Abstract
Increasing the levels of CD20 expression in cells that harbor low CD20 levels may enhance their responsiveness to CD20-specific antibody therapies. Here, we examined the regulation of CD20 expression after treatment with 0.5–2.0 Gy X-irradiation and hydrogen peroxide (H2O2), in the presence or absence of known antioxidants, in the Burkitt lymphoma cell lines Daudi and Raji. Irradiation of cells enhanced cell-surface CD20 expression; the kinetics and extent of this change were cell-type specific and time-dependent. The kinetics of reactive oxygen species generation and changes in mitochondrial membrane potential after irradiation were also correlated with changes in CD20 expression. Raji and Daudi cells treated with H2O2 showed a 2-to 2.5-fold increase in CD20 expression at 12 and 20 h, respectively. Buthionine sulfoximine, which depletes glutathione, also increased surface CD20, whereas antioxidants, such as PEG-catalase, PEG-SOD, vitamin C, and amifostine, decreased CD20 expression induced by radiation or H2O2. The antioxidant-mediated decrease in CD20 expression induced by radiation or H2O2 suggests a mechanism involving redox regulation. These results demonstrate the critical role of radiation-induced oxidative stress in CD20 expression and may have implications for defining and improving the efficacy of CD20-targeted antibody therapy and radioimmunotherapy.
Keywords: CD20, Ionizing radiation, Reactive oxygen species, Antioxidants, Mitochondria, Mitochondrial membrane potential, Free radicals
The CD20 transmembrane protein is exclusively expressed on B cells. It appears during the pre-B-cell stage, but is absent during the earlier or later stages of B cell differentiation, such as in antibody-secreting plasma cells [1,2]. CD20 has received extensive evaluation as an ideal target for immunotherapy and radioimmunotherapy, in part because of its ubiquitous expression on target cells, stable localization within the cell membrane, and absence from normal stem cells [3]. Rituximab, a chimeric monoclonal anti-CD20 antibody, has been used extensively in the treatment of low-grade B cell lymphomas, such as non-Hodgkin lymphoma (NHL) and other related pathologies. In clinical applications, the efficacy of Rituximab seems to fade after a period of months, leading to Rituximab resistance. The explanation for this therapeutic resistance is not clear, although some reports have implicated a decreased level of surface CD20 expression on malignant B cells as one major contributing factor [4,5]. However, there is general agreement that diseases such as chronic lymphocytic leukemia may display the CD20 cell-surface molecule in fairly low titer and may respond proportionally less well to Rituximab compared to other low-grade B cell malignancies [5–7].
Recent studies have reported that bryostatin-1, interleukin-4, granulocyte–macrophage colony-stimulating factor, tumor necrosis factor-α, and interferon-α are all able to induce CD20 surface expression [8–13]. However, the mechanisms regulating the expression of CD20 remain poorly understood, even though these cytokines are well known to cause robust intracellular oxidative bursts [14,15]. Recently, it was shown that the bryostatin-1-induced increase of CD20 was regulated at the transcriptional level. The effect of bryostatin-1 on CD20 expression in NHL-derived cells was apparently mediated through the MAPK/ERK signal transduction pathway and involved protein kinase C [13]. Our group has previously demonstrated that treatment of cells with actinomycin D inhibited a 10-Gy γ-irradiation-induced increase in cell-surface CD20 expression [16]. As treatment of hematopoietic cells with actinomycin D is known to inhibit RNA synthesis, this suggests a transcriptional regulation of CD20 expression rather than a simple alteration in cell-surface morphology or surface presentation of the target antigen [16,17].
The ability to selectively control CD20 expression may have great therapeutic value in optimizing anti-CD20 immunotherapy and radioimmunotherapy. We have thus undertaken additional experiments to expand our investigation into the mechanistic determination of the radiogenic manipulation of CD20 surface expression on malignant B cells. Here we report data strongly suggesting that the CD20 expression is modulated by reactive oxygen species (ROS) and is thus critically dependent on the redox status of the target cell.
Materials and methods
Cell culture and materials
Daudi and Raji cells were obtained from the American Type Culture Collection and maintained in RPMI 1640 supplemented with 10% FBS (Hyclone, Logan, UT, USA), 5 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete RPMI) at 37°C in a humid atmosphere with 95% air/5% CO2.
Gramicidin, penicillin, streptomycin, DMSO, buthionine sulfoximine (BSO), hydrogen peroxide (H2O2), vitamin C, sodium-EDTA, PEG-catalase, and PEG-superoxide dismutase (PEG-SOD) were obtained from Sigma Chemicals (St. Louis, MO, USA). Fluorescent probes, such as 5-(and 6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) and 3,3′-dihexyloxacarbocyanine iodide, or DiOC6(3), were obtained from Molecular Probes (Eugene, OR, USA). Amifostine (WR-2721) was obtained from Ben Venue, Inc. (Bedford, OH, USA).
Cell survival studies
Daudi and Raji cells (5×105 cells/ml) were exposed to X-rays at a fixed energy of 300 kV, 10 mA (dose rate of 2 Gy/min) using a Pantak HF 320 X-irradiator (East Haven, CT, USA). Cells were then seeded at a density of approximately 20 cells/well (eight replicates) in 30% FBS-containing RPMI medium (200 μl) to assess their colony formation in 96-well plates as described by Chan et al. [18]. To measure survival with H2O2, cells (5×105 cells/ml) were treated with increasing concentrations of H2O2 (0–150 μM) for 24 h and then washed with 1× PBS, centrifuged (600 ×g, 10 min), and resuspended in culture medium. Cells were counted and seeded for colony formation assay in 96-well plates. Plating efficiency of Daudi and Raji cells was 90 and 85%, respectively.
To determine the surviving fraction, the unstained colonies were counted in each well using an inverted microscope (Leica, Allendale, NJ, USA). An average was taken from eight wells and experiments were repeated four times. Plating efficacy (PE) and surviving fraction (SF) were determined as [19] follows: PE=(number of colonies counted/number of cells seeded)× 100; SF= (number of colonies counted/cells seeded) ×(PE/100).
Subcellular fractionation and cytochrome c release measurement
Cells were subfractionated according to the method of Wang et al. [20]. Briefly, Daudi cells (1×108) were irradiated at 0.5 and 1.5 Gy, followed by incubation at 37°C in 5% CO2 and 95% atmosphere. After incubation, cells were washed with ice-cold PBS, suspended in 0.5 ml of a hypotonic buffer (5 mM Tris–HCl, pH 7.2; 5 mM KCl; 1.5 mM MgCl2; 0.1 mM EGTA, pH 8.0; 1 mM dithiothreitol; 0.2 mM phenylmethylsulfonyl fluoride (PMSF); 5 μg/ml leupeptin; 1 μg/ml pepstatin; and 5 μg/ml aprotinin) and incubated for 30 min on ice and homogenized using a hand homogenizer (40 strokes). After homogenization, samples were centrifuged at low speed (1000 ×g, 10 min at 4°C) to remove nuclei. The resultant supernatants were centrifuged (15,000 ×g, 15 min at 4°C) to obtain the mitochondrial fraction (pellet). The remaining supernatants were further kept for centrifugation (150,000 ×g, 1 h at 4°C) to obtain the light membrane (pellet) and cytosolic (supernatant) fractions. For detection of radiation-induced release of cytochrome c from the mitochondria, equal amounts of protein (mitochondrial and cytosolic fraction) were loaded in a 4–15% Tris–HCl gel (Bio-Rad, Hercules, CA; No. 161–1158) and then transferred transfer onto PVDF membrane. The membrane was blocked in 5% skim milk onto Tris-buffered saline (TBS) and 0.1% Tween 20 and incubated with a 1:1000 dilution of anti-cytochrome c (BD Pharmingen, Franklin Lakes, NJ; No. 556433) in the blocking mixture, followed by washing, incubation with appropriate secondary antibody, and chemiluminescence detection using ECLWestern blotting reagent (Amersham, Buckinghamshire, UK). Anti-cytochrome c oxidase subunit IV (Molecular Probes, Carlsbad, CA; No. A-21348) and β-actin (Sigma, St. Louis, MO; No. A-3854) antibodies were used as loading controls for mitochondrial and cytosolic fractions, respectively.
Immunoblotting for poly(ADP ribose) polymerase (PARP)
To study the dose (radiation)-dependent changes in cell death, PARP expression and proteolytic cleavage were determined. Briefly, Daudi and Raji cells were irradiated with increasing doses (0–2 Gy) of X-rays and incubated at 37°C in 5% CO2 and 95% air. After 24 h, cells were washed two times with ice-cold PBS, pelleted, and suspended in 200 μl HEPES (20 mM), NaCl (150 mM), sodium-EDTA (100 mM), NP-40 (1%), and protease inhibitor (1 μg/ml each aprotinin, leupeptin, and pepstatin and 1 mM PMSF). Protein concentrations were measured using the Bio-Rad reagent and equal amounts of protein were loaded in a 4–15% Tris–HCl gel (Bio-Rad; No. 161-1158) followed by transfer to PVDF membrane. The membrane was blocked in 5% skim milk powder, TBS, and 0.1% Tween 20 and incubated with an anti-PARP antibody (Cell Signaling Technology, Danvers, MA; No. 9542; 1:2000) in the blocking mixture, followed by washing and incubation with anti-rabbit HRP-conjugated antibody (Amersham, Piscataway, NJ; 1:5000). PARP was then detected using ECL Western blotting reagent.
Flow-cytometric determination of CD20 cell-surface expression
To study the redox regulation of cell-surface CD20 expression, cells (5 ×105 cells/ml in complete RPMI) were pretreated with a low concentration of vitamin C (1 μg/ml, 2 h, 37°C), amifostine (0.1 μg/ml, 1 h, 37°C), PEG–SOD (100 U/ml, 2 h, 37°C), or PEG-catalase (100 U/ml, 2 h, 37°C), followed by irradiation (0.5–2 Gy), and thereafter cells were washed (to remove antioxidants) and incubated in complete RPMI 1640 at 37°C. In contrast, cells were washed with complete RPMI before treatment with H2O2 (1 μM), as these (amifostine, vitamin C, catalase, and SOD) antioxidants are known to neutralize free radicals. PEG-catalase, PEG-SOD, vitamin C, amifostine, radiation, and H2O2 were used as individual controls to examine their effects on CD20 expression. BSO (100–150 μM), which depletes glutathione (GSH), was also used as an additional control to confirm the redox regulation of CD20 expression. Cell-surface expression of CD20 was measured by flow cytometry. Briefly, after treatment, cells were washed with ice-cold PBS, blocked in FACS buffer (0.5% BSA, 1% heat-inactivated human serum, and 0.1% sodium azide in PBS, pH 7.2), and incubated with anti-CD20 phycoerythrin-conjugated antibody [16,21]. After a 15-min incubation at 4°C (in the dark), 10,000 events were acquired using a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA) and data were analyzed using FlowJo 6.4.1 software (Tree Star, Inc., San Carlos, CA, USA). The results were expressed as fold change in CD20 expression.
The changes in levels of GSH after BSO treatment (24 h) were measured using a kinetic enzyme assay, based on the oxidation of GSH by DTNB to measure the total GSH (tGSH) levels at 405 nM using a total glutathione assay kit (Oxford Biomedical Research, Oxford, MI, USA; No. GT20). The levels of tGSH were expressed as percentage change with respect to control.
Reactive oxygen species generation
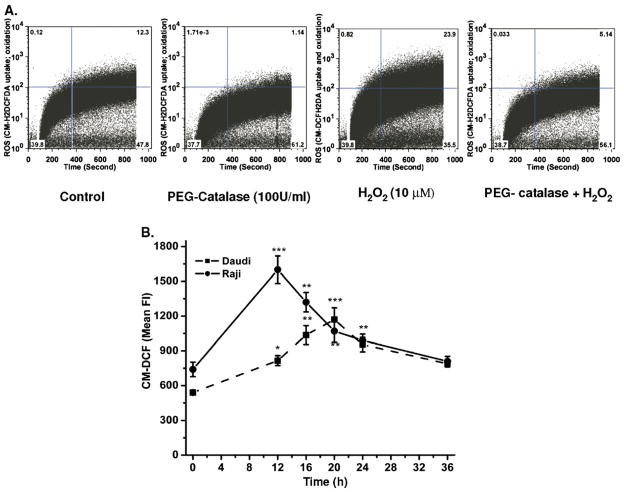
The kinetics of total ROS generation were determined by flow cytometry using CM-H2DCFDA. This molecule passively diffuses into cells and its chloromethyl group reacts with intra-cellular thiols [22–24]. Briefly, cells were treated with either H2O2 (10 μM, 1 h at 37°C as positive control) or PEG-catalase (100 U/ml, 2 h at 37°C), followed by washing and treatment with H2O2 (10 μM, 1 h at 37°C). Autofluorescence data from each cell suspension was acquired for 1 min and thereafter CM-H2DCFDA (30 μM final concentration) was added to determine uptake and free radical-mediated oxidation of dye for 15 min at Ex λ 488±10 nm and Em λ 530±10 nm.
To study the intracellular generation of total ROS after various treatments, cells were washed with PBS and incubated with CM-H2DCFDA (30 μM) for 45–60 min at 37°C. The fluorescence of the oxidized probe was acquired by flow cytometry from 10,000 cells at Ex λ 488±10 nm and Em λ 530± 10 nm. From stained samples, 10,000 counts of an ungated cell population were used to calculate the mean fluorescence using FlowJo 6.4.1. Individual controls of PEG-catalase, PEG-SOD, vitamin C, amifostine, radiation, and H2O2 were used to compensate for interference in ROS measurement, as vitamin C is well known to cause oxidation at higher concentrations, whereas amifostine is known to scavenge ROS and reactive nitrogen species (RNS). Selection of CM-H2DCFDA has the drawback that the chloromethyl group of this probe might react with intracellular thiols and thus alter the intracellular redox balance to some extent and thereby affect CD20 expression. However, radiation or H2O2-mediated changes in CD20 mRNA or protein expression can be observed as early as 4 h (data not shown for mRNA expression), whereas the cells are incubated with CM-H2DCFDA for only 15–60 min, which further decreases the potential effects on alteration of CD20 expression by this probe. Further work is nevertheless desirable with alternative probes.
Mitochondrial membrane potential
The mitochondrial membrane potential (ΔΨm) was measured as described [24]. Briefly, Daudi or Raji cells (5×105 per measurement) were stained with DiOC6(3) (40 nM in PBS) for 15 min at 37°C and thereafter analyzed for DiOC6(3) fluorescence (Ex λ 488±10 nm and Em λ 530±10 nm) by flow cytometry. Nonspecific uptake of DiOC6(3) was measured by treating cells with gramicidin (20 μM for 30 min at 37°C), to abolish the membrane potential of stained cells. The data were analyzed, using FlowJo 6.4.1, from 10,000 cells, with results being expressed as mean fluorescence.
Data analyses and statistical evaluations
Dose–response curves were produced using Prism 3.0 by using a Gaussian fit (GraphPad Software, San Diego, CA, USA), and the percentage survival was calculated using Student’s t test from the graphical analysis (Figs. 1A and B). Fit comparison between survival curves of Daudi and Raji cells were done using the F test. Changes in significance of ROS and ΔΨm were analyzed by Student’s t test, whereas for the combination (Figs. 4A and B and Table 1) ANOVA was used. For the graphical representation of the data, y-axis error bars representing ±SD are depicted and p values are shown at different levels of significance (*p<0.05, **p<0.01, ***p<0.001).
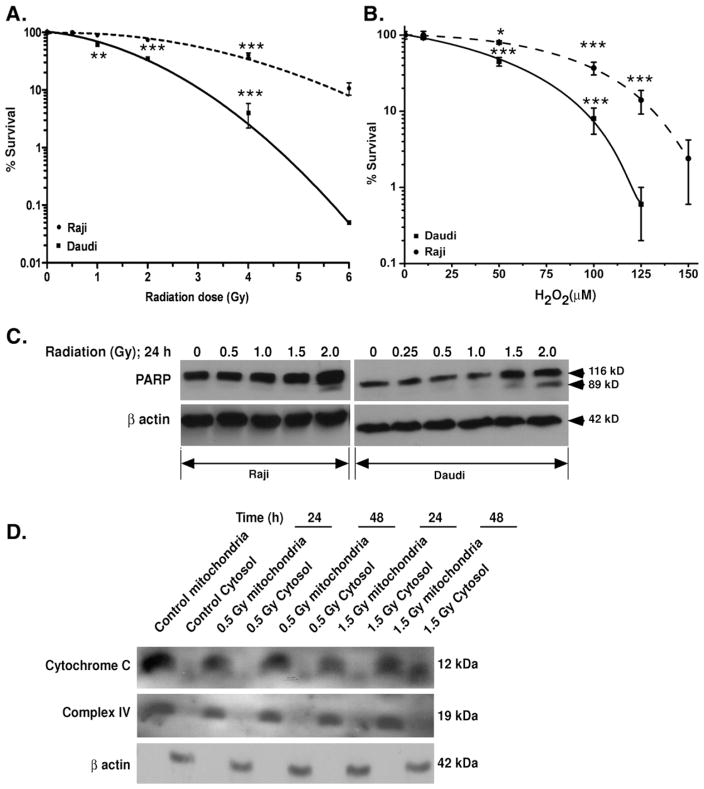
Fig. 1.
Radiation-induced changes in cell survival. (A and B) Radiation and hydrogen peroxide-mediated changes in percentage survival of cells. Daudi and Raji cells were exposed to increasing doses of radiation or treated with increasing concentrations of H2O2. After different treatments, cells were seeded in 30% FBS-containing medium for 7 days in 96-well plates as described under Materials and methods. Results are expressed as the percentage of cells surviving after treatment with respect to control ±SD of three independent experiments. (C) Radiation-induced PARP cleavage. Cells were exposed to increasing doses of radiation and incubated at 37°C for 24 h. Cells were washed and lysed and equal amounts of proteins were separated in a 4–15% Tris–HCl gel, followed by transfer onto PVDF membrane, and immunoblotted with anti-PARP antibodies as described under Materials and methods. (D) Radiation-induced release of cytochrome c. After irradiation of Daudi cells mitochondrial and cytosolic fractions were separated as described under Materials and methods. Equal amounts of proteins were separated, transferred onto PVDF membrane, and blotted with anti-cytochrome c antibody. Cytochrome c oxidase subunit IV was used as cell fractionation control for mitochondria and β-actin for the cytosolic fraction.
Fig. 4.
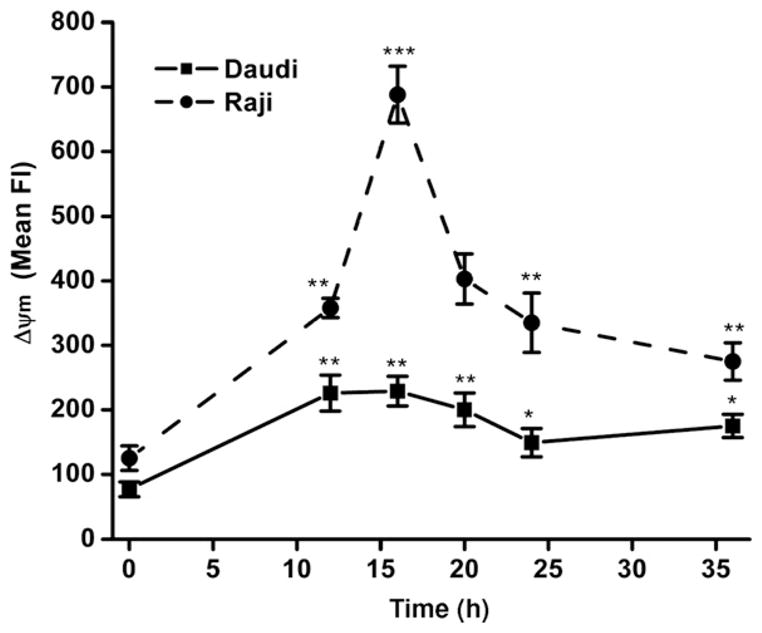
Radiation-mediated changes in mitochondrial membrane potential (ΔΨm). The changes in mitochondrial membrane potential were acquired by flow cytometry at different time intervals after irradiation (0.5 Gy) of Daudi and Raji cells using cyanine dye DiOC6(3). Membrane potential-dependent uptake of DiOC6(3) was measured at an excitation wavelength of 488 nm and emission wavelength of 530 nm. The results are expressed as mean fluorescence±SD of three experiment and significance was analyzed using t test (*p<0.05, **p< 0.01, ***p<0.001).
Table 1.
Summary of cell-surface CD20 expression and its inhibition by antioxidants
| Treatment | CD20 expression (fold change) |
|
|---|---|---|
| Raji (at 12 h) | Daudi (at 20 h) | |
| Control | 1 | 1 |
| Vitamin C (1 μg/ml, 1 h) | 1 | 1 |
| Amifostine (0.1 μg/ml, 1 h) | 1 | 1 |
| H2O2 (1 μM) | 1.65±0.14* | 1.58±0.11* |
| Vitamin C (1 μg/ml, 1 h)+H2O2 (1 μM) | 1.13±0.06# | 1.1±0.08# |
| Radiation (0.5 Gy) | 2.2±0.25* | 1.8±0.3* |
| Vitamin C (1 μg/ml, 1 h)+radiation (0.5 Gy) | 2.25±0.35* | 2.1±0.2* |
| Amifostine+H2O2 | 1.1±0.073## | 0.94±0.08## |
| Amifostine+radiation (0.5 Gy) | 0.9±0.2### | 1.2±0.15### |
Maximum CD20 expression varies from 12 to 20 h, depending on the cell type. Modulation of H2O2-or radiation-(0.5 Gy) induced CD20 expression using antioxidants (amifostine or vitamin C) was measured as described under Materials and methods. Cell-surface CD20 expression was measured by flow cytometry at 12 and 20 h for Raji and Daudi cells, respectively. All results are from three independent experiments and expressed as fold change in expression of CD20±SD.
p<0.05 for control vs radiation or H2O2.
p<0.05 for H2O2 vs vitamin C+H2O2.
p<0.5 for H2O2 vs amifostine+H2O2.
p<0.05 for 0.5 Gy vs amifostine+0.5 Gy.
Results
Cell survival
To determine an appropriate radiation dose range for the present investigations, clonogenic cell survival studies were first performed. Exposure of cells to increasing doses of X-rays (0–6 Gy) reduced the colony formation (Fig. 1A). The observed LD50 values for irradiated Raji and Daudi cells were 3.2±0.4 and 1.7 ±0.2 Gy, respectively. These results indicate that Raji cells are relatively radiation resistant, as reported [25]. Survival studies with H2O2 (0–150 μM) revealed that Raji cells were also more resistant to H2O2 treatment, having an LD50 of 80±5 μM compared to the Daudi cells, which have an LD50 of 38±4 μM (Fig. 1B).
Exposure of Daudi and Raji cells to X-irradiation leads to PARP cleavage in a dose-dependent manner. Daudi cells were found to be more sensitive than Raji and cleaved PARP was observed at 1.5 Gy, whereas in the case of Raji cells it was observed at 2.0 Gy (Fig. 1C). Exposure of Daudi cells to 0.5 Gy did not induce cytochrome c release at either 24 or 48 h. Only at a higher dose (1.5 Gy) was some release of cytochrome c detected at 48 h with respect to control (Fig. 1D).
Cell-surface expression of CD20
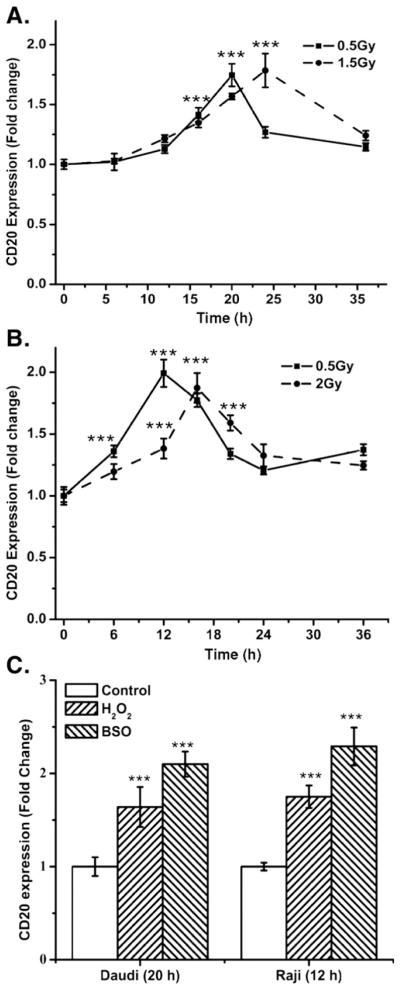
To establish clinical relevance for the present study, a low radiation dose was used to study the kinetics of radiation-induced changes in cell-surface expression of CD20 protein. CD20 expression was measured at different time intervals after exposure to a single dose of 0.5 Gy radiation (Figs. 2A, Daudi, and B, Raji). The increase in cell-surface expression of CD20 was transient and cell-type dependent, as irradiation of logarithmically growing Daudi and Raji cells showed a maximum increase at 20 and 12 h, respectively. The increase in CD20 was followed by a gradual time-dependent decrease, even though the CD20 expression levels remained significantly higher with respect to control (0 h) in both Daudi and Raji cells (p<0.05).
Fig. 2.
The kinetics of changes in cell-surface expression of CD20 by radiation. The changes in CD20 expression were measured by flow cytometry using phycoerythrin-conjugated anti-CD20 antibody and the fold change in cell-surface CD20 expression was calculated with respect to 0 h. (A) Daudi cells. (B) Raji cells. (C) Changes in cell-surface CD20 expression caused by BSO or H2O2. Daudi or Raji cells were treated with BSO (100 or 150 μM, respectively) or H2O2 (1 μM) for 20 or 12 h, respectively, and thereafter fold change±SD in cell-surface CD20 expression was measured from four independent experiments. Statistical analysis was done using Student’s t test (***p<0.001).
To study the relationship between radiation dose and CD20 expression, Daudi and Raji cells were exposed to a higher dose of radiation (37% cell death; 1.5 and 2.0 Gy, respectively). Exposure of Daudi and Raji cells to 1.5 and 2.0 Gy, respectively, also increased cell-surface CD20 expression significantly (Figs. 2A and B; p<0.001). This increase was quite similar to that observed after 0.5 Gy, although the maximal increase in the levels of CD20 by higher radiation doses was delayed in both cell lines. After irradiation, maximal cell-surface expression of CD20 was observed at 24 h for Daudi and at 16 h for Raji cells.
Exposure of cells to radiation is known to induce intracellular oxidative stress [26]. To determine the role of intracellular redox changes or oxidative stress on the expression of CD20, cells were treated with two oxidative agents, H2O2 and BSO; BSO increases intracellular oxidative stress by depleting glutathione. Treatment of Raji cells for 12 h with H2O2 (1 μM) or BSO (150 μM) resulted in a 1.65±0.24-and a 2.3±0.21-fold increase in CD20 expression, respectively (Fig. 2C). Moreover Daudi cells treated for 20 h with 1 μM H2O2 or 100 μM BSO showed a 1.58±0.18-and 2.16±0.24-fold increase in CD20 levels, respectively. The induction of CD20 expression by H2O2 and BSO strongly supports a redox regulation of CD20 expression (Fig. 2C). Daudi or Raji cells treated with BSO showed 79.8±4.73 and 74±3.12% decrease in levels of tGSH, respectively, compared to untreated control.
ROS and mitochondrial membrane potential
The kinetics of time-dependent uptake and oxidation of CM-H2DCFDAwas measured using flow cytometry. Cells pretreated with PEG-catalase showed inhibition of H2O2-mediated oxidation of probe, which suggests that the intracellular oxidation of dye is ROS dependent (Fig. 3A). The kinetics of radiation-induced changes in levels of ROS (Fig. 3B) and mitochondrial membrane potential (Fig. 4) was also measured to examine the role of intracellular redox changes in CD20 expression. Exposure of Daudi and Raji cells to 0.5 Gy radiation showed maximal increase in ROS generation of 2.8±0.3-fold at 12 h and 2.2±0.18-fold at 20 h, respectively (Fig. 3B). Radiation-induced changes in ΔΨm were maximal at 16 and 20 h in Raji (4.5±0.37-fold) and Daudi (2.6±0.3-fold) cells, respectively (Fig. 4). The increases in both ROS and ΔΨm were transient and their levels were restored by 36 h to those seen before treatment, indicating that the viability of the cells was maintained.
Fig. 3.
CM-H2DCFDA uptake and its ROS- mediated oxidation. (A) Measurement of uptake and oxidation of CM-H2DCFDA. (A) Cells were pretreated with PEG-catalase followed by washing and treatment with H2O2. The changes in uptake and free radical-mediated oxidation of CM-H2DCFDA were acquired for 15 min as described under Materials and methods. (B) Radiation-induced changes in intracellular ROS generation. The kinetics of radiation-induced changes in generation of ROS was measured at different time intervals after 0.5 Gy radiation by flow cytometry. After irradiation, cells were washed with PBS and incubated with CM-H2DCFDA for 1 h at 37°C in the dark. Fluorescence (Fl) of CM-DCF was acquired at 488-nm excitation wavelength and 535-nm emission wavelength by flow cytometry and data were analyzed using FlowJo 6.4.1. Results are expressed as the mean fluorescence±SD of three independent experiments and statistical analysis was performed using Student’s t test (*p<0.05, **p<0.01, ***p<0.001).
Redox regulation of CD20 expression
To determine the role of redox changes in cell-surface CD20 expression, cells were treated with antioxidants (PEG-SOD, PEG-catalase, vitamin C, or amifostine) before irradiation or treatment with H2O2. Daudi or Raji cells pretreated with PEG-catalase and vitamin C showed significant inhibition of H2O2-mediated increase in CD20 expression (Figs. 5A, 6, and Table 1; p<0.001). However, PEG-SOD, PEG-catalase, and vitamin C partially inhibited the radiation-induced increase in cell-surface CD20 expression in either Daudi or Raji cells. Nevertheless, preirradiation treatment with amifostine significantly inhibited the radiation-induced increase in CD20 expression at both lower (0.5 Gy; Table 1) and higher (1.5 Gy for Daudi and 2.0 Gy for Raji) doses of irradiation (Fig. 5A). However, cells treated with PEG-catalase or vitamin C showed complete inhibition of H2O2-mediated increase in CD20 expression (Fig. 6).
Fig. 5.
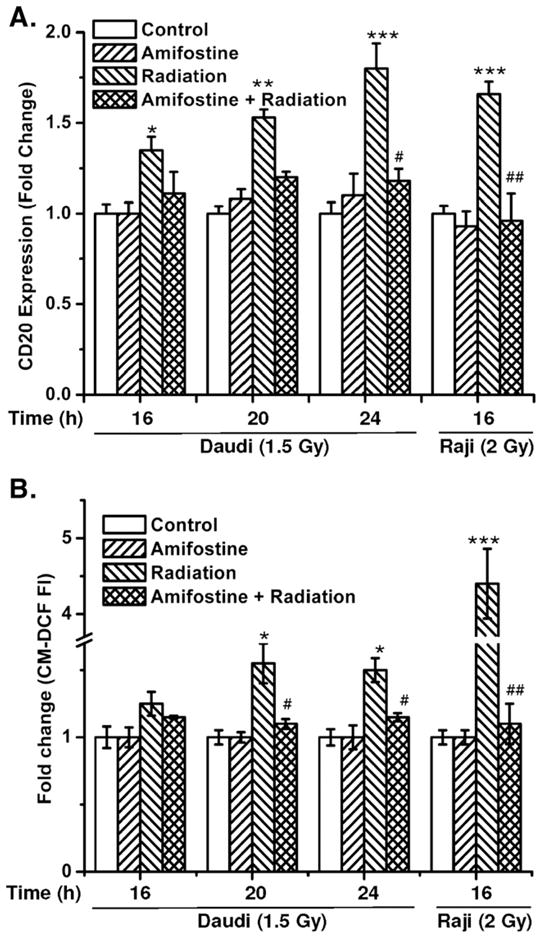
Antioxidants prevent radiation-mediated increase in CD20 expression and ROS generation. (A) The kinetics of CD20 expression and (B) changes in ROS generation were measured with respect to untreated control by flow cytometry after different treatments with amifostine, radiation, or a combination of amifostine with radiation. Cells were treated with amifostine (0.1 μg/ml; 1 h) followed by irradiation (Daudi, 1.5 Gy; Raji, 2.0 Gy) and thereafter cells were washed to remove the amifostine as described under Materials and methods. Expression of CD20 and ROS was measured by flow cytometry using anti-CD20 phycoerythrin-conjugated antibody and CM-H2DCFDA, respectively. The data were analyzed using FlowJo 6.4.1 and the results are expressed as the mean fluorescence for ROS generation or CD20 expression. The statistical comparisons were done using ANOVA (*p<0.05, **p<0.01, ***p<0.001 for control vs radiation, #p<0.05, ##p<0.001 for radiation vs amifostine+radiation).
Fig. 6.
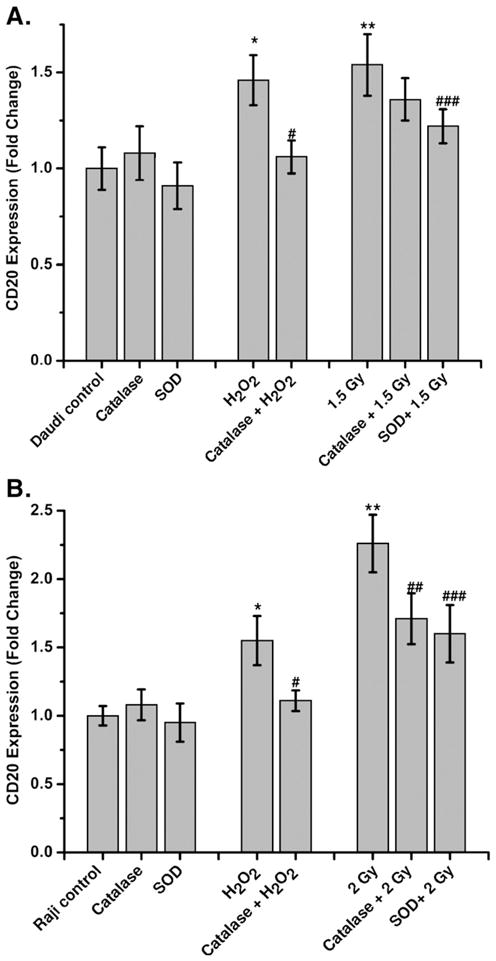
Antioxidants prevent the radiation-mediated increase in CD20 expression. The kinetics of CD20 expression was measured with respect to untreated control by flow cytometry after treatment with either PEG-SOD or PEG-catalase, radiation, or H2O2, or a combination of PEG-SOD or PEG-catalase with radiation or H2O as described under Materials and methods. CD20 expression was determined in (A) Daudi and (B) Raji cells using anti-CD20 phycoerythrin-conjugated antibody at 20 and 12 h, respectively. The data were analyzed using FlowJo 6.4.1 and the results expressed as fold change of CD20 expression with respect to control. Statistical comparison was performed using ANOVA (*p<0.05 for control vs H2O2, **p<0.05 for control vs radiation, #p<0.05 for PEG-catalase vs H2O2, ##p < 0.05 for PEG-catalase vs radiation, ###p < 0.05 PEG-SOD vs radiation).
The results of radiation-induced changes in the generation of ROS also correlated with the expression of CD20 in both Raji and Daudi cells (Fig. 5). Preirradiation treatment of cells with amifostine significantly inhibited radiation-induced alterations in ROS generation in both Daudi and Raji cells (p<0.001; Fig. 5B). The inhibition of H2O2 and/or radiation-induced increase in CD20 levels by antioxidants further suggests a possible regulatory role of redox changes in CD20 expression.
Discussion
In the present investigation, radiation doses ranging from 0.5-to 2.0 Gy were used to mimic the clinical conditions of radiotherapy and/or radioimmunotherapy used in the management of B cell NHL. Cells exposed to 0.5 Gy radiation showed a significant increase in CD20 expression (Figs. 2A and B). The enhanced expression of CD20 antigen was associated with transcriptional upregulation of CD20 mRNA and CD20 regulatory transcription factors (D. Gupta et al., unpublished data). The increase in CD20 surface antigen was found to be correlated with overall changes in oxidative stress (Fig. 3B) and ΔΨm (Fig. 4). The role of oxidative stress in low-dose radiation-induced CD20 expression was confirmed by similar findings in cells treated with low concentrations of H2O2 (Fig. 2C). Moreover, the increased expression of CD20 was also observed after increasing endogenous levels of oxidative stress by BSO (Fig. 2C), a well-known agent that decreases the levels of the major endogenous molecular antioxidant glutathione, by inhibiting the synthesis of the glutathione S-transferase enzyme [27].
Mitochondria consume over 85% of molecular oxygen during cellular oxidative phosphorylation and, therefore, constitute the major site of oxidative stress. The presence of a high amount of unsaturated fatty acids and metallo-proteins in mitochondrial membranes makes them more vulnerable to radiation (ROS)-induced damage, which can lead to the release of cytochrome c and induction of apoptosis [28,29]. Release of cytochrome c is an indicator of apoptosis; however, its extent depends on radiation exposure [30]. Exposure of cells to low dose radiation alters the intracellular redox balance by inducing oxidative stress and thereby depletion of intracellular antioxidants [14,31]. Therefore, extraneous supplementation of antioxidants serves as a useful buffer to protect cells from radiation (ROS)-induced injury [32]. Antioxidants, such as SOD, catalase, and vitamin C, are known to neutralize free radicals. Pretreatment of cells with PEG-catalase or vitamin C showed significant inhibition of H2O2-induced increase of CD20 expression (Fig. 6 and Table 1). This suggests an important role for oxidative stress in CD20 expression. However, PEG-catalase, PEG-SOD, and vitamin C were inefficient in inhibiting the radiation-induced increase in CD20 expression. This may be due to their specificity for neutralizing a more limited range of free radicals than what is produced by radiation, which generates superoxide, H2O2, hydroxyl radical, nitric oxide, and peroxynitrite [15,28,31]. Strikingly, amifostine could completely inhibit the radiation-induced increase in CD20 levels. Amifostine (WR-2721) is an FDA-approved phosphothiol prodrug, which forms the pharmacologically active metabolite WR-1065 upon dephosphorylation [32,33]. WR1065 is known for its free radical scavenging potential and stability. WR-1065 enters the cell predominantly by passive diffusion or via the polyamine transport system [34]. The dephosphorylation of WR-2721 may initiate the thiol/disulfide exchange reaction and thereby influences intracellular thiols viz. cysteine and GSH [35,36]. Upon entry of WR–1065 into the cells, its naturalizing potential of redox modifiers viz. ROS and RNS may be responsible for inhibiting the radiation-or ROS-mediated expression of CD20.
The increase in CD20 expression/cell was independent of radiation dose in the range of 0.5 to 2.0 Gy (sublethal or lethal; Figs. 1A–1C), suggesting that the low-dose radiation delivered during radioimmunotherapy may be sufficient to achieve significant changes in CD20 levels. This effect, conceivably, may be responsible for certain clinical benefits of the targeted radio-pharmaceuticals. There are two main therapeutic implications of the present investigation. First, one might imagine pretreating areas of known CD20+ tumor with low doses of external beam radiation immediately before anti-CD20 immunotherapy to increase CD20 availability on the target surface. Because the half-life of the antibody in serum is greater than 6 weeks, and the duration of the CD20 hyperstimulation effect is only 1–2 days, this pretreatment might be repeated at some reasonable clinical interval. In essence, targeted external beam radiation could be used to provide a geographic mechanism for selective target enhancement of the immunotherapy effect in cases in which one desires to widen the therapeutic window between normal B cells and the malignant target tissue. Clinical trials are now in progress in which bulky CD20+ lymphoma sites in patients being prepared to undergo Y-90 Ibritumomab therapy are pretreated with focal external beam radiation in an attempt to increase disease responsiveness or response duration without negatively impacting hematopoietic tolerance. One might also extrapolate these findings to help explain the enhanced efficacy of anti-CD20 Ibritumomab compared to Rituximab antibody treatment alone [37]. The relatively low dose of radiation delivered during the process of Y-90 Ibritumomab infusion might concurrently function to increase CD20 expression on target cells, thereby increasing the likelihood of effective binding during the initial pharmacologic loading phase in which much of the radiolabeled antibody is still in the cardiac blood pool and vascular channels.
Moreover, recent clinical trials in follicular NHL patients indicate that low doses of external beam radiotherapy may result in a delayed but dramatic response with gradual but ultimately complete clearance of tumor in both treated and untreated areas of the body (the poorly understood “abscopal” effect) [38,39]. Part of this effect might involve the same radiation-related changes discussed in this report. If so, then understanding how to control the radiogenic CD20 stimulation effect may produce both short-term and long-term anti-tumor activity.
In conclusion, we provide the first report implicating redox signaling in the enhanced expression of CD20. The data presented here strongly suggest that changes in the cellular redox environment are responsible for the enhanced expression of CD20 antigen in cells exposed to low doses of radiation. These findings may improve our understanding of the molecular signaling events leading to the expression of the CD20 antigen. The ability of low-dose radiation to enhance the expression of CD20 in B-cell malignancies, together with its geographically selective cytotoxic effects, warrant the further exploration of the use of focal low doses of radiation and CD20-targeted radioimmunotherapy in patients with CD20-positive B cell malignancies.
Acknowledgments
The authors thank the anonymous reviewers for their insightful comments. These studies were supported by funds from the Legros Memorial Cancer Research Fund (R.M.M.) and the National Institutes of Health (CA81504 to A.A.). We are grateful for the valuable support of the Flow Cytometry Facilities staff at the Lerner Research Institute, Cleveland Clinic.
Abbreviations
- ΔΨm
Mitochondrial membrane potential
- NHL
Non-Hodgkin lymphoma
- ROS
Reactive oxygen species
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-regulated kinase
- FBS
Fetal bovine serum
- DMSO
Dimethyl sulfoxide
- PEG–catalase
Polyethyleneglycol catalase
- PEG–SOD
Polyethyleneglycol superoxide dismutase
- CM–H2DCFDA
5–(and 6)–chloromethyl–2′7′–dichlorodihydrofluorescein diacetate acetyl ester
- DiOC6(3)
3,3′–dihexyloxacarbocyanine iodide
- PBS
Phosphate-buffered saline
- PE
Plating efficacy
- SF
Surviving fraction
- BSO
buthionine sulfoximine
- GSH
Glutathione
- DTNB
5,5′–dithiobis(2–nitrobenzoic acid)
- RNS
reactive nitrogen species
References
- 1.Tedder TF, Disteche CM, Louie E, Adler DA, Croce CM, Schlossman SF, Saito H. The gene that encodes the human CD20 (B1) differentiation antigen is located on chromosome 11 near the t(11;14) (q13; q32) translocation site. J Immunol. 1989;142:2555–2559. [PubMed] [Google Scholar]
- 2.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 3.Pohlman B, Sweetenham J, Macklis RM. Review of clinical radio-immunotherapy. Expert Rev Anticancer Ther. 2006;6:445–461. doi: 10.1586/14737140.6.3.445. [DOI] [PubMed] [Google Scholar]
- 4.Manshouri T, Do KA, Wang X, Giles FJ, O’Brien SM, Saffer H, Thomas D, Jilani I, Kantarjian HM, Keating MJ, Albitar M. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101:2507–2513. doi: 10.1182/blood-2002-06-1639. [DOI] [PubMed] [Google Scholar]
- 5.Lundin J, Osterborg A. Advances in the use of monoclonal antibodies in the therapy of chronic lymphocytic leukemia. Semin Hematol. 2004;41:234–245. doi: 10.1053/j.seminhematol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, Rambaldi A, Introna M. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–3389. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 7.Huhn D, von Schilling C, Wilhelm M, Ho AD, Hallek M, Kuse R, Knauf W, Riedel U, Hinke A, Srock S, Serke S, Peschel C, Emmerich B. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98:1326–1331. doi: 10.1182/blood.v98.5.1326. [DOI] [PubMed] [Google Scholar]
- 8.Dancescu M, Wu C, Rubio M, Delespesse G, Sarfati M. IL-4 induces conformational change of CD20 antigen via a protein kinase C-independent pathway: antagonistic effect of anti-CD40 monoclonal antibody. J Immunol. 1992;148:2411–2416. [PubMed] [Google Scholar]
- 9.Sivaraman S, Venugopal P, Ranganathan R, Deshpande CG, Huang X, Jajeh A, Gregory SA, O’Brien T, Preisler HD. Effect of interferon-alpha on CD20 antigen expression of B-cell chronic lymphocytic leukemia. Cytokines Cell Mol Ther. 2000;6:81–87. doi: 10.1080/13684730050515804. [DOI] [PubMed] [Google Scholar]
- 10.Sivaraman S, Deshpande CG, Ranganathan R, Huang X, Jajeh A, O’Brien T, Huang RW, Gregory SA, Venugopal P, Preisler HD. Tumor necrosis factor modulates CD 20 expression on cells from chronic lymphocytic leukemia: a new role for TNF alpha? Microsc Res Tech. 2000;50:251–257. doi: 10.1002/1097-0029(20000801)50:3<251::AID-JEMT9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal P, Sivaraman S, Huang XK, Nayini J, Gregory SA, Preisler HD. Effects of cytokines on CD20 antigen expression on tumor cells from patients with chronic lymphocytic leukemia. Leuk Res. 2000;24:411–415. doi: 10.1016/s0145-2126(99)00206-4. [DOI] [PubMed] [Google Scholar]
- 12.Chow KU, Schneider B, Mitrou PS, Weidmann E. Influence of various cytokines on the expression of CD20 on the surface of CLL–cells in vitro. Leuk Res. 2001;25:99–100. doi: 10.1016/s0145-2126(00)00098-9. [DOI] [PubMed] [Google Scholar]
- 13.Wojciechowski W, Li H, Marshall S, Dell’Agnola C, Espinoza-Delgado I. Enhanced expression of CD20 in human tumor B cells is controlled through ERK-dependent mechanisms. J Immunol. 2005;174:7859–7868. doi: 10.4049/jimmunol.174.12.7859. [DOI] [PubMed] [Google Scholar]
- 14.Turpaev KT. Reactive oxygen species and regulation of gene expression. Biochemistry (Moscow) 2002;67:281–292. doi: 10.1023/a:1014819832003. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annu Rev Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- 16.Kunala S, Macklis RM. Ionizing radiation induces CD20 surface expression on human B cells. Int J Cancer. 2001;96:178–181. doi: 10.1002/ijc.1018. [DOI] [PubMed] [Google Scholar]
- 17.Mazumder S, Gong B, Almasan A. Cyclin E induction by genotoxic stress leads to apoptosis of hematopoietic cells. Oncogene. 2000;19:2828–2835. doi: 10.1038/sj.onc.1203623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan HT, Hughes D, French RR, Tutt AL, Walshe CA, Teeling JL, Glennie MJ, Cragg MS. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into Triton X-100 insoluble membrane rafts. Cancer Res. 2003;63:5480–5489. [PubMed] [Google Scholar]
- 19.Crosby ME, Jacobberger J, Gupta D, Macklis RM, Almasan A. E2F4 regulates a stable G2 arrest response to genotoxic stress in prostate carcinoma. Oncogene. 2007;26:1897–1909. doi: 10.1038/sj.onc.1209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 21.Treon SP, Pilarski LM, Belch AR, Kelliher A, Preffer FI, Shima Y, Mitsiades CS, Mitsiades NS, Szczepek AJ, Ellman L, Harmon D, Grossbard ML, Anderson KC. CD20-directed serotherapy in patients with multiple myeloma: biologic considerations and therapeutic applications. J Immunother. 2002;(25):72–81. doi: 10.1097/00002371-200201000-00008. 1997. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 23.Grant ER, Dubin AE, Zhang SP, Zivin RA, Zhong Z. Simultaneous intracellular calcium and sodium flux imaging in human vanilloid receptor 1 (VR1)-transfected human embryonic kidney cells: a method to resolve ionic dependence of VR1-mediated cell death. J Pharmacol Exp Ther. 2002;300:9–17. doi: 10.1124/jpet.300.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ. 2003;10:323–334. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustonen R, Bouvier G, Wolber G, Stohr M, Peschke P, Bartsch H. A comparison of gamma and neutron irradiation on Raji cells: effects on DNA damage, repair, cell cycle distribution and lethality. Mutat Res. 1999;429:169–179. doi: 10.1016/s0027-5107(99)00123-2. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drew R, Miners JO. The effects of buthionine sulphoximine (BSO) on glutathione depletion and xenobiotic biotransformation. Biochem Pharmacol. 1984;33:2989–2994. doi: 10.1016/0006-2952(84)90598-7. [DOI] [PubMed] [Google Scholar]
- 28.Somosy Z. Radiation response of cell organelles. Micron. 2000;31:165–181. doi: 10.1016/s0968-4328(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, Taketani S. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- 30.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 32.Grdina DJ, Murley JS, Kataoka Y, Zhou D, Seed TM. Radio-protectors: current status and new directions. Radiat Res. 2005;163:704–705. [PubMed] [Google Scholar]
- 33.Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63(Suppl 2):2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- 34.Dest VM. Radioprotectants: adding quality of life to survivorship? Semin Oncol Nurs. 2006;22:249–256. doi: 10.1016/j.soncn.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Dennis MF, Stratford MR, Wardman P, Watfa RR. Increase in intracellular cysteine after exposure to dithiothreitol: implications in radiobiology. Int J Radiat Biol. 1989;56:877–883. doi: 10.1080/09553008914552351. [DOI] [PubMed] [Google Scholar]
- 36.Dennis MF, Stratford MRL, Wardman P, White J. Thiols and antioxidants in radiobiology: chemical and bioanalytical problems. In: Seymour CB, Mothersill C, editors. New developments in fundamental and applied radiobiology. London: Taylor & Francis; 1991. pp. 328–333. [Google Scholar]
- 37.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104:2635–2642. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 38.Safwat A. The role of low-dose total body irradiation in treatment of non-Hodgkin’s lymphoma: a new look at an old method. Radiother Oncol. 2000;56:1–8. doi: 10.1016/s0167-8140(00)00167-5. [DOI] [PubMed] [Google Scholar]
- 39.Safwat A. The immunobiology of low-dose total-body irradiation: more questions than answers. Radiat Res. 2000;153:599–604. doi: 10.1667/0033-7587(2000)153[0599:tioldt]2.0.co;2. [DOI] [PubMed] [Google Scholar]