SUMMARY
The identities and roles of proteins associated with human telomerase remain poorly defined. To gain insight, we undertook an affinity purification of endogenously assembled human telomerase complexes. We show that specific subsets of H/ACA, Sm, and hnRNP proteins associate with active and inactive telomerase RNPs, while two NTPase proteins associate preferentially with active enzyme. All three core H/ACA-motif binding proteins are telomerase holoenzyme components essential for RNP accumulation. On the other hand, telomerase RNPs lacking interaction with Sm proteins or hnRNP C remain fully functional for telomere elongation. Curiously, overexpression of either associated hnRNP protein (hnRNP C and hnRNP U) or either NTPase protein (NAT10 and GNL3L) induced telomere shortening. Our findings suggest that endogenous human telomerase complexes are more heterogeneous than those of single-celled eukaryotes, have predominantly shared rather than telomerase-specific proteins, and make numerous regulatory interactions.
INTRODUCTION
Telomerase extends chromosome 3′ ends by synthesis of telomeric repeats. Telomerase reverse transcriptase (TERT) and telomerase RNA can reconstitute telomeric repeat synthesis activity in vitro, but much larger multicomponent telomerase holoenzyme complexes are assembled in vivo (Collins, 2006). Chromatography of endogenously assembled ciliate telomerases suggests an apparent molecular mass of ~250 kDa using glycerol gradient sedimentation or ~500 kDa using gel filtration, with yet-larger complexes induced in a developmentally regulated manner. The endogenously assembled yeast and mammalian telomerase holoenzymes also fractionate in cell extract as large particles. However, because the globular protein standards used for calibration are not representative of RNA-scaffolded telomerase RNP architecture, chromatography assays do not necessarily predict the total subunit mass of a telomerase holoenzyme.
Telomerase-associated proteins have been best characterized in single-celled eukaryotes. Affinity-purified ciliate telomerase RNPs include a telomerase-specific La-motif protein that folds telomerase RNA into a conformation recognized by TERT (Collins, 2006). The Tetrahymena thermophila holoenzyme contains at least two additional subunits, p45 and p75, that are not essential for TERT RNP assembly but are required for telomere elongation. This general role is played in Saccharomyces cerevisiae by the telomerase proteins Est1p and Est3p (Lingner et al., 1997). Human proteins with this function have not been discovered, but known interaction partners of human telomerase RNA (hTR) include the H/ACA-motif RNA binding proteins dyskerin, NHP2, NOP10, and GAR1. These four proteins assemble with hTR and with large families of H/ACA-motif small nucleolar (sno) RNAs and small Cajal body (sca) RNAs. Amino acid substitutions in dyskerin reduce hTR accumulation and give rise to the X-linked form of dyskeratosis congenita (DC), a bone marrow failure syndrome (Wong and Collins, 2003). Proteomics of highly purified active human telomerase led to the suggestion that only hTERT and dyskerin are associated with hTR (Cohen et al., 2007). However, this conclusion is challenged by previous studies showing that dyskerin possesses minimal RNA binding affinity in the absence of its H/ACA-motif binding partners NHP2 and NOP10 (see the Discussion).
Altered telomerase biogenesis and function are implicated in human disease. New insights about human telomerase holoenzyme interactions could have high medical significance but are hindered by the technical difficulties inherent in studying nonabundant complexes. Here we describe a rapid and gentle affinity purification of human telomerase holoenzyme complexes containing hTERT and hTR. For each protein identified to copurify specifically with hTERT and hTR, we assessed interaction specificity and addressed the influence of protein-RNA interaction and/or protein overexpression on telomere length maintenance. This effort provides a framework for understanding the biogenesis and regulation of endogenous human telomerase.
RESULTS
Establishing a Cell Line for Affinity Purification of Telomerase Holoenzyme Complexes
A rapid and gentle tandem affinity purification (TAP) strategy was used to purify T. thermophila telomerase holoenzyme (Witkin and Collins, 2004). We adopted a similar approach to purify endogenously assembled human telomerase complexes. A HeLa human cervical carcinoma cell line was established that expressed a relatively low level of N-terminal TAP-tagged human TERT (hTERT) from an integrated retroviral vector (HeLa TAP-hTERT cells). To increase the level of telomerase holoenzyme, a second retroviral expression vector that produces mature hTR was introduced (HeLa TAP-hTERT+hTR cells). Antibody against the TAP tag detected an ~150 kDa polypeptide in HeLa TAP-hTERT cell lysates, but not the parental HeLa cell lysates used as a mock purification control (Figure 1A). Northern blots revealed an ~2.5-fold increase in hTR in HeLa-TAP-hTERT+hTR cells compared to parental HeLa or HeLa TAP-hTERT cells (Figure 1B). Telomerase catalytic activity assayed using the telomeric repeat amplification protocol (TRAP) method increased by more than 6-fold in extracts prepared from HeLa TAP-hTERT+hTR cells relative to parental HeLa or HeLa TAP-hTERT cells (Figure 1C; extract dilutions are 2.5-fold steps). We also compared telomeric restriction fragment (TRF) lengths by in-gel hybridization with a telomeric-repeat oligonucleotide. Strikingly, the parental HeLa cell line maintained TRF lengths of ~2–6 kb, the HeLa TAP-hTERT cell line maintained TRF lengths of ~9–12 kb, and the HeLa TAP-hTERT+hTR cell line maintained TRF lengths of substantially greater than 12 kb (Figure 1D). These results indicate that TAP-hTERT and hTR expressed from integrated retroviral expression vectors will induce telomere elongation in HeLa cells, consistent with findings described subsequent to this cell line construction (Cristofari and Lingner, 2006).
Figure 1. Human Telomerase Affinity Purification.

(A) Nuclear extracts were analyzed by immunoblot to detect the TAP tag or hnRNP C loading control (LC).
(B) Total RNA was analyzed by northern blot to detect hTR and a cross-reacting LC.
(C) Nuclear extracts were analyzed for telomerase activity by TRAP using a series of 2.5-fold dilutions normalized by total protein.
(D) Telomere lengths were analyzed by in-gel hybridization.
(E) Nuclear extract from HeLa TAP-hTERT+hTR cells was fractionated in a 15%–35% glycerol gradient. RC denotes the recovery control added before RNA extraction.
(F and G) RNA from 0.005% of the input nuclear extracts or 1% of the final elutions was analyzed by northern blot. Elutions were analyzed by TRAP using a series of 5-fold dilutions.
(H) Proteins identified by mass spectrometry in TAP-hTERT samples are summarized.
The HeLa TAP-hTERT+hTR cell line provided starting material for a rapid and gentle purification of endogenously assembled telomerase complexes. It is important to note that both tagged hTERT and ectopically expressed hTR have biological function and accumulate to only modest levels. A variety of subcellular fractionation and lysate preparation methods were tested with the goal of producing a HeLa TAP-hTERT+hTR cell extract containing the minimal amount of RNP-free hTERT. This optimization was intended to focus our initial effort on the identification of factors that interact with telomerase holoenzyme, not just hTERT. We assayed the relative sedimentation of hTERT and hTR in an optimized nuclear extract using a glycerol gradient (Figure 1E). Both hTERT and hTR were detected in complexes of greater apparent molecular mass than the H/ACA snoRNA U64 or the protein standards ferritin (440 kDa) and catalase (232 kDa). Importantly, the distributions of hTERT and hTR partially but incompletely overlap. This derives in large part from a substantial amount of hTR lacking hTERT in this type of nuclear extract, although some RNP-free hTERT was detected as well (data not shown).
Affinity Purification and Mass Spectrometry of Telomerase-Associated Proteins
Nuclear extract derived from ~3 × 1010 HeLa TAP-hTERT+hTR cells was used as the input for rapid tandem steps of affinity purification. We followed the conventional TAP protocol of binding to IgG resin, elution with tobacco etch virus (TEV) protease, binding to calmodulin resin, and elution with EGTA. As a specificity control, a parallel mock purification was performed using nuclear extract from the same number of HeLa cells without tagged hTERT. As expected, TAP-hTERT copurified hTR and telomerase activity while the mock purification did not (Figures 1F and 1G). By SDS-PAGE, several polypeptides were detected specifically in the purified TAP-hTERT sample; however, a background of nonspecific polypeptides was also enriched by calmodulin agarose (see Figure S1 available online). The final elutions from parallel TAP-hTERT and mock affinity purifications were processed as complex mixtures for mass spectrometry (Supplemental Experimental Procedures). Using strict criteria for reliable protein identifications (Supplemental Experimental Procedures), we compiled an inventory of proteins recovered specifically with TAP-hTERT. Subsequent analysis was restricted to proteins identified specifically in association with TAP-hTERT by two or more unique peptides or proteins identified specifically with a single peptide but present in both of two independent large-scale TAP-hTERT purifications (Figure 1H; peptide sequences are listed in Table S1).
Numerous peptide sequences confirmed the recovery of tagged hTERT itself. Proteomic identification of the three core H/ACA-motif binding proteins (dyskerin, NHP2, NOP10) established the success of the intended RNP affinity purification (Figure 1H), because these proteins associate with hTERT indirectly through a bridging molecule of hTR. Additional unique peptides from the H/ACA-motif binding proteins were identified in pilot mass spectrometry assays of purified TAP-hTERT complexes with less precisely matched mock purification controls (not shown in Figure 1H). In addition to the H/ACA-motif binding proteins, the TAP-hTERT sample also contained RNA binding proteins from the Sm and hnRNP protein families (Figure 1H). The association of SmB and SmD3 with active telomerase requires the hTR CAB box (Fu and Collins, 2006), a motif required for the steady-state Cajal body localization of scaRNAs (Jády et al., 2004). The association of hnRNP C with human telomerase holoenzyme has been noted previously (Ford et al., 2000; Lee et al., 2003), while association of hnRNP U has not. The sequenced peptides of hnRNP C did not differentiate the C1 and C2 isoforms encoded by the same gene. Mass spectrometry also identified two relatively uncharacterized proteins enriched specifically by TAP-hTERT: human N-acetyltransferase number 10 (NAT10) and guanine nucleotide binding protein-like 3 (nucleolar)-like (GNL3L), a paralog of the stem cell-enriched GTP binding protein GNL3 also known as nucleostemin (Tsai and McKay, 2002; Misteli, 2004). Molecular chaperones and histones were identified in the TAP-hTERT samples that were not detected in the paired mock affinity purifications, but these proteins have appeared as nonspecific background in unrelated TAP-tagged protein purifications in the laboratory and so were not analyzed further in the studies described below (data not shown).
Specificity and Significance of hTR Interaction with H/ACA-Motif Binding Proteins
The yeast orthologs of dyskerin, NHP2, and NOP10 are essential for H/ACA-motif RNA accumulation in vivo, while the fourth H/ACA-motif binding protein, GAR1, is required for RNP nucleolar localization and function. Each of the four human H/ACA-motif RNA binding proteins has been reported to interact with hTR (Collins, 2006). Curiously, we identified only three of these four proteins (Figure 2A) in the TAP-hTERT sample. The absence of GAR1 among the TAP-hTERT copurifying proteins could be due to a technical caveat of the affinity purification or protein identification, or GAR1 may be substoichiometric in human telomerase complexes in vivo. To compare human telomerase association with each of the H/ACA-motif binding proteins and the biogenesis factor NAF1, which assembles dyskerin, NHP2, and NOP10 on a nascent transcript (Darzacq et al., 2006), we transfected human 293T cells to express N-terminal TAP-tagged versions of each protein. Immunoblot analysis confirmed that the tagged proteins except NOP10 were expressed at comparable levels (Figure 2B). Complexes harboring the tagged proteins were affinity purified from whole-cell extracts using IgG resin, followed by RNA extraction and northern blot hybridization to detect hTR, the H/ACA-motif snoRNA U64, and the control small nuclear (sn) RNA U2 (Figure 2C). Each H/ACA-motif binding protein except NAF1 copurified hTR, while no hTR copurified with the TAP tag alone (Figure 2C, lanes 7–12). At a higher expression level of NAF1, some association with hTR could be detected (data not shown). NOP10 copurified less hTR than dyskerin or NHP2 due to the low level of tagged protein expression and a competitive disadvantage imposed by epitope tagging of this very small polypeptide (Kittur et al., 2006). Notably, the ratio of hTR to U64 recovered with dyskerin and NHP2 was much greater than that recovered with GAR1 (Figure 2C, compare lanes 8 and 10 to lane 11). This observation is consistent with the mass spectrometry results and suggests that, unlike other H/ACA RNPs, at least some of the endogenous human telomerase RNP population lacks GAR1 or associates with GAR1 in a less stable manner.
Figure 2. Roles for H/ACA-Motif Binding Proteins in Telomerase Biogenesis.

(A) Sizes and domain structures of the H/ACA-motif binding proteins are illustrated.
(B and C) 293T cells were transfected to express TAP tag alone or TAP-tagged protein. Whole-cell extracts were analyzed by immunoblot. Following affinity purification on IgG resin, RNA from 5% of input lysates or 100% of bound fractions was analyzed by northern blot.
(D) HeLa cells transfected with shRNA constructs were harvested at 96 hr. Asterisk indicates a nonspecific protein. Signal intensity was normalized to HSP70 protein or U2 RNA and divided by signal intensity in the control lane; substantially reduced levels are highlighted with a box.
To characterize the physiological significance of the human H/ACA-motif binding proteins for telomerase RNP biogenesis and stability, we employed small hairpin (sh) RNA constructs to reduce protein levels by RNA interference. As a control, we included a shRNA construct that targets the heterologous luciferase mRNA. Immunoblots revealed that the targeted H/ACA-motif binding protein was reduced in level but still detectable, as would be expected for a protein with essential functions (Figure 2D, top). Depletion of dyskerin, NHP2, or NOP10 reduced the levels of hTR and U64 but not U2 RNA in vivo (Figure 2D, bottom). The modest extent of GAR1 depletion that could be accomplished did not substantially alter the accumulation of any RNA tested, consistent with the phenotype of Gar1p depletion in yeast. Decreased dyskerin accumulation led to reduced biological stability of NOP10, as noted previously (Hoareau-Aveilla et al., 2006), and reduced biological stability of NHP2 as well (Figure 2D, dyskerin column; protein level was quantified relative to the control luciferase lane and if depleted was highlighted by a surrounding box). Also, depletion of NHP2 reduced the biological stability of dyskerin and NOP10 (Figure 2D, NHP2 column). This interdependence of protein levels supports the conclusion that snoRNP biogenesis occurs by concerted assembly of H/ACA-motif binding proteins (Darzacq et al., 2006). These results demonstrate crucial roles for three of the four H/ACA-motif RNA binding proteins in the accumulation of human telomerase RNP in vivo.
Specificity and Significance of hTR Interaction with Sm and hnRNP Proteins
Early in the progress of this work, we were surprised to find an association of SmB and SmD3 with scaRNAs and with hTR dependent on its CAB box for Cajal body localization (Fu and Collins, 2006). Another Sm and Like Sm (Lsm) protein was identified by mass spectrometry in subsequent TAP-hTERT sample analysis (Figure 1H), which would not be surprising nonspecific contamination in an affinity purification from RNP-enriched nuclear extract. We further investigated the specificity of Sm protein association with hTR using an expanded panel of Sm and Lsm proteins with N- or C-terminal epitope tags (Figure S2). We verified the association of SmB and SmD3 with hTR; also, we detected a very low level of hTR copurification by SmD1, but only when SmD1 was tagged at its C terminus (Fu and Collins, 2006; Figure S2C). No other Sm or Lsm protein associations with hTR or scaRNAs were detected. Among the Sm proteins, only SmB, SmD3, and SmD1 have an extended C-terminal tail hypermodified with symmetric dimethylarginine. We found that deletion of the SmB tail disrupted its association with both hTR and snRNAs, but deletion of the SmD3 or SmD1 tail disrupted only hTR association (Figure S2D). These results suggest a speculative model in which the CAB box interacts with an extended tail of SmB, SmD3, or SmD1 rather than with the multimerized core domains of a Sm protein ring. Our data do not distinguish whether such an interaction would be direct or indirect or would occur in a stable or transient manner. Because SmB, SmD3, and SmD1 can be imported into the nucleus without assembly as a Sm protein ring (Girard et al., 2004), these Sm proteins could assist in transport and/or localization of telomerase RNPs. However, we find that stable Sm protein association and Cajal body localization of hTR are not essential for telomere elongation (see below).
The hnRNP proteins package nascent mRNA and also have independent functions. The hnRNP C and hnRNP U proteins recovered with TAP-hTERT (Figure 3A) harbor distinct types of RNA binding domains and differ in known functions. To confirm the interaction of hnRNP C and hnRNP U with human telomerase holoenzyme, we immunopurified endogenous hnRNP proteins and assayed for copurification of telomerase activity. TRAP assays of hnRNP antibody-bound complexes revealed the coenrichment of telomerase activity with hnRNP C or hnRNP U, but not with the several isoforms of hnRNP Q/R or non-specific rabbit IgG (Figure 3B). Immunoblots confirmed the expected recovery specificity of hnRNP protein (data not shown). As a second approach, we expressed a panel of TAP-tagged hnRNP proteins in 293T cells, recovered the hnRNP complexes by binding to IgG agarose, and examined hTR copurification by northern blot (Figure S3). Again, only hnRNP C and hnRNP U copurified hTR. As a third approach, we assayed hnRNP protein copurification with hTERT. Extracts of cells expressing FLAG-tagged hTERT were incubated with FLAG antibody resin to recover hTERT-containing complexes, and the hTERT-associated hnRNP proteins were analyzed by immunoblot (Figure 3C). Consistent with the other assay results, we found that hTERT copurified hnRNP U and the two isoforms of hnRNP C. These interactions were specific because hTERT did not copurify any other hnRNP protein tested (Figure 3C; darker exposures and additional data not shown).
Figure 3. Telomerase RNP Association with hnRNP C and hnRNP U.
(A) Sizes and domain structures of hnRNP C and hnRNP U are illustrated. RRM and RGG are RNA binding domains, and SAP is the scaffold attachment protein motif.
(B) Immunopurifications were performed from HeLa cell nuclear extract using nonspecific polyclonal antibody, hnRNP U polyclonal antibody, or monoclonal antibody against hnRNP C or hnRNP Q/R. Bound samples were tested by TRAP using 5-fold dilutions. IC denotes the internal amplification control.
(C) 293T cells were transfected to express the FLAG tag alone or FLAG-hTERT. Following affinity purification, protein from 2.5% of the input lysates and 100% of the bound fractions was analyzed by immunoblot.
(D and E) VA13 hTR cells were transfected to express TAP-tagged hnRNP C1 or hnRNP U in the presence or absence of hTERT; proteins were analyzed as Figure 2C.
(F) 293T cells were transfected to express tagged wild-type (WT) hTR or hTR U39-42A along with the TAP tag or TAP-tagged protein. Following affinity purification on IgG resin, RNA from 5% of the input lysates or 100% of bound fractions was analyzed by northern blot.
We also determined whether the association of hnRNP C and hnRNP U with hTR was dependent on hTERT. Human VA13 lung fibroblast cells do not express endogenous hTERT or hTR. We created a VA13 cell line stably expressing retrovirally encoded hTR and transfected these cells to express TAP-tagged hnRNP C1 or hnRNP U with or without coexpression of hTERT. Immunoblot analysis confirmed that each component was expressed and accumulated as expected (data not shown). Tagged hnRNP C1 (Figure 3D) or hnRNP U (Figure 3E) was recovered from whole-cell extract using IgG agarose, and copurification of hTR was examined by northern blot. Specific hTR coenrichment with hnRNP C1 or hnRNP U was observed in the presence or absence of hTERT (Figures 3D and 3E, lanes 5–8). A consensus uridinerich hnRNP C binding site has been proposed in hTR that encompasses nucleotides 38–43 (Ford et al., 2000). We constructed an hTR variant with the four uridines at positions 39–42 substituted for adenosines (hTR U39-42A, Figure 3F). Wild-type or U39-42A hTR was coexpressed with TAP-tagged hnRNP C1 or hnRNP U in 293T cells, and then tagged protein complexes were purified from whole-cell extract using IgG agarose. Wild-type and U39-42A hTR accumulated to comparable levels (Figure 3F, lanes 1–6), and both bound hnRNP U (lanes 11 and 12), yet hTR U39-42A was substantially reduced in association with hnRNP C (lanes 9 and 10). These results suggest that hnRNP C association with telomerase holoenzyme occurs predominantly by interaction with the consensus hnRNP C binding site 5′ of the template (Figure 3F).
Several hnRNP proteins have been proposed to productively bridge telomerase holoenzyme to telomeres, based on their ability to interact with single-stranded telomerase RNA and/or telomeric-repeat DNA. A stimulatory role for hnRNP C was predicted by a correlation between loss of telomerase holoenzyme association with hnRNP C and loss of telomerase function at telomeres (Ford et al., 2000). We exploited a recently established system for assaying hTR function in vivo to test whether reducing hTR association with hnRNP C also reduced telomerase function at telomeres. The short telomeres of X-linked DC patient primary fibroblasts expressing hTERT can be restored to normal length by increasing the level of wild-type hTR (Wong and Collins, 2006). If hnRNP C interaction with hTR activates telomerase for telomere elongation, then U39-42A hTR compromised for hnRNP C interaction would be functionally deficient. In parallel, we introduced wild-type hTR, U39-42A hTR, or empty expression vector into X-linked DC patient primary fibroblasts constitutively expressing hTERT.
Expression of either wild-type or U39-42A hTR increased the overall level of hTR accumulation and increased telomerase catalytic activity in cell extract (Figures 4A and 4B). Both wild-type and U39-42A hTR also induced a rapid gain in telomere length (Figure 4C). In a similar set of experiments, we compared the biological function of wild-type hTR and a hTR variant with a single-nucleotide change in the CAB box (G414C) that abrogates its Cajal body localization and association with Sm proteins (Jády et al., 2004; Fu and Collins, 2006). Expression of either wild-type or G414C hTR increased the overall level of hTR accumulation and increased telomerase catalytic activity in cell extract (Figures 4D and 4E). Both wild-type and G414C hTR also induced a rapid gain in telomere length (Figure 4F; Figure S4). As a negative control, a catalytically inactive hTR variant failed to elongate telomeres in the same assay (Figure S4). These unexpected results suggest that neither hTR association with hnRNP C nor its steady-state enrichment in Cajal bodies has a crucial stimulatory role for telomerase holoenzyme function at telomeres, at least in the primary cells assayed here.
Figure 4. Lack of Impact of hTR Interactions with hnRNP C and Sm Proteins on Telomere Length.
Retroviral constructs expressing no hTR, wild-type (WT) hTR, or an hTR variant were integrated in human X-linked DC patient fibroblasts expressing hTERT. Following selection, cells were analyzed for hTR by northern blot (A and D), telomerase activity by TRAP of 2.5-fold dilutions of cell extract (B and E), and TRF length by in-gel hybridization (C and F). Genomic DNA used for the TRF analysis shown was harvested from cells within 10 PDL of release from selection. The two panels merged in (D) were cropped from the same image.
Change in Telomere Length Homeostasis Induced by Overexpression of hnRNP C or hnRNP U
Short-term depletion of hnRNP C or hnRNP U using shRNA vectors had no impact on the level of endogenous hTR accumulation (Figure S3C), consistent with the wild-type accumulation level of hTR U39-42A shown above. To further investigate the influence of hnRNP C and hnRNP U on telomerase function in vivo, we turned to regulated protein overexpression. We generated polyclonal lines of HTC75 human fibrosarcoma cells (van Steensel and de Lange, 1997) that harbor an integrated vector for expression of FLAG-tagged hnRNP C1 or hnRNP U or empty vector as a control. The cell lines were expanded in the presence of doxycycline to minimize recombinant protein expression. Subsequent removal of doxycycline from the culture media induced protein overexpression at a level substantially higher than that of the endogenous protein (Figure 5A). Protein overexpression was stably maintained for more than 100 population doublings (PDL) of each cell culture (data not shown). Importantly, no detectable change in culture growth rate or cell morphology occurred upon induction of protein overexpression.
Figure 5. Impact of hnRNP C or hnRNP U Overexpression on Telomere Length.
HTC75 cells were selected for integration of FLAG-tagged hnRNP C1 or hnRNP U.
(A) Antibodies specific for hnRNP C or hnRNP U were used for immunoblots.
(B) Cells were collected at the indicated PDL of protein overexpression for telomere length analysis.
TRF length was examined by in-gel hybridization using a telomeric-repeat oligonucleotide probe for cells harvested over 100 PDL of continuous growth (Figure 5B). Under our culture conditions, the parental HTC75 cell line and cell cultures selected for integration of empty vector reproducibly showed a gradual increase in telomere length (Figure 5B, vector). In contrast, cell cultures overex-pressing hnRNP C1 or hnRNP U experienced progressive telomere shortening (Figure 5B). This decrease in telomere length was reproduced in independently generated polyclonal cell cultures (data not shown). The telomere shortening induced by overexpression of hnRNP C1 or hnRNP U contrasts with the telomere elongation reported upon overexpression of hnRNP A1 (LaBranche et al., 1998). Because hnRNP proteins are multifunctional, there are many possible explanations for the impact of hnRNP protein overexpression on telomere length (see the Discussion).
Specificity of Telomerase Holoenzyme Association with NAT10 and GNL3L
NAT10 is a 116 kDa protein with a putative ATPase domain of unknown function (DUF699) and an associated N-acetyltransferase domain from the GCN5 superfamily of acetyl-CoA transferases (Figure 6A). GNL3L is a 66 kDa protein from the HSR1_MMR1 subfamily of GTP binding proteins (Figure 6A), which has an atypical primary sequence order of the conserved GTPase motifs (Leipe et al., 2002). The endogenous functions of human NAT10 and GNL3L are uncharacterized. Provocatively, proteomic and/or fluorescence localization studies place both proteins in the nucleolus (Leung et al., 2006; Du et al., 2006). We have confirmed that both proteins are predominantly nucleolar in localization when expressed with a fluorescent-protein tag (data not shown). Databases indicate that the mRNAs encoding NAT10 and GNL3L are expressed ubiquitously and at relatively high levels, suggesting that NAT10 and GNL3L have functions that extend beyond any role in regulation of telomerase.
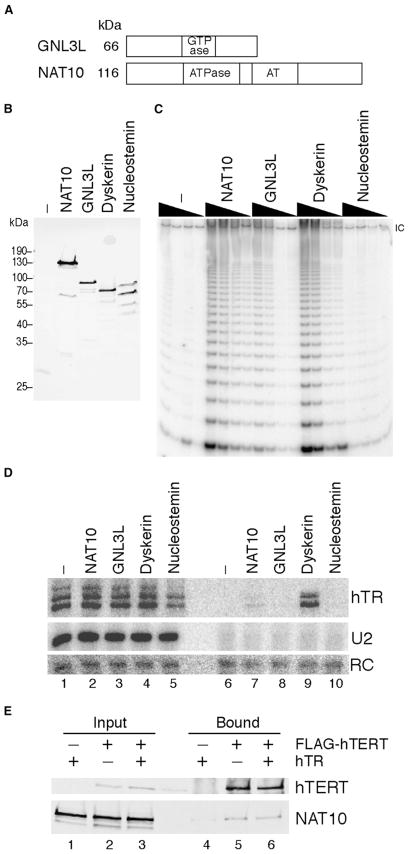
Figure 6. NAT10 and GNL3L Association with Telomerase Holoenzyme and hTERT.
(A) Sizes and domain structures of NAT10 and GNL3L are illustrated. AT indicates N-acetyltransferase domain.
(B–D) 293T cells were transfected to express the TAP tag alone or protein tagged at the N terminus with a TAP tag (NAT10, dyskerin) or C terminus with protein A domains (GNL3L, nucleostemin). (B) Whole-cell extracts were analyzed by immunoblot. (C) Following affinity purification on IgG resin, the bound fraction was assayed by TRAP in a series of 5-fold dilutions. IC denotes the internal amplification control. (D) RNA from 5% of the input lysates or 100% of bound fractions was analyzed by northern blot.
(E) Constructs expressing N-terminal RFP-tagged NAT10 or FLAG-tagged hTERT were transfected into VA13 cells in the presence or absence of hTR. Following purification on FLAG resin, protein from 2.5% of the input lysates and 100% of the bound fractions was used for immunoblot.
To confirm an association of NAT10 and GNL3L with human telomerase holoenzyme, we expressed each protein in tagged form in 293T cells. NAT10 was tagged at its N terminus with the TAP tag as was done for the other proteins described above, and GNL3L was tagged with tandem protein A domains at its C terminus based on prior localization studies (Du et al., 2006). In parallel, as positive and negative controls for interaction specificity, we expressed N-terminal-tagged dyskerin and C-terminal-tagged nucleostemin. Nucleostemin is a GNL3L paralog that is also concentrated in the nucleolus; it is most abundant in stem and cancer cells and plays a role in regulating cell proliferation (Tsai and McKay, 2002; Misteli, 2004). Proper expression of each epitope-tagged protein was confirmed by immunoblot (Figure 6B). Affinity purification of dyskerin, NAT10, and GNL3L using IgG agarose coenriched telomerase catalytic activity, while affinity purification of nucleostemin or the TAP tag alone did not (Figure 6C). NAT10 copurified hTR above the background level recovered with the TAP tag alone, but it did not co-purify as much hTR as dyskerin (Figure 6D; compare lanes 6, 7, and 9). A low but reproducibly detectable amount of hTR was also copurified by GNL3L, but not nucleostemin (additional data not shown). We readily detected GNL3L copurification with TAP-hTERT by mass spectrometry (Figure 1H), but reciprocal copurification of hTR or hTERT with various tagged versions of GNL3L seemed less efficient. A basis for this difference could be the use of nuclear extract for large-scale holoenzyme-affinity purification but whole cell extract for small-scale interaction assays, because nuclear extract production would have enriched nucleolar complexes and depleted soluble GTP.
Notably, the telomerase RNP population copurified with NAT10 or GNL3L had higher specific activity than the population of telomerase RNP copurified with dyskerin (compare Figures 6C and 6D). This enrichment suggests that, unlike the H/ACA, Sm, and hnRNP proteins described above, NAT10 and GNL3L are preferentially associated with the catalytically active RNP pool. The relatively robust association of NAT10 with telomerase holoenzyme allowed us to characterize its interaction specificity in greater detail. VA13 cells were transfected to express FLAG-tagged hTERT and red fluorescent protein (RFP)-tagged NAT10 in the presence or absence of hTR. Tagged hTERT was purified from cell extract using FLAG antibody resin, and copurification of NAT10 was assessed by immunoblot (Figure 6E). We found that affinity purification of hTERT coenriched NAT10 in the presence or absence of hTR. We next assayed the copurification of hTR with tagged NAT10 or dyskerin in the presence or absence of hTERT. VA13 cells stably producing hTR were transfected to express TAP-tagged NAT10 or dyskerin, with or without coexpression of hTERT. We found that affinity purification of dyskerin coenriched hTR in the presence or absence of hTERT, but purification of NAT10 coenriched hTR only in the presence of hTERT (Figure S5). These results support the model that NAT10 associates with telomerase holoenzyme through hTERT.
A Change in Telomere Length Homeostasis Induced by Overexpression of GNL3L or NAT10
Depletion of GNL3L or nucleostemin has been described to halt cell proliferation (Tsai and McKay, 2002; Du et al., 2006), precluding an analysis of changes in telomere length homeostasis. Short-term depletion of GNL3L or NAT10 using shRNA vectors did not substantially impact hTR accumulation or telomerase activity in cell extract, but the significance of this finding is limited by the partial extent of mRNA depletion that was accomplished (~50%; data not shown). To investigate the potential influence of GNL3L and NAT10 on telomerase function in vivo, we again turned to protein overexpression. In parallel, we established stable polyclonal HTC75 cell cultures inducibly overexpressing GNL3L, nucleostemin, or empty vector. In a separate set of parallel experiments, we established stable polyclonal HTC75 cell cultures inducibly overexpressing empty vector or NAT10. The mRNA levels of GNL3L and NAT10 increased substantially upon removal of doxycycline from the culture media (Figure 7A), and nucleostemin level assayed using an available antibody was increased by ~6-fold throughout the time course of analysis (Figure S6). In a remarkable coincidence, the only previous study of NAT10 isolated its cDNA from a one-hybrid screen for proteins that bind to the hTERT promoter to stimulate transcription (Lv et al., 2003). A very modest increase in hTERT mRNA was reported to be induced by NAT10 overexpression (Lv et al., 2003), which we did not observe (Figure 7A). Curiously, despite the lack of change in hTERT or hTR transcript levels, NAT10 overexpression did increase the level of telomerase catalytic activity in cell extract by ~2.5-fold (Figure 7B).
Figure 7. Impact of GNL3L, NAT10, or Nucleostemin Overexpression on Telomere Length.
HTC75 cells were selected for integration of the indicated expression vector followed by induction of protein overexpression.
(A) Total RNA was used to assay mRNA by RT-PCR or hTR by northern blot. Band intensities were normalized to GAPDH mRNA for RT-PCR or a non-specific crossreacting RNA for hTR and then divided by band intensity in the vector control.
(B) Telomerase activity was measured by TRAP using a series of 2.5-fold dilutions of whole-cell extract.
(C and D) Cells were collected at the indicated PDL of protein overexpression for telomere length analysis.
(E) Model for regulation of human telomerase holoenzyme.
TRF lengths were monitored for each cell culture over more than 100 PDL following protein overexpression. Importantly, all cultures grew at similar rates without a detectable change in cell morphology (data not shown). Because a previous study reported growth inhibition by overexpressed nucleostemin (Tsai and McKay, 2002), the consequences of nucleostemin overexpression may be cell-type specific. As described above, the cell cultures selected for integration of empty vector showed a modest increase in telomere length (Figures 7C and 7D, vector). Parallel increase was observed for the cell culture overexpressing nucleostemin (Figure 7C). In striking contrast, telomere length progressively decreased in cells overexpressing GNL3L or NAT10 (Figures 7C and 7D). As a control, we verified that the telomere shortening imposed by GNL3L or NAT10 was reversed by readdition of doxycycline to culture media (data not shown). These results reveal that, without decreasing telomerase activity in extract, GNL3L and NAT10 can reduce telomere length at homeostasis. We propose that these NTPase proteins regulate telomerase function by affecting the balance of telomerase subunit assembly, disassembly, and localization (Figure 7E; see the Discussion).
DISCUSSION
Complexity in the Endogenous Assemblies of hTERT and hTR
The intent of the work described here was to purify and characterize an endogenously assembled complex (or complexes) containing both hTERT and hTR. This discovery process is a critical step toward understanding the physiological principles of telomerase biogenesis, function, and regulation beyond the minimal requirements for enzyme activity in vitro. To preserve a potential complexity of telomerase-interacting proteins, we employed a relatively nondisruptive affinity purification strategy. We also exploited the tolerance of cancer cell lines such as HeLa to a relatively high level of hTR. It is important to note that these details of our approach restrict the discovery of telomerase-interacting proteins. For example, the subunit composition of telomerase assemblies may differ in normal primary cells versus a cancer cell line. Also, detection of telomerase-associated proteins was limited by a background of proteins enriched nonspecifically by the TAP protocol. Furthermore, by using a purification tag on hTERT and an RNP-enriched nuclear extract as starting material, we biased against recovery of hTERT-free RNP or RNP-free hTERT populations of potential physiological relevance. Clearly, future purifications of hTERT and hTR will be necessary to refine and expand the understanding of telomerase-interacting factors.
We originally expected to isolate hTERT RNPs containing the four human H/ACA-motif RNA binding proteins (dyskerin, NHP2, NOP10, and GAR1) and unknown proteins with functions analogous to S. cerevisiae Est1p and Est3p or T. thermophila p45 and p75. Instead, results above suggest that a substantial fraction of hTERT RNP harbors the three core H/ACA-motif RNA binding proteins (dyskerin, NHP2, and NOP10) without GAR1 or any telomerase-specific protein other than hTERT. It seems possible that, in human cells, the roles played in yeasts and ciliates by Est1p and Est3p or by p45 and p75 are not fulfilled by devoted telomerase holoenzyme proteins. In addition, telomerase holoenzyme complexes appear more prone to disassembly as TERT and RNP subcomplexes in human cells than in single-celled eukaryotes. Perhaps the vertebrate diversification of cell types was accompanied by an increase in the complexity of telomerase-negative regulation in order to optimally restrict enzyme function in time and place.
Our conclusions differ from those of a recently published study describing telomerase affinity purification by hTERT antibody binding, peptide elution, binding to substrate oligonucleotide, and activity-dependent elution (Cohen et al., 2007). In this study, which used several steps of peptide selection prior to mass spectrometry, only hTERT and dyskerin were identified specifically in purified telomerase samples. The conclusion that an endogenous human telomerase holoenzyme has just one subunit, dyskerin, in addition to the two required for catalytic activity, is attractive in its simplicity. However, our studies indicate that the situation is likely to be more complex. The increased protein complexity detected in this study may be due to the use of more rapid, gentle affinity purification and more direct analysis of the purified sample by mass spectrometry. Several lines of evidence support our proteomic evidence that human telomerase holoenzyme harbors more than a single H/ACA-motif binding protein, including at least NHP2 and NOP10 as well as dyskerin. RNA binding by dyskerin is known to require its coassembly with NHP2 and NOP10 (Darzacq et al., 2006). We show here that all three of these proteins are essential for hTR and H/ACA-motif snoRNA accumulation, consistent with recent independent studies targeting dyskerin (Hoareau-Aveilla et al., 2006) or NOP10 (Walne et al., 2007). Furthermore, a mutation in the NOP10 gene has been shown to cause DC (Walne et al., 2007), paralleling previous discoveries of DC-linked mutations in the genes encoding dyskerin, hTR, and hTERT.
Human Telomerase Regulation by Interacting Proteins
Proteins that interact with human telomerase have been more challenging to characterize in function than the proteins associated with yeast or ciliate telomerases. This may reflect a greater complexity of human telomerase-interacting proteins with regulatory roles rather than direct function in telomere elongation. If human telomerase complexes require only H/ACA proteins for biological stability and hTERT for catalytic activity, then all other associated proteins could be regulatory. The other associated proteins described here seem likely to be substoichiometric, consistent with a regulatory function. Also consistent with a regulatory function, long-term overexpression of each protein reduced telomere length by a mechanism not linked to reduction of telomerase catalytic activity in extract. We note that there are many uncertainties in the interpretation of our protein depletion and overexpression results, particularly because none of the newly characterized telomerase-interacting proteins are telomerase specific. Each protein is known or likely to have multiple functions, and the change in telomere length upon protein overexpression could arise from altered regulation of any one or more of these functions. Furthermore, the change in telomere length upon protein overexpression could be indirect, due to altered balance of the protein of interest with a competing or interacting protein that independently affects telomere length homeostasis.
One likely role for telomerase-interacting RNA binding proteins is to direct the dynamics of telomerase RNP localization. Localization of hTR changes across the cell cycle (Jády et al., 2006; Tomlinson et al., 2006). Determining the relationship between hTR localization and its association with GAR1, Sm, and hnRNP proteins is a challenging but important direction for future studies. Curiously, the two telomerase-interacting hnRNP proteins hnRNP C and hnRNP U share the distinction of nuclear retention rather than shuttling between nucleus and cytoplasm (Dreyfuss et al., 2002). As one of many possible models, these hnRNP proteins could function to retain telomerase RNP in the nucleus. Unlike yeast or ciliate telomerase RNPs, vertebrate telomerase RNPs would need to be reassembled with or reimported into the nucleus following each mitosis. If hTR interactions with hnRNP C, hnRNP U, and/or Cajal bodies are partially redundant in function, simultaneous abrogation of all interactions may be necessary to detect a phenotype. However, overexpression of hnRNP C or hnRNP U alone could induce telomere shortening by sequestering more of the pool of telomerase RNP away from telomeres at the intranuclear sites of hnRNP tethering.
The paucity of previous studies examining GNL3L or NAT10 restricts models for their roles to a speculative nature. As a speculative working model, we propose that GNL3L and NAT10 regulate hTERT RNP assembly and/or localization by a mechanism that involves physical sequestration (Figure 7E). This form of negative regulation could depend on cell integrity and thus not be detectable as a change in catalytic activity in cell extract. Negative regulation by sequestration of inactive hTERT and hTR subcomplexes would account for why either subunit can be limiting for telomerase function at a copy number far in excess of telomeres (Wong and Collins, 2006; Cristofari and Lingner, 2006). In future studies, it will be of primary interest to test the potential link between nucleolar-localized NAT10 and GNL3L proteins and the stress- and cell-cycle-dependent changes in hTERT localization (Wong et al., 2002).
EXPERIMENTAL PROCEDURES
Telomerase Affinity Purification
Nuclear extract derived from ~3 × 1010 cells was split among three tubes containing 0.25 ml of IgG agarose resin (Sigma) and rotated for 2 hr. The beads were washed four times with 20 volumes of HGN150 (10 mM HEPES [pH 7.9], 10% glycerol, 0.1% NP-40, 150 mM NaCl) and resuspended in 0.25 ml HGN150 supplemented with 30 μg/mL TEV protease, 1 μg/mL tRNA, 1 μg/mL BSA, and 0.1 mM PMSF. Following TEV protease cleavage for 1 hr, eluted material was recovered and incubated with 25 μl of calmodulin Sepharose 4B for 1 hr, then washed three times with 10 volumes of HGN150. Bound complexes were eluted at room temperature with 2 volumes of HGN150 supplemented with 2 mM EGTA. For mass spectrometry, samples were eluted with two additions of 2 volumes of 80% acenonitrile/5% formic acid.
Affinity Purification of Other Tagged or Endogenous Complexes
Transient transfection and whole-cell extract production were performed as previously described (Fu and Collins, 2006). Whole-cell extract from transiently transfected cells (1 mg of total protein) was rotated with 6 μl of IgG resin or FLAG M2 antibody resin for 2 hr at 4°C in lysis buffer (20 mM HEPES [pH 7.9], 2 mM MgCl2, 0.2 mM EGTA, 10% glycerol, 1 mM DTT, 0.1 mM PMSF, 0.1% NP-40) with 150 mM NaCl. Resin was washed extensively using the same buffer. For endogenous hnRNP protein immunopurification, rabbit polyclonal antibodies against hnRNP U (H94, Santa Cruz) or monoclonal antibodies against hnRNP C (4F4, Abcam) or hnRNP Q/R (I8E4, Abcam) were incubated with nuclear extract (1 mg of total protein) prepared from HeLa S3 cells. Following rotation at 4°C for 1 hr, protein G Sepharose was added to the extract and rotation was continued for another 1 hr.
RNA, DNA, and Telomerase Activity Assays
RNA was extracted using TRIzol and analyzed by northern blot with oligonucleotide probes. Transient hTR expression assays used wild-type or variant hTR with a 5′ tag to distinguish recombinant hTR from endogenous, and telomerase activity was analyzed by modified TRAP assay (Fu and Collins, 2006). Genomic DNA was harvested from cells using the Qiamp genomic DNA extraction kit. For TRF analysis, genomic DNA was digested with RsaI and HinfII overnight, recovered by phenol/chloroform extraction and ethanol precipitation, resolved in a 0.5% agarose gel for 16 hr, dried, and subjected to in-gel hybridization with end-labeled telomeric-repeat oligonucleotide (Wong and Collins, 2006).
Additional details are provided in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Judy Wong for pioneering the X-linked DC patient cell assay for hTR function and for contributing her expertise, Witold Filipowicz and Michael Terns for antibodies against human H/ACA RNP proteins, Gideon Dreyfuss and Jeff Wilusz for hnRNP protein antibodies, and Titia de Lange for HTC75 cells. We also thank Bobby Hogg and Greg Hannon for generous donations of expression constructs. We thank Ann Fischer for tissue culture assistance and Lori Kohlstaedt, Marco Blanchette, and Don Rio for mass spectrometry assistance. This work was funded by a grant from the National Institutes of Health (HL079585).
Footnotes
Supplemental Data include Supplemental Experimental Procedures, Supplemental References, six figures, and one table and can be found with this article online at http://www.molecule.org/cgi/content/full/28/5/773/DC1/.
References
- Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat Rev Mol Cell Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 2006;25:565–574. doi: 10.1038/sj.emboj.7600952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq X, Kittur N, Roy S, Shav-Tal Y, Singer RH, Meier UT. Stepwise RNP assembly at the site of H/ACA RNA transcription in human cells. J Cell Biol. 2006;173:207–218. doi: 10.1083/jcb.200601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol. 2002;3:195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- Du X, Rao MR, Chen XQ, Wu W, Mahalingam S, Balasundaram D. The homologous putative GTPases Grn1p from fission yeast and the human GNL3L are required for growth and play a role in processing of nucleolar pre-rRNA. Mol Biol Cell. 2006;17:460–474. doi: 10.1091/mbc.E05-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LP, Suh JM, Wright WE, Shay JW. Heterogeneous nuclear ribonucleoproteins C1 and C2 associate with the RNA component of human telomerase. Mol Cell Biol. 2000;20:9084–9091. doi: 10.1128/mcb.20.23.9084-9091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Collins K. Human telomerase and Cajal body ribo-nucleoproteins share a unique specificity of Sm protein association. Genes Dev. 2006;20:531–536. doi: 10.1101/gad.1390306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard C, Mouaikel J, Neel H, Bertrand E, Bordonne R. Nuclear localization properties of a conserved protuberance in the Sm core complex. Exp Cell Res. 2004;299:199–208. doi: 10.1016/j.yexcr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Hoareau-Aveilla C, Bonoli M, Caizergues-Ferrer M, Henry Y. hNaf1 is required for accumulation of human box H/ACA snoRNPs, scaRNPs, and telomerase. RNA. 2006;12:832–840. doi: 10.1261/rna.2344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády BE, Bertrand E, Kiss T. Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal. J Cell Biol. 2004;164:647–652. doi: 10.1083/jcb.200310138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády BE, Richard P, Bertrand E, Kiss T. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol Biol Cell. 2006;17:944–954. doi: 10.1091/mbc.E05-09-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur N, Darzacq X, Roy S, Singer RH, Meier UT. Dynamic association and localization of human H/ACA RNP proteins. RNA. 2006;12:2057–2062. doi: 10.1261/rna.249306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBranche H, Dupuis S, Ben-David Y, Bani M, Wellinger RJ, Chabot B. Telomere elongation by hnRNP A1 and a derivative that interacts with telomeric repeats and telomerase. Nat Genet. 1998;19:199–202. doi: 10.1038/575. [DOI] [PubMed] [Google Scholar]
- Lee SR, Wong JM, Collins K. Human telomerase reverse transcriptase motifs required for elongation of a telomeric substrate. J Biol Chem. 2003;278:52531–52536. doi: 10.1074/jbc.M311359200. [DOI] [PubMed] [Google Scholar]
- Leipe DD, Wolf YI, Koonin EV, Aravind L. Classification and evolution of P-loop GTPases and related ATPases. J Mol Biol. 2002;317:41–72. doi: 10.1006/jmbi.2001.5378. [DOI] [PubMed] [Google Scholar]
- Leung AK, Trinkle-Mulcahy L, Lam YW, Andersen JS, Mann M, Lamond AI. NOPdb. Nucleolar Proteome Database Nucleic Acids Res. 2006;34:D218–D220. doi: 10.1093/nar/gkj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingner J, Cech TR, Hughes TR, Lundblad V. Three ever shorter telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci USA. 1997;94:11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Liu H, Wang Q, Tang Z, Hou L, Zhang B. Molecular cloning of a novel human gene encoding histone acetyltransferase-like protein involved in transcriptional activation of hTERT. Biochem Biophys Res Commun. 2003;311:506–513. doi: 10.1016/j.bbrc.2003.09.235. [DOI] [PubMed] [Google Scholar]
- Misteli T. Going in GTP cycles in the nucleolus. J Cell Biol. 2004;168:177–178. doi: 10.1083/jcb.200412038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol Biol Cell. 2006;17:955–965. doi: 10.1091/mbc.E05-09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991–3003. doi: 10.1101/gad.55671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin KL, Collins K. Holoenzyme proteins required for the physiological assembly and activity of telomerase. Genes Dev. 2004;18:1107–1118. doi: 10.1101/gad.1201704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JMY, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, Kusdra L, Collins K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat Cell Biol. 2002;4:731–736. doi: 10.1038/ncb846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.