: 10.1016/s0090-4295(99)80349-5. [DOI] [PubMed] [Google Scholar]
- 50.Chong KT, Hampson NB, Corman JM. Early hyperbaric oxygen therapy improves outcome for radiation-induced hemorrhagic cystitis. Urology. 2005 Apr;65(4):649–653. doi: 10.1016/j.urology.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 51.Liu YK, Harty JI, Steinbock GS, Holt HA, Jr., Goldstein DH, Amin M. Treatment of radiation or cyclophosphamide induced hemorrhagic cystitis using conjugated estrogen. J Urol. 1990 Jul;144(1):41–43. doi: 10.1016/s0022-5347(17)39361-8. [DOI] [PubMed] [Google Scholar]
- 52.Hampson SJ, Woodhouse CR. Sodium pentosanpolysulphate in the management of haemorrhagic cystitis: experience with 14 patients. Eur Urol. 1994;25(1):40–42. doi: 10.1159/000475245. [DOI] [PubMed] [Google Scholar]
- 53.Geisler JP, Linnemeier GC, Manahan KJ. Recombinant factor VIIa to treat late radiation-induced hemorrhagic cystitis. J Reprod Med. 2008 May;53(5):350–352. [PubMed] [Google Scholar]
- 54.Ashrani AA, Gabriel DA, Gajewski JL, Jacobs DR, Jr, Weisdorf DJ, Key NS. Pilot study to test the efficacy and safety of activated recombinant factor VII (Novoseven) in the treatment of refractory hemorrhagic cystitis following high dose chemotherapy. Bone Marrow Transplant. 2006 Dec;38(12):825–828. doi: 10.1038/sj.bmt.1705535. [DOI] [PubMed] [Google Scholar]
- 55.Donahue LA, Frank IN. Intravesical formalin for hemorrhagic cystitis: analysis of therapy. J Urol. 1989 Apr;141(4):809–812. doi: 10.1016/s0022-5347(17)41016-0. [DOI] [PubMed] [Google Scholar]
- 56.Koc S, Hagglund H, Ireton RC, Perez-Simon JA, Collins SJ, Appelbaum FR. Successful treatment of severe hemorrhagic cystitis with cystectomy following matched donor allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2000 Oct;26(8):899–901. doi: 10.1038/sj.bmt.1702611. [DOI] [PubMed] [Google Scholar]
- 57.Harris CF, Cooper CS, Hutcheson JC, Snyder HM., 3rd Appendicovesicostomy: the mitrofanoff procedure-a 15-year perspective. J Urol. 2000 Jun;163(6):1922–1926. doi: 10.1016/s0022-5347(05)67599-4. [DOI] [PubMed] [Google Scholar]
- 58.Metcalfe PD, Casale Aj, Kaefer MA, et al. Spontaneous Bladder Perforations: A Report of 500 Augmentations in Children and Analysis of Risk. J Urol. 2006 Apr;175(4):1466–1470. doi: 10.1016/S0022-5347(05)00672-5. [DOI] [PubMed] [Google Scholar]
- 59.Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004 Oct;172(4 Pt 2):1649–1651. doi: 10.1097/01.ju.0000140194.87974.56. discussion 1651-1642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


