Abstract
Two decades after introducing threaded titanium dental implants, Dr. Per-Ingvar Brånemark used a similar technique in the 1980s to pioneer the direct skeletal attachment (DSA) of limb prostheses. He and his colleagues used convincing clinical experience to overcome the skepticism of their peers, affording a new dimension of prosthetic rehabilitation to almost 100 individuals with amputation. As a result, more research has been initiated worldwide to move DSA to a level of greater safety, longevity, and reliability. This review highlights the trends and milestones in current DSA development. It also identifies ideas from previous studies in various fields that may be useful in future DSA development.
Keywords: amputation, arthroplasty, bone, direct skeletal attachment, infection, limb amputation, osseointegration, prosthetic rehabilitation, skin, transcutaneous devices
INTRODUCTION
As of 2008, about 100 individuals with amputation have had abutments for attaching leg prostheses implanted in their residuum [1]. Dr. Per-Ingvar Brånemark developed the technology for direct skeletal attachment (DSA) of limb prostheses after discovering that a firm bond exists between the titanium implant and the surrounding bone. He called the bond “osseointegration” [2]. Brånemark discovered osseointegration in an orthopedic model by implanting the titanium device into the medullary canal of an animal tibia [3], but the first translation of the research to human applications occurred in the field of dentistry [4]. Brånemark introduced his method for DSA of limb prostheses after almost 2 decades of continuous development and refinement of his successful method in dental implantology [5].
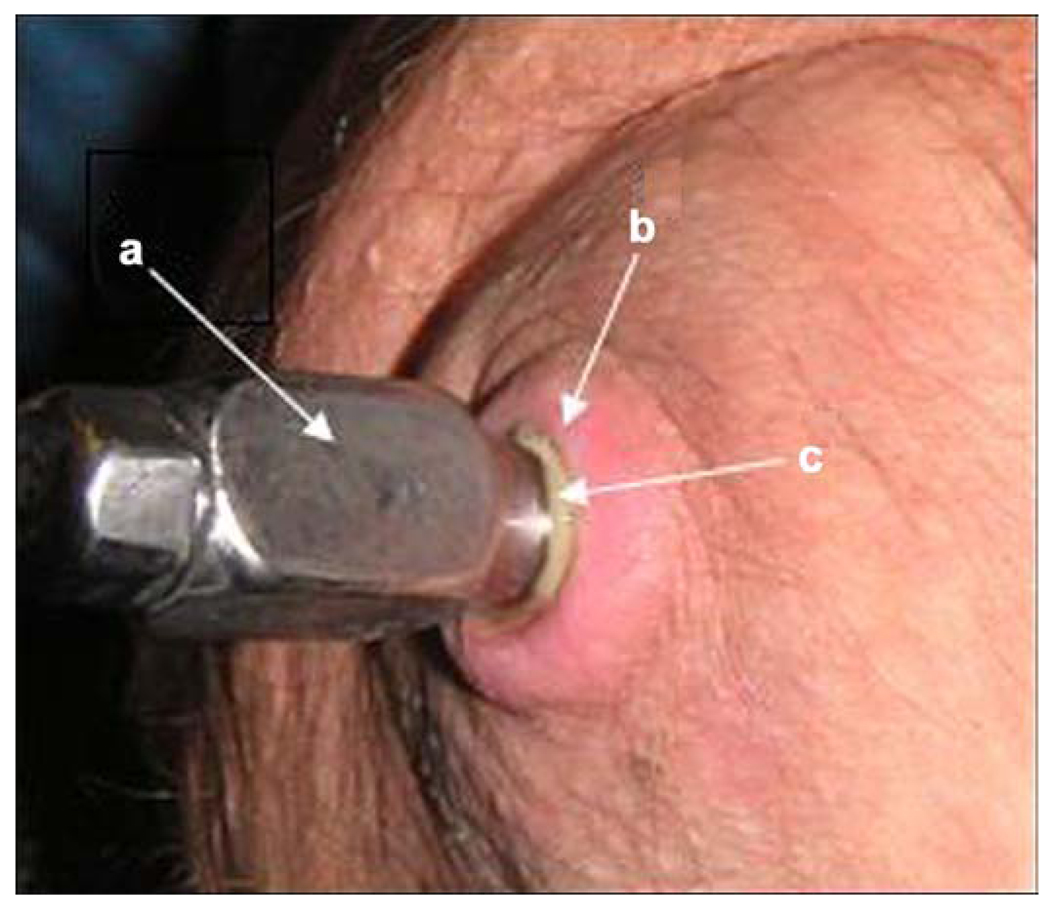
At the 2004 International Society for Prosthetics and Orthotics World Congress in Hong Kong, one of Brånemark’s patients demonstrated how his transfemoral prosthesis could be attached to and detached from the abutment implanted in his residuum. I was impressed by the demonstrator’s quality of ambulation and high level of satisfaction with and enthusiasm for DSA technology. However, the demonstrator pointed out the layer of pus between the abutment and skin of the residuum (Figure 1), which was an obvious indicator that an infection-free seal did not exist around the abutment. This observation prompted me to consider integrating the abutment with the surrounding skin. Thus, a project was commenced to explore the hypothesis that a porous implant would create the proper conditions for the skin to form an infection-free seal [6]. A safe skin-device interface for DSA is part of the broader problem of infection-free transcutaneous devices, and this article presents a review of different approaches to the problem.
Figure 1.
(a) Titanium abutment penetrating residuum skin, (b) surrounding skin, and (c) layer of pus between skin and abutment. Source: Reprinted from Pitkin M, Raykhtsaum G, Galibin OV, Protasov MV, Chihovskaya J V, Belyaeva I G. Skin and bone integrated prosthetic pylon: A pilot animal study. J Rehabil Res Dev. 2006;43(4):573–80. [PMID: 17123195] DOI:10.1682/JRRD.2005.05.0160
Another aspect of DSA that will be presented and discussed here is the risk of loosening of the implant in the medullary canal and the existing approaches to addressing this problem.
CHALLENGE OF LONGEVITY FOR DIRECT SKELETAL ATTACHMENT
In 2007, approximately 1.7 million persons were living with limb loss in the United States [7]. The main cause of acquired limb loss is poor circulation in the limb as a result of arterial disease, with more than half of all amputations occurring among people with diabetes mellitus. Amputation of a limb may also occur after a traumatic event or for the treatment of bone cancer [7].
The U.S. Department of Defense reported that between September 2001 and January 12, 2009, 1,286 individuals underwent amputations, of whom 935 had major limb amputations during Operation Iraqi Freedom in Iraq, Operation Enduring Freedom in Afghanistan, and unrelated conflicts. Of the 1,286 total persons with amputation, 77 percent sustained their injury while in the Army, 19 percent while in the Marines, 2 percent while in the Navy, and 2 percent while in the Air Force [8]. Expanded military operations have decreased the mean age at which amputation occurs. The survival rate after severe gunshot and land mine trauma has concurrently increased significantly. The increased survival rate has been made possible by improved immediate medical care on the battlefield, fast transporting of the wounded soldiers to definitive care facilities, better instrumentation that allows advanced external fixation of the traumatized limbs, and new surgical materials and techniques. As a consequence, the ratio of “number of amputees to number of death casualties” has increased to 30 percent from 7 percent during World War II and from 15 percent during the former Soviet Union’s 1979–1989 war in Afghanistan [9].
DSA of limb prostheses is a promising alternative to the traditional “socket-residuum” attachment. DSA can provide actual osseoperception, improving all locomotor activities of a patient, and eliminates the problems associated with donning and using a socket [10]. In light of the growing attention to neural prosthetics [11–12], DSA may provide in the future a platform for reliable transmission of signals from peripheral nerves to controllers outside the body [13]. However, prosthetics and rehabilitation professionals face the double challenges of rising number and rising life expectancy of amputation survivors. One must ensure that the skin-device interface is safe and sustainable and that the bone-device bond is sufficiently long-lasting. For these new challenges to be met, the existing or potential problems related to all components of the DSA technique and instrumentation should be carefully examined and addressed.
DIRECT SKELETAL ATTACHMENT OF LIMB PROSTHESIS: BRÅNEMARK’S METHOD
To eliminate the negative outcomes of the socket-residuum attachment, such as discomfort, pain, and secondary trauma [14], Brånemark and his colleagues introduced a method of attaching a prosthesis directly to the bone of the residuum [10,15]. Reasonable indications for DSA include a short residuum, an excessive volume of soft tissues, skin problems, and pain, which make the traditional residuum-socket attachment a hardly viable option. In upper-limb prosthetics, DSA can better restore grasp in patients with thumb amputations [16].
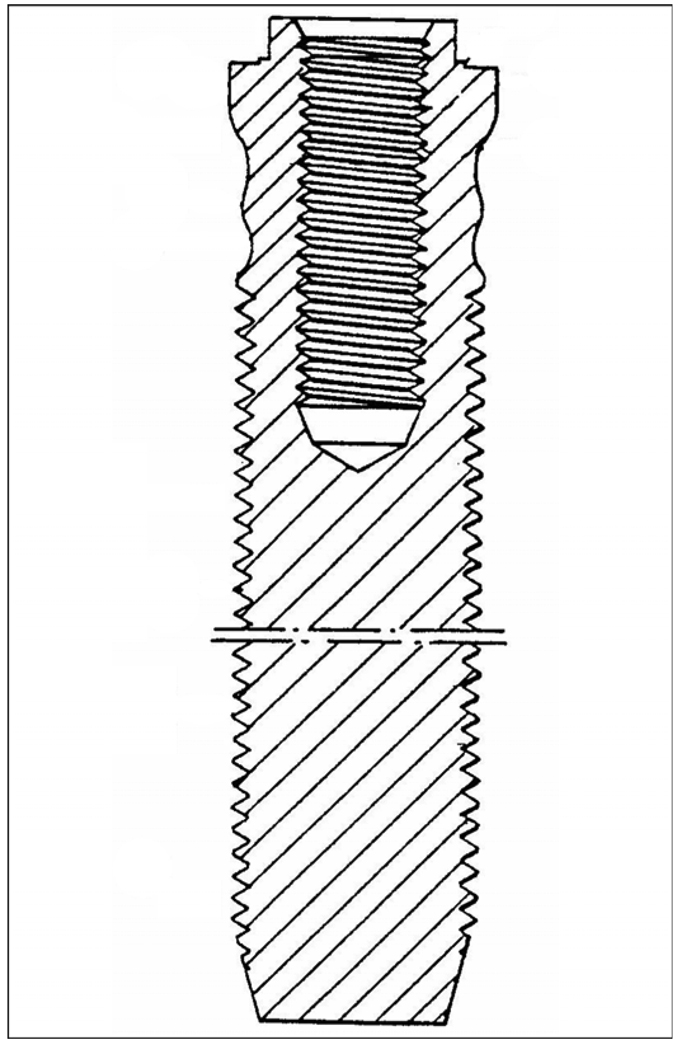
The DSA technique is similar to that developed by Brånemark for dental implantology (Figure 2): a titanium threaded fixture is inserted into the bone remnant of the residuum, and the prosthesis is attached by an abutment. Albrektsson and colleagues reported that the threaded fixtures, manufactured of pure titanium and without any coating, were removed for various reasons from 18 patients after loading up to 90 months and demonstrated direct chemical bonding between the bone and titanium [17].
Figure 2.
Implant fixture for tooth prosthesis by Brånemark. Source: Adapted from Brånemark P. Implant fixture for tooth prosthesis. United States patent US 4988299. 1991 Jan 29.
DSA requires a long-lasting bond between the implant and the hosting bone and an infection-free transcutaneous passage. These requirements are also critical in the other areas of implantation. Experts in arthroplasty (total joint replacement) have sought to improve the bone-device bond for decades [18–20]. A safe skin-device interface is of great importance for dialysis [21–23], trachoeostomy [24–26], and feeding and drug delivery in chronically ill patients [27–28]. Therefore, DSA may benefit from research and ideas from other fields.
SKIN-IMPLANT INTERFACE
The problem of infection in the device-skin interface remains unsolved; infection as an adverse effect is reported in a high percentage of cases [29–31]. Studies were undertaken to promote and increase the binding junction with the skin by testing different materials with various circumferential components [32]. Attempts have been made to enhance the interface between the implants and skin cells by creating open pores on the surface of the implant [33], making fiber metal composites [34], treating the surface of the implants with calcium phosphates [35–36], varying thermal conditions [37], and coating the metal with specific agents to decrease mechanical mismatch between the implant and skin or bone [38]. The interface has been evaluated by measuring adhesion, proliferation, and differentiation of cells [39–40]. Cell morphology and cytoskeleton have been evaluated with scanning or confocal microscopy by the synthesis of the proteins of the extracellular matrix and proteoglycans [41]. In vivo, biocompatibility has been evaluated histologically [42–43].
EFFECTS OF POROSITY
Porosity was considered a logical means for improving the integration of devices with hosting biological tissues like tubular bone [44–50], spine [51–53], skin [54–58], and tendon [59–61]. Empirically, the volumetric porosity of an implant should be at least 30 to 50 percent for surrounding tissues to grow inside. Increased porosity, however, significantly decreases the strength of the implant. New technologies combining powder metallurgy and polymer foam technologies demonstrated some progress in manufacturing titanium and tantalum foam with strength close to trabecular bone but not exceeding compact bone [62–63].
Porosity of percutaneous implants has been a point of interest for many researchers. Von Recum summarizes the areas of application of the percutaneous implants, including internal/external prosthetic devices, blood access devices, tissue access devices, body cavity access devices, and power and signal conduits [64]. He also describes the principal failure modes in the applications of the percutaneous devices, as identified by Lee et al. [65], Hall et al. [66], and Winter [67]. These modes are (1) extrusion due to marsupialization, (2) extrusion due to permigration, (3) extrusion due to infection and abscess formation, (4) extrusion due to avulsion, and (5) extrusion due to any combination of the just listed failure modes.
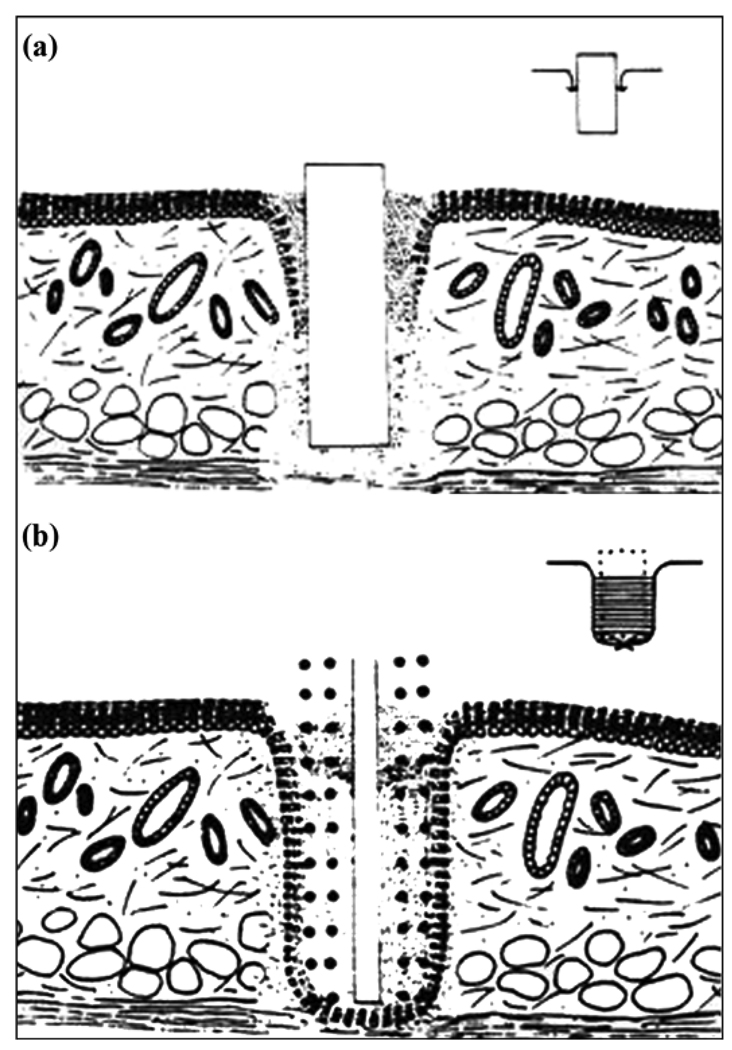
Marsupialization is shown in Figure 3(a), with the epidermis migrating and proliferating inward along the solid implant. Also, when a penetrating implant has no pores or pores with diameter less than 40 µm, a sinus tract is formed [66]. The use of a porous implant surface was proposed to avoid marsupialization and the sinus tract infection identified in a study with Dacron velour [68]. In that study in pigs, a percutaneous implant for attaching an artificial limb had an intramedullar stem with porous surface and Dacron velour was used at the soft tissue interface. The ingrowth of both bone and soft tissue was seen. However, the authors reported that velour was unable to maintain adequate epithelial adhesion to form an anatomical seal and a barrier to bacteria. The failure of the permigration mode (Figure 3(b)) in the use of Dacron velour has been confirmed by von Recum [64].
Figure 3.
(a) Schematic of histological section of skin with smooth surface implant. Epidermis migrates and proliferates inward along implant causing marsupialization. (b) Epidermal migration through porosity or permigration. Source: Adapted by permission from Von Recum AF. Applications and failure modes of percutaneous devices: A review. J Biomed Mater Res. 1984;18(4):323–36. [PMID: 6234317] DOI:10.1002/jbm.820180403
In 1970, Hahn and Palich reported positive results using porous titanium in orthopedic applications [69]. Clemow et al. have examined the interfacial shear properties of bone tissue growth into porous-coated Ti6A14V femoral implants as a function of the pore size of the porous surface [70]. Cylindrical implants with powders of three particle-size ranges (297, 420–500, and 595– 707 µm) were manufactured. After sintering, the fine, medium, and coarse powders resulted in pore sizes of approximately 75, 225, and 325 pm, respectively. The implants were inserted for 6 months into the femoral medullary canal of dogs. Push-out tests on the removed femurs revealed that the interfacial shear strength and stiffness of the bond decreased as pore diameter increased within the range of 175–325 µm.
The antimicrobial feature of titanium oxide (TiO2) has been demonstrated and used to explain the low failure rate (0.07% per month) of hearing aids percutaneously anchored to bone with Brånemark’s procedure [71]. The antimicrobial properties of TO2, along with fibroblast adhesion to it, have been confirmed on Dacron fibers coated with a thin layer of TO2 [72]. In a study on implanted biosensors, the anti-inflammatory properties of TO2 were demonstrated with a small amount of TO2 covering only 23 percent of the surface area [73].
The effectiveness of a barrier to infection in a skin-implant interface depends on the quality of the proliferation and attachment of keratinocytes to the surface of the implant. The attachment of the skin and bone cells to titanium surfaces is so strong because a very thin layer of titanium peroxy compounds is in contact with the living cells [10,74]. Surprisingly, Pendegrass and colleagues postulated that surfaces with a smooth topography of the titanium alloy implant at the point of epithelium-implant contact could increase attachment in vivo, producing an effective barrier against infection [75].
If we go back to the instance of pus between the abutment and skin (Figure 1), the failure to prevent chronic inflammation can explained by avulsion,* which is the other failure mode described earlier [64].
Avulsion is a major problem for limb DSA, since the mobility of skin reaches its maximum in the distal zone of a residuum. The average mobility in patients with excessive volume of soft tissues in that zone is even higher.
To minimize avulsion, the mobility of skin around the implant has to be significantly reduced. A surgical technique developed by Brånemark and his colleagues for this purpose includes removing the fat and thinning the surrounding skin to the thickness of a split-skin graft, allowing for skin adhesion to the bone end [16]. Devices for reducing avulsion have included a percutaneous bar with a flexible mesh collar, holes at the subcutaneous perimeter [76–77], and a collar made of a stainless steel spring or nylon hooks [78]. Animal studies with these devices produced promising results. However, the implants with a connecting collar are sensitive to its positioning relative to the derma and subcutaneous tissues and may not tolerate junction shifting when the distance from the bone to the skin-binding junction changes [78].
Another approach was positioning of a bar with a porous flange in the dermal tissues immediately below the epithelium [79–80]. While it may reduce the mobility of skin in the plane parallel to the flange, the attachment to the solid bar still remains fragile, similar to the prior art.
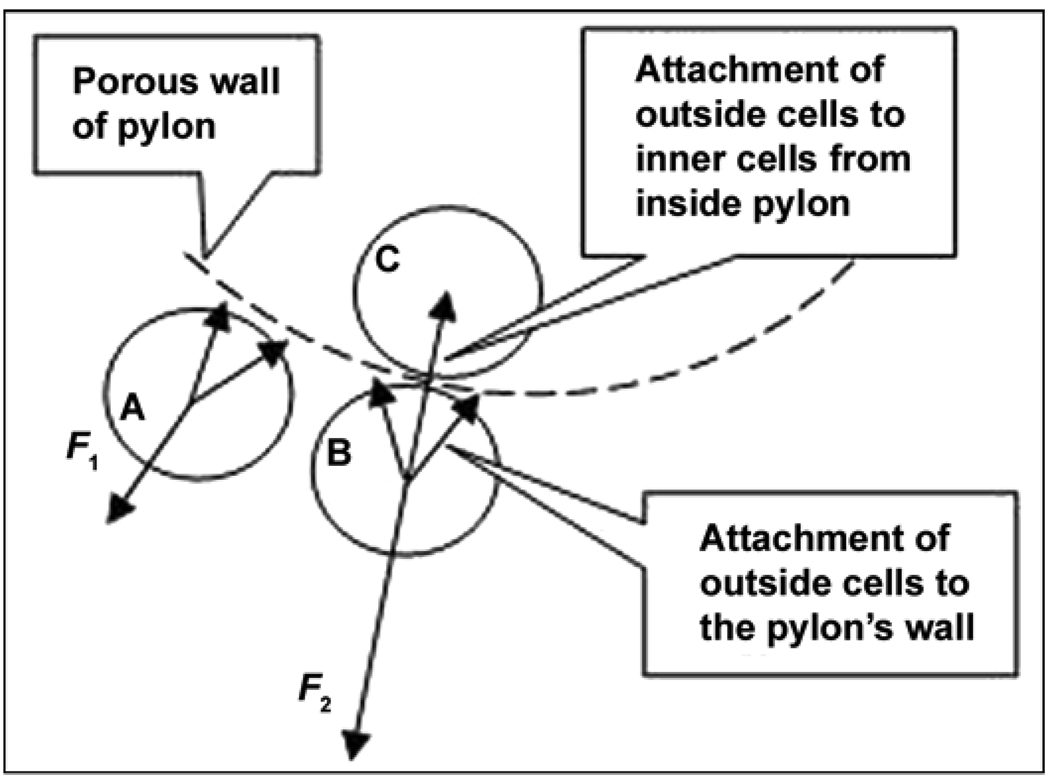
In 2004, we initiated in vitro and in vivo studies on a totally porous pylon for DSA [81]. We suggested that resistance to detachment (avulsion [64]) will be increased because of the natural bond of the cells outside the pylon with the cells inside the pylon. This resistance is illustrated by a schematic in Figure 4, in which F1 and F2 are the minimal forces needed to detach a skin cell A from the pylon with the traditional “cell-to-wall” and a cell B with the “cell-to-wall-to-inner cell C” attachments, respectively. If a natural bond is permitted between the cells outside and inside the pylon through a pore in the pylon wall, an additional “cell-to-cell” attachment (adhesion) force contributes to the resistance to the cell’s detachment, thus providing F2 > F1 [82].
Figure 4.
Minimal force needed for detachment of skin cells A, B, and C from pylon. F1 = “cell-to-wall” detachment (prior art), F2 = “cell-to-wall-to-inner cell” detachment in skin and bone integrated pylon design. Source: Adapted from Pitkin M, Raykhtsaum G, Pilling J, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG, Blinova MI, Yudintseva NM, Potokin IL, Pinaev GP, Moxson V, Duz V. Porous composite prosthetic pylon for integration with skin and bone. J Rehabil Res Dev. 2007;44(5):723–38. [PMID: 17943684] DOI:10.1682/JRRD.2006.12.0160
A recent histopathology study demonstrated deep ingrowth of skin and bone throughout the novel pylon (i.e., skin and bone integrated pylon 2) [83], whose strength exceeds that of human bone [84].
BONE-IMPLANT INTERFACE
The literature tends to perceive the interface of the implanted fixture with the bone as a less serious problem than its interface with the skin [10,38,85]. We believe that this perception might be true in the short term. Long-term effects must be considered, since DSA is intended for lifelong use and present reports on the long-term applications of the DSA are still limited [86].
Current DSA technology uses threaded titanium fixtures that are screwed into the medullary canal of the residuum bone [5]. Bone remodeling has been observed inside the threads and on the surface of the metal fixture [86]. However, the procedure of screwing damages endosteum† integrity, resulting in endosteal absorption and consequent medullary canal widening, which may cause clinically unstable implants [87]. The medullary canal also widens naturally: as young people age, the osteoclasts in the endosteum break down bone on the internal bone surface, around the medullary cavity [88]. The increase in the medullary canal diameter was reported as a risk factor for implant loosening in younger patients after arthroplasty [89].
I therefore look at results from arthroplasty procedures because the bone-implant interface has been investigated intensively and because the medullary canal is used for implantation in both arthroplasty and DSA.
The loosening of the implanted shaft inside the canal has always been a concern in arthroplasty [90]. Loosening occurs mainly as a result of the cyclical application of axial loads and bending moments during locomotion, which eventually destroy the bond between the implant and the bone [91–92].
A drill bores the tube bone to prepare an area in the medullary canal into which a DSA fixture or prosthesis stem fits exactly. The bone remodeling proceeds from the outer walls toward the interior walls of the medullary canal [93]. Such ossification keeps the implant inside the bone canal by developing multiple microlocks [10].
Different theories, including the genome-based theory [94], have tried to explain the biological mechanism of loosening of the implanted prostheses, but none are satisfactory [95–97]. Various design modifications of the stems and techniques, with or without use of cement, have been introduced and examined, including taper slip stems with a polished surface, fixation by intramedullary nails, and the use of high-pressure saline to inflate the diameter of a cylindrical implant [98]. However, all known approaches depend on the ability of the medullary canal to act as a holding cavity for the stem of the prosthesis.
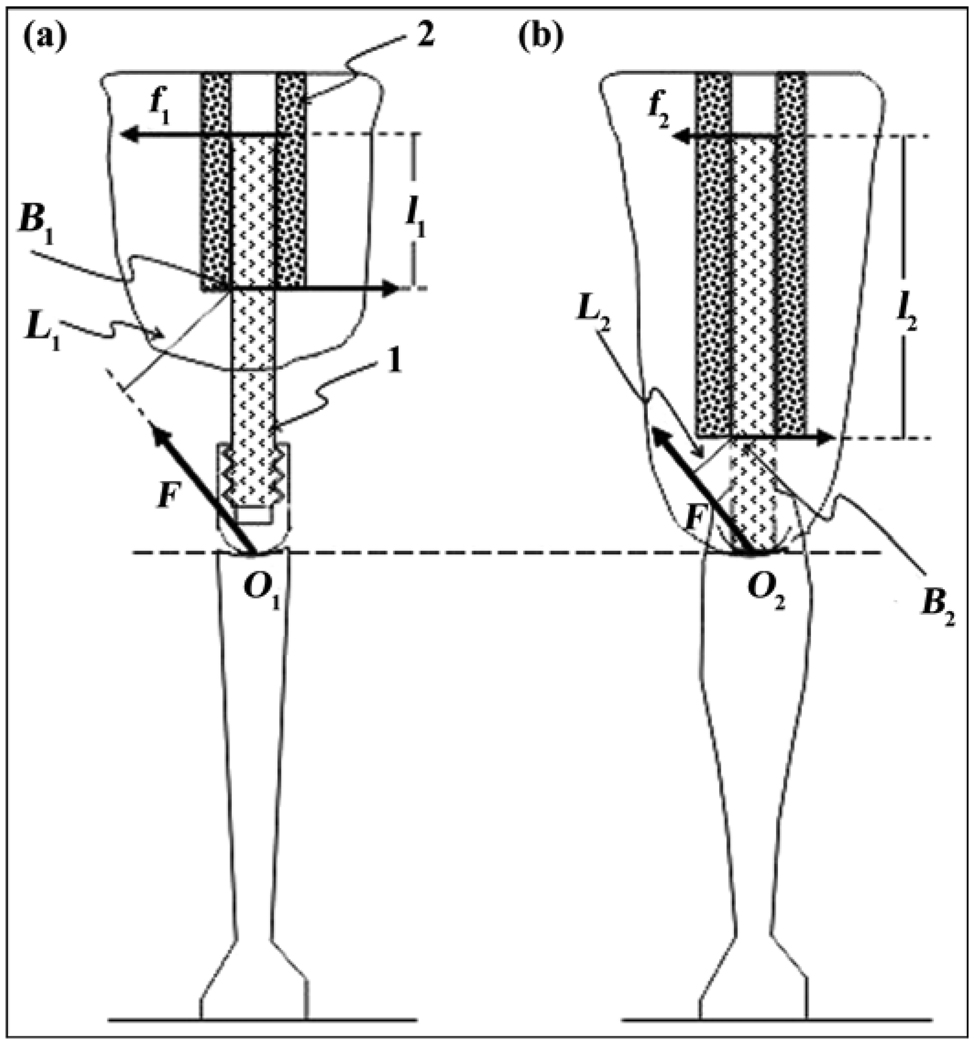
One important reason exists why even the most promising technologies addressing loosening in arthroplasty may not be translated successfully to osseointegration. Because a hosting bone is shortened after amputation, its ability to retain a DSA implant is less than a full-length bone’s ability to retain a prosthetic stem in arthroplasty. Therefore, the depth of insertion in osseointegration is always less than that following arthroplasty. A model of loading/resistance in total joint replacement and DSA is presented elsewhere [99] and illustrated in Figure 5. The model demonstrated that normal loads from the implanted shaft to the bone walls following DSA will always be greater than following total joint replacement.
Figure 5.
Modeling of moment of resistance to bending of pylon (1) implanted to medullary canal (2) via (a) osseointegration and (b) arthroplasty. L1 = lever arm with respect to point B1 of bending force F applied to point O1, L2 = lever arm with respect to point B2 of force F applied to point O2, l1 = lever arm of reaction force f1 with respect to point B1, l2 = lever arm of reaction force f2 with respect to point B2. Source: Reprinted from Pitkin M. One lesson from arthroplasty to osseointe-gration in search for better fixation of in-bone implanted prosthesis. J Rehabil Res Dev. 2008;45(4):vii–xiv. [PMID: 18712634]
For patients with a long residuum, devices with a solid core and a porous collar for osseointegration could be a solution. Two of these devices were applied to attach leg prostheses in dogs. One, designed by Dr. Ola Harrisson and his colleagues [100], had a porous collar on an interlocking nail. The implant was secured with screws inserted from the outside of the bone into the implanted nail, which had a porous collar at the level where it penetrated the skin.
A similar system with a porous tantalum sleeve on the titanium core was applied to a dog with bilateral transtibial amputations. The implants were 123 mm in length with a 43 mm-long tapered threaded stem ranging in diameter from 4 to 6 mm, which was implanted to the tibial medullary canal. The tantalum sleeve was 70 to 80 percent porous by volume, with an average pore diameter of 500 mm. A stem was press-fitted to the tibia with the sleeve providing a stop. At 26 months after initial surgery and 17 months after revision of one of the two implants, the dog’s function was restored at a walk, trot, and run [85].
The anchoring and locking approach requires additional operation time and techniques for exact positioning of the screws relative to the holes of the shaft implanted into the medullary canal. Another potential disadvantage of the technique is a predetermined orientation of the porous collar relative to the skin of the residuum, which replicates that seen in the device with the perforated flange [79]. On the other hand, it is a one-step procedure compared with the classical two-step Brånemark procedure, which could be beneficial for patients in the future.
An important argument in favor of the classical two-step procedure is that the after fixture is initially implantated, it remains under the skin of the residuum and is totally “immersed” to the bone. The bone walls keep the fixture unloaded for about 3 months before the second step (installation of the abutment), which is essential for osseointegration. Eliminating the mobility of an implant relative to the bone for the period of bone remodeling is imperative for successful osseointegration. A study by Pilliar and colleagues demonstrated that bone ingrowth can occur in the presence of movement up to 28 µm, while excess movement (150 µm or more) can result in attachment by mature fibrous connective tissue ingrowth [101].
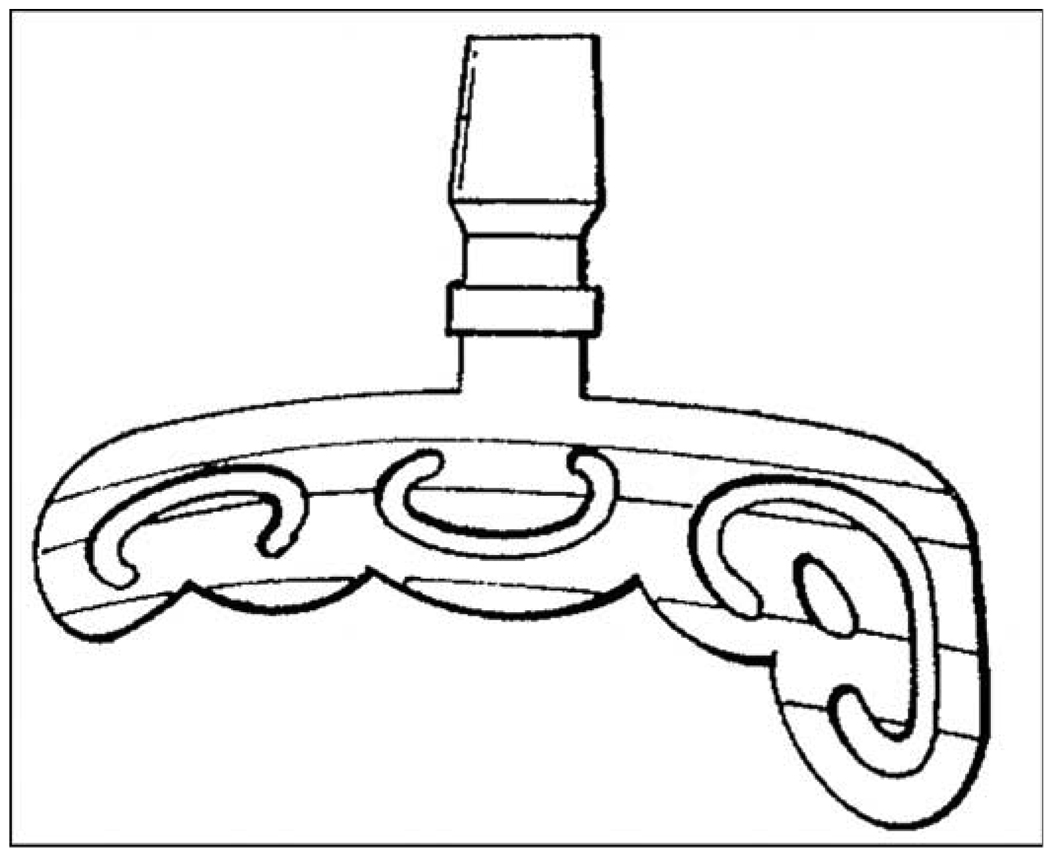
Beyond Brånemark’s threaded titanium fixtures for dental implantology (Figure 2), different devices with holes for bone ingrowth to achieve a firm device-bone composite structure have been developed and implemented. In 1913, Dr. Edward J. Greenfield designed implants with cylindrical wire baskets of iridio-platinum. The bone could grow into the implant basket body, and it would be secured [102]. A similar approach can be found in Ashukian’s Artificial Tooth design [103]. In 1963, Dr. Leonard L. Linkow introduced the “VentPlant” with an open cagelike design that went into the bone with a few threads on a solid body at the top. He then developed a blade implant [104–105]. Linkow’s device has a long thin blade that would be surgically placed into a groove in the bone (Figure 6). The long hollowed base of Linkow’s implant allows ingrowth of bone, which adds to the device-bone integration [106–107]. Among the negative aspects of the long base is that it requires a lot of space even for a single tooth prosthesis. If the implant has to be removed, a very big portion of bone has to be taken out, which complicates or makes impossible new implantation [108].
Figure 6.
Linkow’s bone adapting tissue packing post system. Source: Adapted from Linkow LI, inventor; Oratronics, Inc, assignee. Bone adapting tissue packing post system. United States patent US 3849888. 1974 Nov 26.
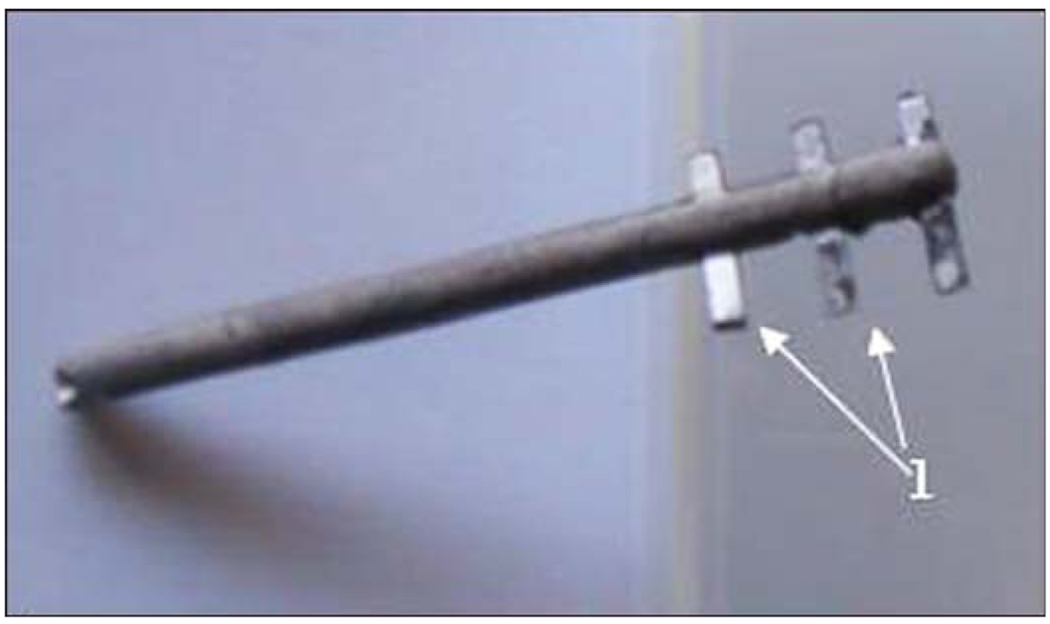
No reports, to my knowledge, have tested hollowed systems like Linkow’s for DSA of limb prostheses. However, for implanting into tubular bone, the bladelike design can be revisited. I suggested [99] that using the medullary canal contradicts the biological purpose of the canal, namely its role as a designated functional cavity for bone marrow [109]. I also noted that inserting a stem into the canal destroys the endosteum, a thin layer of connective tissue filled with cortical capillaries that lines the medullary cavity. I further developed a hypothesis, to be experimentally verified, that an implant with specially added side elements called “fins” could initiate bone regeneration in the circular direction [110]. I believe that regeneration in the circular direction is biologically more efficient than regeneration in the direction toward the center of the bone canal, which is exploited for cylindrical implants [99]. As a result, the pylon with fins for DSA was recently introduced by Poly-Orth International [110]. The design (Figure 7) remotely replicates Linkow’s blade implants [111] and the implants for total hip joint replacement with Wagner’s longitudinal ribs [112].
Figure 7.
Poly-Orth International pylon with three pairs of fins (1).
OTHER APPROACHES
The work by Brånemark and his group inspired many animal studies that used the skeleton as a site for modeling different attachment technologies [43,113–114]. As an alternative to titanium, vitallium (alloy of chromium, cobalt, and molybdenum) has been tried extensively since the 1970s [115]. After medullary implantation, porous wafers made of vitallium and carbon showed more fibrous connective tissue than bone ingrowth at the bone-implant interface [116]. With hydroxyapatite (HA)-coating, vitallium demonstrated better bone regeneration and osseointegration than pure titanium [117]. Implants coated with a bone-graft substitute derived from reef-building sea coral coralline replamineform HA were placed in canine subcutaneous tissues to clarify whether the HA matrix acted as a passive matrix for osseous ingrowth when placed in some inherent bone-induction capacity. The implants were well tolerated and elicited no deleterious host response. Connective tissue rapidly infiltrated the pores, but no evidence of bone formation was noted in any of the specimens. It was concluded that this implant material does not act to induce bone formation [118]. Another material tested for percutaneous implantation was nongraphitizing (vitreous) carbon, which has high strength, hardness, and low porosity and permeability [119]. Implants of vitreous carbon with pore diameters 200 to 500 µm were surgically placed in rabbits and pigs [120]. Staphylococcus aureus and Escherichia coli were inoculated in a test area adjacent to the implant and a remote control area. Results showed that despite a temporarily high rate of colonization and obvious binding of the bacteria to the carbon, the skin-implant interface resists infection by both normal and pathogenic flora.
Mismatch in moduli of elasticity in the bone and the implant is one reason for the loosening and further failure of the implant. The least mismatch so far was achieved by the use of different polymers. Reasonable biointegration and cellular ingrowth were reported, with highporosity expanded polytetrafluoroethylene for facial plastic surgery [121]. Positive results were reported with the use of porous polyethylene (Medpor) implants for nasal reconstruction [122].
Many attempts were made to improve or speed up creation of a cell-implant bond by HA coating. HA is a form of calcium phosphate closely related to the mineral phase of bone and teeth, having a similar crystalline structure [123]. Attachment of the bone to the coated titanium implants occurs faster than to the uncoated implants [124]. Mechanical properties and adhesion of the cells to the layer of coating depend on a calcium phosphoric ratio in HA and on the method of coating. The plasma-spraying technique is commonly used to coat the orthopedic implants with HA, but it provides a low bond strength compared with the electron beam deposition method [125]. Different coating techniques result in different bond strengths between the implant and the bone. Coating degrades with time, leading to detachment and subsequent loosening of the prosthesis [126].
While highly biocompatible, HA-coated titanium implants more easily developed a more severe infection in the presence of bacteria than non-HA-coated titanium implants [127].
To improve interface with the cells, titanium implants have been treated with an enamel matrix derivative (EMD) and a vehicle gel propylene glycol alginate [128]. All osteoblast activity indicators were significantly greater in the porous coated region than in the host bone region [129]. The results obtained showed that the EMD treatment did not benefit the bone formation around titanium implants.
Remodeling of extracellular matrix-associated tissue promotes angiogenesis, recruitment of circulating progenitor cells, rapid scaffold degradation and constructive remodeling of damaged tissues, and adhesion and migration of cells [130–131]. Since treating the surface of the implant by the extracellular matrix components has the potential for better cell growth [132], it may be applied in the future for the implantable prosthetic pylon as well. An in vitro study with titanium foam for spine fusion showed the ingrowth of human osteoblasts to the metal structure [53]. To prevent bacteria from colonizing implants and forming biofilms, Antoci and colleagues have covalently attached antibiotics to implant surfaces. They reported that vancomycin-modified implants showed better inhibition of bacterial attachment and proliferation than control titanium surfaces [133].
Porous circumferential components used in total joint replacement, which are advantageous in the bone-device interface [129,134], can be a source of infection for the skin-device zone [135]. Efforts to optimize the roughness of the abutment to achieve a reliable and infection-free skin-device interface were unsuccessful [42], and current clinical studies use abutments with a smooth surface [86].
DISCUSSION
At present, no follow-up data on skin seals around porous percutaneous implants have been collected for more than several months. Reports of successful DSA in humans refer to implanted abutments with a smooth surface [10–86], data on which have been collected for many years. Potential skin mobility is addressed surgically during the second step of the two-step DSA procedure: the removal of the fat layer induces skin ingrowth into the open end of the residuum bone. Patients then get instructions for daily hygienic care of the area where the abutment goes through the skin. Nevertheless, this approach should not be considered the ultimate solution, since it does not reliably protect against skin infection and sinus tract infection affecting bone and other subcutaneous tissues.
Deep ingrowth of skin through the porous pylon may create a safer and more sustainable seal. A potential mechanical advantage exists as well. The total integration of the skin with a porous implant reduces skin mobility and therefore reduces the probability of avulsion. Moreover, once the implant is invaded with host tissue, the bacteria are less apt to establish infection [136].
At least two immediate issues exist with total skin integration. The first is the mismatch between the moduli of skin and the implant. The second is the dynamics of initial ingrowth: before its completion bacteria can invade the host tissue [136].
The mismatch issue requires a mediator or mediators between the skin and the implant to be found, with the modulus or moduli gradually increasing within the skinimplant gap [38,137]. Ideally, the mediator layer would function as the eponychium (cuticle) and hyponychium that comprise the junction between the skin stratum corneum and the base of the nail plate [138]. To facilitate the initial ingrowth, various growth factors can be considered [139–142].
The bone-implant interface is another area requiring more study. The current DSA technology, introduced by Brånemark, and arthroplasty use the medullary canal of residual bone as the hosting cavity for implantation. The principal difference between the two procedures lies in the prosthesis design. The prosthesis used for total joint replacement is asymmetric relative to its longitudinal axis, since it has a portion that models the bone head. Two parameters must be controlled for precise installation: axial translation into the canal and angular position of the head of the artificial bone. For that reason, the placement of the shaft of the prosthesis is a press-fit type. In contrast, DSA implants are symmetrical relative to the longitudinal axis and only the depth of installation needs to be controlled, as with dental implants. Therefore, the implants for DSA can be screwed into the canal, a technique that has proven successful in dental implantology.
A screw is a more reliable fastener than a nail. So, too, the screw-in device-bone connection is more mechanically effective than the press-fit type. The screw-in type requires a smaller zone of contact with the hosting bone than the press-fit type for the same value of resistance to detachment. Such a consideration is especially important for DSA in the case of a short residuum.
To summarize the origins of current DSA technology [143]: DSA uses the screw-in method adopted from dental implantology [108] and uses the medullary canal for implantation like arthroplasty [144].
The bond between the bone and all existing implants relies on ossification inside the canal in the inward direction, which is the least efficient when compared with ossification in the longitudinal and circular directions [99]. Inward ossification is induced by damage to the inner walls of the canal during installation of the implant but is limited by the space designated for bone marrow. Longitudinal ossification, introduced by Ilizarov, can lengthen the bone up to 30 percent via distractional osteogenesis [145]. Circular ossification is a mechanism for fracture healing and can widen the bone up to more than two initial diameters [145]. A hypothesis was developed that circular ossification could be used for a more reliable fixation of the implants for DSA [99], and a design of such an implant was introduced [110]. If confirmed experimentally, this approach may decrease loosening both in DSA and arthroplasty. Specifically, circular ossification will counter the increase in diameter of the medullary canal, which diminishes the long-term reliability of the bond between the inner walls of the canal and the implant. The increase in diameter is a natural process in bone development and can be accelerated by a high level of physical activity [146].
CONCLUSIONS
The increased number of young active persons with amputation due to recent wars has brought more attention to the technology of DSA of limb prostheses. A multidisciplinary revisiting of all aspects of the technology responsible for lifelong safe functioning is now required. For DSA implants, titanium and tantalum remain the most promising materials. They maximize cell attachment, both at the interface between the implant and skin at the transcutaneous passage and between the implant and the residuum bone. Porous implants have a strong potential for improved skin and bone integration if problems of strength and material mismatch can be resolved.
ACKNOWLEDGMENTS
Funding/Support: This material was based on work supported in part by the National Institutes of Health (grant 1R43HD057492-01A1).
Abbreviations
- DSA
direct skeletal attachment
- EMD
enamel matrix derivative
- HA
hydroxyapatite
- TiO2
titanium oxide
Footnotes
A tearing away or forcible separation. Stedman’s Medical Dictionary. 25th ed. Baltimore (MD): Williams & Wilkins; 1990. Avulsion; p. 159.
The thin layer of cells lining the medullary cavity of a bone.
Financial Disclosures: The author has declared that no competing interests exist.
REFERENCES
- 1.Frossard L, Stevenson N, Smeathers J, Häggström E, Hagberg K, Sullivan J, Ewins D, Gow DL, Gray S, Brånemark R. Monitoring of the load regime applied on the osseointegrated fixation of a trans-femoral amputee: A tool for evidence-based practice. Prosthet Orthot Int. 2008;32(1):68–78. doi: 10.1080/03093640701676319. [PMID: 18330805] [DOI] [PubMed] [Google Scholar]
- 2.Brånemark PI. Vital microscopy of bone marrow in rabbit. Scand J Clin Lab Invest. 1959;11 Suppl 38:1–82. [PMID: 13658913] [PubMed] [Google Scholar]
- 3.Brånemark R, Ohrnell LO, Nilsson P, Thomsen P. Biomechanical characterization of osseointegration during healing: An experimental in vivo study in the rat. Biomaterials. 1997;18(14):969–978. doi: 10.1016/s0142-9612(97)00018-5. [PMID: 9212192] [DOI] [PubMed] [Google Scholar]
- 4.Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PMID: 356184] [PubMed] [Google Scholar]
- 5.Robinson K, Brånemark R, Ward D. Future developments: Osseointegration in transfemoral amputees. In: Smith D, Michael J, Bowker J, editors. Atlas of amputations and limb deficiencies: Surgical, prosthetic and rehabilitation principles. 3rd ed. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2004. pp. 673–681. [Google Scholar]
- 6.Pitkin M, Raykhtsaum G, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG. Skin and bone integrated prosthetic pylon: A pilot animal study. J Rehabil Res Dev. 2006;43(4):573–580. doi: 10.1682/jrrd.2005.05.0160. [PMID: 17123195] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amputee Coalition of America [home page on the Internet] Knoxville (TN): Amputee Coalition of America; c2009 [updated 2007]. National Limb Loss Information Center fact sheet: Limb loss in the United States; [about 4 screens]. Available from: http://www.amputee-coalition.org/fact_sheets/limbloss_us.pdf. [Google Scholar]
- 8.Fischer H. United States military casualty statistics: Operation Iraqi Freedom and Operation Enduring Freedom. Washington (DC): Congressional Research Service, Library of Congress; 2009. [Google Scholar]
- 9.Nechaev EA, Gritsanov AI, Fomin NF, Minnulin IP. In: Mine blast trauma: Experience from the war in Afghanistan. Khlunovskaya GP, translator. Varnamo (Sweden): Russian Ministry of Public Health and Medical Industry; 1995. p. 463. [Google Scholar]
- 10.Brånemark R, Brånemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev. 2001;38(2):175–181. [PMID: 11392650] [PubMed] [Google Scholar]
- 11.O’Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am. 2008;90(2):393–400. doi: 10.2106/JBJS.G.00268. [PMID: 18245601] [DOI] [PubMed] [Google Scholar]
- 12.Edell DJ. A peripheral nerve information transducer for amputees: Long-term multichannel recordings from rabbit peripheral nerves. IEEE Trans Biomed Eng. 1986;33(2):203–214. doi: 10.1109/TBME.1986.325892. [PMID: 3007332] [DOI] [PubMed] [Google Scholar]
- 13.Pitkin M, Raykhtsaum G, inventors. Skin integrated device. 20070071788. United States patent US. 2007 Mar 29;
- 14.Levy SW. Skin problems of amputee. St. Louis (MO): W. H. Green; 1983. [Google Scholar]
- 15.Eriksson E, Brånemark PI. Osseointegration from the perspective of the plastic surgeon. Plast Reconstr Surg. 1994;93(3):626–637. [PMID: 8115525] [PubMed] [Google Scholar]
- 16.Lundborg G, Brånemark PI, Rosén B. Osseointegrated thumb prostheses: A concept for fixation of digit prosthetic devices. J Hand Surg [Am] 1996;21(2):216–221. doi: 10.1016/s0363-5023(96)80103-1. [PMID: 8683049] [DOI] [PubMed] [Google Scholar]
- 17.Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52(2):155–170. doi: 10.3109/17453678108991776. [PMID: 7246093] [DOI] [PubMed] [Google Scholar]
- 18.Pittman GT, Peters CL, Hines JL, Bachus KN. Mechanical bond strength of the cement-tibial component interface in total knee arthroplasty. J Arthroplasty. 2006;21(6):883–888. doi: 10.1016/j.arth.2005.10.006. [PMID: 16950044] [DOI] [PubMed] [Google Scholar]
- 19.Knahr K, Karamat L, Loho G, Pospischill M. Tribological considerations for a new hip system. Acta Chir Orthop Traumatol Cech. 2005;72(2):116–121. Czech, English. [PMID: 15890144] [PubMed] [Google Scholar]
- 20.Fornasier VL, Goodman SB, Protzner K, Kamel M, Song Y, Shojaci A. The role of implant alignment on stability and particles on periprosthetic osteolysis—A rabbit model of implant failure. J Biomed Mater Res B Appl Biomater. 2004;70(2):179–186. doi: 10.1002/jbm.b.20038. [PMID: 15264298] [DOI] [PubMed] [Google Scholar]
- 21.Nolph KD. Access problems plague both peritoneal dialysis and hemodialysis. Kidney Int Suppl. 1993;40:S81–S84. [PMID: 8445843] [PubMed] [Google Scholar]
- 22.Herbrig K, Pistrosch F, Gross P, Palm C. Resumption of peritoneal dialysis after transcutaneous treatment of a peritoneal leakage using fibrin glue. Nephrol Dial Transplant. 2006;21(7):2037–2038. doi: 10.1093/ndt/gfl080. [PMID: 16520352] [DOI] [PubMed] [Google Scholar]
- 23.Ash SR. Chronic peritoneal dialysis catheters: Challenges and design solutions. Int J Artif Organs. 2006;29(1):85–94. doi: 10.1177/039139880602900108. [PMID: 16485243] [DOI] [PubMed] [Google Scholar]
- 24.Campanini A, De Vito A, Frassineti S, Vicini C. Role of skinlined tracheotomy in obstructive sleep apnoea syndrome: Personal experience. Acta Otorhinolaryngol Ital. 2004;24(2):68–74. [PMID: 15468994] [PubMed] [Google Scholar]
- 25.McGee L. Case study: Maintaining skin integrity during the use of tracheostomy ties. Ostomy Wound Manage. 1990;30:37–40. [PMID: 2080975] [PubMed] [Google Scholar]
- 26.Bressler K, Coladipietro L, Holinger LD. Protection of the cervical skin in the pediatric patient with a recent tracheostomy. Otolaryngol Head Neck Surg. 1997;116(3):414–415. doi: 10.1016/S0194-59989770286-9. [DOI] [PubMed] [Google Scholar]
- 27.Udomsawaengsup S, Brethauer S, Kroh M, Chand B. Percutaneous transesophageal gastrostomy (PTEG): A safe and effective technique for gastrointestinal decompression in malignant obstruction and massive ascites. Surg Endosc. 2008;22(10):2314–2318. doi: 10.1007/s00464-008-9984-y. [PMID: 18622539] [DOI] [PubMed] [Google Scholar]
- 28.Patel PH, Thomas E. Risk factors for pneumonia after percutaneous endoscopic gastrostomy. J Clin Gastroenterol. 1990;12(4):389–392. doi: 10.1097/00004836-199008000-00006. [PMID: 2398246] [DOI] [PubMed] [Google Scholar]
- 29.Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res A. 2006;78(3):595–604. doi: 10.1002/jbm.a.30789. [PMID: 16752397] [DOI] [PubMed] [Google Scholar]
- 30.Bubenik LJ. Infections of the skeletal system. Vet Clin North Am Small Anim Pract. 2005;35(5):1093–1109. doi: 10.1016/j.cvsm.2005.05.001. [PMID: 16129134] [DOI] [PubMed] [Google Scholar]
- 31.Sullivan J, Uden M, Robinson KP, Sooriakumaran S. Rehabilitation of the trans-femoral amputee with an osseointegrated prosthesis: The United Kingdom experience. Prosthet Orthot Int. 2003;27(2):114–120. doi: 10.1080/03093640308726667. [PMID: 14571941] [DOI] [PubMed] [Google Scholar]
- 32.Murphy EF. History and philosophy of attachment of prostheses to the musculo-skeletal system and of passage through the skin with inert materials. J Biomed Mater Res. 1973;7(3):275–295. doi: 10.1002/jbm.820070319. [PMID: 4577874] [DOI] [PubMed] [Google Scholar]
- 33.Lueck RA, Galante J, Rostoker W, Ray RD. Development of an open pore metallic implant to permit attachment to bone. Surg Forum. 1969;20:456–457. [PMID: 5383115] [PubMed] [Google Scholar]
- 34.Galante J, Rostoker W, Lueck R, Ray RD. Sintered fiber metal composites as a basis for attachment of implants to bone. J Bone Joint Surg Am. 1971;53(1):101–114. [PMID: 5540151] [PubMed] [Google Scholar]
- 35.Pilliar RM, Filiaggi MJ, Wells JD, Grynpas MD, Kandel RA. Porous calcium polyphosphate scaffolds for bone substitute applications—In vitro characterization. Biomaterials. 2001;22(9):963–972. doi: 10.1016/s0142-9612(00)00261-1. [PMID: 11311015] [DOI] [PubMed] [Google Scholar]
- 36.Chang YL, Stanford CM, Wefel JS, Keller JC. Osteoblastic cell attachment to hydroxyapatite-coated implant surfaces in vitro. Int J Oral Maxillofac Implants. 1999;14(2):239–247. [PMID: 10212541] [PubMed] [Google Scholar]
- 37.Fujibayashi S, Neo M, Kim HM, Kokubo T, Nakamura T. Osteoinduction of porous bioactive titanium metal. Biomaterials. 2004;25(3):443–450. doi: 10.1016/s0142-9612(03)00551-9. [PMID: 14585692] [DOI] [PubMed] [Google Scholar]
- 38.Aaron RK, Morgan JR. Biohybrid limbs: New materials and new properties. Med Health R I. 2007;90(1):4–6. [PMID: 17487026] [PubMed] [Google Scholar]
- 39.Serro AP, Fernandes AC, Saramago B, Lima J, Barbosa MA. Apatite deposition on titanium surfaces—The role of albumin adsorption. Biomaterials. 1997;18(14):963–968. doi: 10.1016/s0142-9612(97)00031-8. [PMID: 9212191] [DOI] [PubMed] [Google Scholar]
- 40.Vehof JW, Spauwen PH, Jansen JA. Bone formation in calcium-phosphate-coated titanium mesh. Biomaterials. 2000;21(19):2003–2009. doi: 10.1016/s0142-9612(00)00094-6. [PMID: 10941922] [DOI] [PubMed] [Google Scholar]
- 41.Ciolfi VJ, Pilliar R, McCulloch C, Wang SX, Grynpas MD, Kandel RA. Chondrocyte interactions with porous titanium alloy and calcium polyphosphate substrates. Biomaterials. 2003;24(26):4761–4770. doi: 10.1016/s0142-9612(03)00373-9. [PMID: 14530073] [DOI] [PubMed] [Google Scholar]
- 42.Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22(1):87–96. doi: 10.1016/s0142-9612(00)00174-5. [PMID: 11085388] [DOI] [PubMed] [Google Scholar]
- 43.Cho SA, Jung SK. A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials. 2003;24(26):4859–4863. doi: 10.1016/s0142-9612(03)00377-6. [PMID: 14530083] [DOI] [PubMed] [Google Scholar]
- 44.Iesaka K, Jaffe WL, Kummer FJ. Integrity of the stem-cement interface in THA: Effects of stem surface finish and cement porosity. J Biomed Mater Res B Appl Biomater. 2008;87(1):77–82. doi: 10.1002/jbm.b.31071. [PMID: 18386841] [DOI] [PubMed] [Google Scholar]
- 45.Mann KA, Damron LA, Miller MA, Race A, Clarke MT, Cleary RJ. Stem-cement porosity may explain early loosening of cemented femoral hip components: Experimental-computational in vitro study. J Orthop Res. 2007;25(3):340–350. doi: 10.1002/jor.20330. [PMID: 17149748] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baleani M, Fognani R, Toni A. The influence of stem insertion rate on the porosity of the cement mantle of hip joint replacements. Proc Inst Mech Eng [H] 2003;217(3):199–205. doi: 10.1243/095441103765212695. [PMID: 12807160] [DOI] [PubMed] [Google Scholar]
- 47.Tepic S, Soltész U. Fatigue of bone cement with simulated stem interface porosity. J Mater Sci Mater Med. 1998;9(12):707–709. doi: 10.1023/a:1008942600230. [PMID: 15348926] [DOI] [PubMed] [Google Scholar]
- 48.Galibin OV, Protasov MV, Chikhovskaya YV, Belyaeva IG, Pitkin MP. Study of growth processes in bone and skin tissues in porous implants designed for fixation of external prosthesis after amputation of extremities. Cell and Tissue Biol. 2007;1(3):272–275. doi: 10.1134/s1990519x07030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bünger MH, Foss M, Erlacher K, Li H, Zou X, Langdahl BL, Bünger C, Birkedal H, Besenbacher F, Pedersen JS. Bone nanostructure near titanium and porous tantalum implants studied by scanning small angle x-ray scattering. Eur Cell Mater. 2006;12:81–91. doi: 10.22203/ecm.v012a10. [PMID: 17136679] [DOI] [PubMed] [Google Scholar]
- 50.Likibi F, Assad M, Coillard C, Chabot G, Rivard CH. Ann Chir. 4. Vol. 130. French: 2005. [Bone integration and apposition of porous and non porous metallic orthopaedic biomaterials] pp. 235–241. [PMID: 15847858] [DOI] [PubMed] [Google Scholar]
- 51.Minamide A, Yoshida M, Kawakami M, Yamasaki S, Kojima H, Hashizume H, Boden SD. The use of cultured bone marrow cells in type I collagen gel and porous hydroxyapatite for posterolateral lumbar spine fusion. Spine. 2005;30(10):1134–1148. doi: 10.1097/01.brs.0000162394.75425.04. [PMID: 15897826] [DOI] [PubMed] [Google Scholar]
- 52.Alitalo I. Ventral interbody implantation for fusion of the lumbar spine using polytetrafluoroethylene-carbonfiber and porous high density polyethylene. An experimental study in growing pigs. Acta Vet Scand Suppl. 1979;(71):1–49. [PMID: 395840] [PubMed] [Google Scholar]
- 53.Müller U, Imwinkelried T, Horst M, Sievers M, Graf-Hausner U. Do human osteoblasts grow into open-porous titanium? Eur Cell Mater. 2006;11:8–15. doi: 10.22203/ecm.v011a02. [PMID: 16425146] [DOI] [PubMed] [Google Scholar]
- 54.Chin CD, Khanna K, Sia SK. A microfabricated porous collagen-based scaffold as prototype for skin substitutes. Biomed Microdevices. 2008;10(3):459–467. doi: 10.1007/s10544-007-9155-2. [PMID: 18213520] [DOI] [PubMed] [Google Scholar]
- 55.Auxenfans C, Builles N, Andre V, Lequeux C, Fievet A, Rose S, Braye FM, Fradette J, Janin-Manificat H, Nataf S, Burillon C, Damour O. [Porous matrix and primary-cell culture: A shared concept for skin and cornea tissue engineering.] Pathol Biol (Paris) doi: 10.1016/j.patbio.2008.04.014. Epub 2008 Jul 2. French. [PMID: 18602223] [DOI] [PubMed] [Google Scholar]
- 56.Galibin OV, Protasov MV, Chikhovskaya JV, Beliaeva IG, Pitkin M. Skin and bone integrated prosthetic technology. III. An exposed implantation of a porous titanium pellet into the skin; Proceedings of the 9th Russian National Congress, People and Health; Nov 22–26; St. Petersburg (Russia): Russian National Congress; 2005. [Google Scholar]
- 57.Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C. Collagen/chitosan porous scaffolds with improved biostabil-ity for skin tissue engineering. Biomaterials. 2003;24(26):4833–4841. doi: 10.1016/s0142-9612(03)00374-0. [PMID: 14530080] [DOI] [PubMed] [Google Scholar]
- 58.Pitkin M, Raykhtsaum G, Pilling J, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG, Blinova MI, Yudintseva NM, Potokin IL, Pinaev GP, Moxson V, Duz V. Porous composite prosthetic pylon for integration with skin and bone. J Rehabil Res Dev. 2007;44(5):723–738. doi: 10.1682/jrrd.2006.12.0160. [PMID: 17943684] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omae H, Mochizuki Y, Yokoya S, Adachi N, Ochi M. Augmentation of tendon attachment to porous ceramics by bone marrow stromal cells in a rabbit model. Int Orthop. 2007;31(3):353–358. doi: 10.1007/s00264-006-0194-8. [PMID: 16909253] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itälä A, Heijink A, Leerapun T, Reach JS, An KN, Lewallen DG. Successful canine patellar tendon reattachment to porous tantalum. Clin Orthop Relat Res. 2007;463:202–227. [PMID: 17987673] [PubMed] [Google Scholar]
- 61.Muneta T, Yamamoto H, Ishibashi T, Asahina S, Murakami S, Furuya K. The effects of tibial tunnel placement and roofplasty on reconstructed anterior cruciate ligament knees. Arthroscopy. 1995;11(1):57–62. doi: 10.1016/0749-8063(95)90089-6. [PMID: 7727013] [DOI] [PubMed] [Google Scholar]
- 62.Lefebvre L, Thomas Y, Gauthier M, inventors. Method of making open cell material. 20050100470. United States patent US. 2005 May 12;
- 63.Cohen R. A porous tantalum trabecular metal: Basic science. Am J Orthop. 2002;31(4):216–217. [PMID: 12008853] [PubMed] [Google Scholar]
- 64.Von Recum AF. Applications and failure modes of percutaneous devices: A review. J Biomed Mater Res. 1984;18(4):323–336. doi: 10.1002/jbm.820180403. [PMID: 6234317] [DOI] [PubMed] [Google Scholar]
- 65.Lee H, Ocumpaugh DE, Cupples AL, Culp GW. Final report. Washington (DC): National Technical Information Services; 1970. Development of satisfactory long-term percutaneous leads. National Institutes of Health, Report No. PH43-67-1108-1, Accession No. PB196985. [Google Scholar]
- 66.Hall CW, Adams LM, Ghidoni JJ. Development of skin interfacing cannula. Trans Am Soc Artif Intern Organs. 1975;21:281–288. [PMID: 124976] [PubMed] [Google Scholar]
- 67.Winter GD. Transcutaneous implants: Reactions of the skin-implant interface. J Biomed Mater Res. 1974;8(3):99–113. doi: 10.1002/jbm.820080311. [PMID: 4616966] [DOI] [PubMed] [Google Scholar]
- 68.Fernie GR, Kostuik JP, Lobb RJ. A percutaneous implant using a porous metal surface coating for adhesion to bone and a velour covering for soft tissue attachment: Results of trials in pigs. J Biomed Mater Res. 1977;11(6):883–891. doi: 10.1002/jbm.820110608. [PMID: 145440] [DOI] [PubMed] [Google Scholar]
- 69.Hahn H, Palich W. Preliminary evaluation of porous metal surfaced titanium for orthopedic implants. J Biomed Mater Res. 1970;4(4):571–577. doi: 10.1002/jbm.820040407. [PMID: 5487557] [DOI] [PubMed] [Google Scholar]
- 70.Clemow AJ, Weinstein AM, Klawitter JJ, Koeneman J, Anderson J. Interface mechanics of porous titanium implants. J Biomed Mater Res. 1981;15(1):73–82. doi: 10.1002/jbm.820150111. [PMID: 7348706] [DOI] [PubMed] [Google Scholar]
- 71.Holgers KM, Tjellström A, Bjursten LM, Erlandsson BE. Soft tissue reactions around percutaneous implants: A clinical study of soft tissue conditions around skin-penetrating titanium implants for bone-anchored hearing aids. Am J Otol. 1988;9(1):56–59. [PMID: 3364537] [PubMed] [Google Scholar]
- 72.Feldman DS, Von Recum AF. Non-epidermally induced failure modes of percutaneous devices. Biomaterials. 1985;6(5):352–356. doi: 10.1016/0142-9612(85)90091-2. [PMID: 4052549] [DOI] [PubMed] [Google Scholar]
- 73.Sahlin H, Contreras R, Gaskill DF, Bjursten LM, Frangos JA. Anti-inflammatory properties of micropatterned titanium coatings. J Biomed Mater Res A. 2006;77(1):43–49. doi: 10.1002/jbm.a.30642. [PMID: 16345099] [DOI] [PubMed] [Google Scholar]
- 74.Linder L, Carlsson A, Marsal L, Bjursten LM, Brånemark PI. Clinical aspects of osseointegration in joint replacement. A histological study of titanium implants. J Bone Joint Surg Br. 1988;70(4):550–555. doi: 10.1302/0301-620X.70B4.3403596. [PMID: 3403596] [DOI] [PubMed] [Google Scholar]
- 75.Pendegrass CJ, Gordon D, Middleton CA, Sun SN, Blunn GW. Sealing the skin barrier around transcutaneous implants: In vitro study of keratinocyte proliferation and adhesion in response to surface modifications of titanium alloy. J Bone Joint Surg Br. 2008;90(1):114–121. doi: 10.1302/0301-620X.90B1.19580. [PMID: 18160512] [DOI] [PubMed] [Google Scholar]
- 76.Yu C, Sun Y, Bradfield J, Fiordalisi I, Harris GD. A novel percutaneous barrier device that permits safe subcutaneous access. ASAIO J. 1999;45(6):531–534. doi: 10.1097/00002480-199911000-00005. [PMID: 10593682] [DOI] [PubMed] [Google Scholar]
- 77.Yu C, Harris GD. The LPD-II: A modified locked percutaneous device that permits safe subcutaneous access. ASAIO J. 2001;47(1):25–29. doi: 10.1097/00002480-200101000-00007. [PMID: 11199309] [DOI] [PubMed] [Google Scholar]
- 78.Yu C, Harris GD, Sun Y. An alternative design of locked percutaneous device for skeletal extension through skin. Artif Organs. 2003;27(3):267–271. doi: 10.1046/j.1525-1594.2003.07094.x. [PMID: 12662214] [DOI] [PubMed] [Google Scholar]
- 79.Pendegrass CJ, Goodship AE, Blunn GW. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials. 2006;27(23):4183–4191. doi: 10.1016/j.biomaterials.2006.03.041. [PMID: 16618500] [DOI] [PubMed] [Google Scholar]
- 80.The BBC [home page on the Internet] London (United Kingdom): The BBC; c2009 [updated 2006 Jul 30]. ‘Bionic’ limb breakthrough made; [about 3 screens]. Available from: http://news.bbc.co.uk/go/pr/fr/-/1/hi/health/5140090.stm/ [Google Scholar]
- 81.Pitkin M, Blinova MI, Yudintseva NV, Potokin IL, Raykhtsaum G, Pinaev GP. Skin and bone integrated prosthetic technology. I. Characterization and morphology of human cells cultivated on titanium implants of different structures [abstract]; Proceedings of the 9th Russian National Congress, People and Health; Nov 22–26; St. Petersburg (Russia): Russian National Congress; 2004. p. 217. [Google Scholar]
- 82.Pitkin M, Raykhtsaum G, Pilling J, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG, Blinova MI, Yudint-seva NM, Potokin IL, Pinaev GP, Moxson V, Duz V. Porous composite prosthetic pylon for integration with skin and bone. J Rehabil Res Dev. 2007;44(5):723–738. doi: 10.1682/jrrd.2006.12.0160. [PMID: 17943684] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitkin M, Raykhtsaum G, Pilling J, Shukeylo Y, Moxson V, Duz V, Lewandowski J, Connolly R, Kistenberg S, Dalton J, Prilutsky B, Jacobson S. Mathematical modeling, mechanical and histopathological testing of porous prosthetic pylon for direct skeletal attachment. J Rehabil Res Dev. 2009;46(3):315–330. doi: 10.1682/jrrd.2008.09.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pitkin M, Raykhtsaum G, Pilling J, Galibin O, Protasov M, Chihovskaya J, Belyaeva I, Blinova M, Yudintseva M, Potokin I, Pinaev G, Moxson V, Duz V. Porous composite prosthetic pylon for integration with skin and bone of the residuum; Proceedings of the 4th Conference of the US-Russian Program in Prosthetics and Rehabilitation; 2007 Jun; Sloughton: Massachusetts; 2007. pp. 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drygas KA, Taylor R, Sidebotham CG, Hugate RR, McAlexander H. Transcutaneous tibial implants: A surgical procedure for restoring ambulation after amputation of the distal aspect of the tibia in a dog. Vet Surg. 2008;37(4):322–327. doi: 10.1111/j.1532-950X.2008.00384.x. [PMID: 18564255] [DOI] [PubMed] [Google Scholar]
- 86.Palmquist A, Jarmar T, Emanuelsson L, Brånemark R, Engqvist H, Thomsen P. Forearm bone-anchored amputation prosthesis: A case study on the osseointegration. Acta Orthop. 2008;79(1):78–85. doi: 10.1080/17453670710014806. [PMID: 18283577] [DOI] [PubMed] [Google Scholar]
- 87.Frei H, O’Connell J, Masri BA, Duncan CP, Oxland TR. Biological and mechanical changes of the bone graftcement interface after impaction allografting. J Orthop Res. 2005;23(6):1271–1279. doi: 10.1016/j.orthres.2005.03.021.1100230606. [PMID: 15964167] [DOI] [PubMed] [Google Scholar]
- 88.Stevens A, Lowe JS. Human histology. 3rd ed. Philadelphia (PA): Elsevier Mosby; 2005. p. 428. [Google Scholar]
- 89.Robinson D, Hendel D, Halperin N. Changes in femur dimensions in asymptomatic non-cemented hip arthro-plasties. 20 cases followed for 5–8 years. Acta Orthop Scand. 1994;65(4):415–417. doi: 10.3109/17453679408995482. [PMID: 7976287] [DOI] [PubMed] [Google Scholar]
- 90.Karachalios T, Hartofilakidis G, Zacharakis N, Tsekoura M. A 12- to 18-year radiographic follow-up study of Charnley low-friction arthroplasty. The role of the center of rotation. Clin Orthop Relat Res. 1993;(296):140–147. [PMID: 8222417] [PubMed] [Google Scholar]
- 91.Nakamura S, Kusuzaki K, Murata H, Takeshita H, Hirata M, Hashigushi S, Hirasawa Y. Bone reaction induced by femoral stem of titanium alloy endoprosthesis for malignant bone tumors at the distal femur. Oncol Rep. 2001;8(4):877–881. doi: 10.3892/or.8.4.877. [PMID: 11410802] [DOI] [PubMed] [Google Scholar]
- 92.Healy WL, Wasilewski SA, Takei R, Oberlander M. Patellofemoral complications following total knee arthroplasty. Correlation with implant design and patient risk factors. J Arthroplasty. 1995;10(2):197–201. doi: 10.1016/s0883-5403(05)80127-5. [PMID: 7798101] [DOI] [PubMed] [Google Scholar]
- 93.Salter RB. Textbook of disorders and injuries of the musculoskeletal system: An introduction to orthopaedics, fractures, and joint injuries, rheumatology, metabolic bone disease, and rehabilitation. 3rd ed. Baltimore (MD): Wilkins & Wilkins; 1999. p. 687. xxxiv. [Google Scholar]
- 94.Malik MH, Jury F, Bayat A, Ollier WE, Kay PR. Genetic susceptibility to total hip arthroplasty failure: A preliminary study on the influence of matrix metalloproteinase 1, interleukin 6 polymorphisms and vitamin D receptor. Ann Rheum Dis. 2007;66(8):1116–1120. doi: 10.1136/ard.2006.062018. [PMID: 17363400] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prendergast PJ, Taylor D. Design of intramedullary prostheses to prevent bone loss: Predictions based on damagestimulated remodelling. J Biomed Eng. 1992;14(6):499–506. doi: 10.1016/0141-5425(92)90103-r. [PMID: 1434573] [DOI] [PubMed] [Google Scholar]
- 96.How good are knee replacements? Lancet. 1991;338(8765):477–478. [PMID: 1678447] [PubMed] [Google Scholar]
- 97.Reininga IH, Wagenmakers R, Van den Akker-Scheek I, Stant AD, Groothoff JW, Bulstra SK, Zijlstra W, Stevens M. Effectiveness of computer-navigated minimally invasive total hip surgery compared to conventional total hip arthroplasty: Design of a randomized controlled trial. BMC Musculoskelet Disord. 2007;8:4. doi: 10.1186/1471-2474-8-4. [PMID: 17214906] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guyen O, Chen QS, Bejui-Hugues J, Berry DJ, An KN. Unconstrained tripolar hip implants: Effect on hip stability. Clin Orthop Relat Res. 2007;455:202–208. doi: 10.1097/01.blo.0000238796.59596.1f. [PMID: 17279045] [DOI] [PubMed] [Google Scholar]
- 99.Pitkin M. One lesson from arthroplasty to osseointegration in search for better fixation of in-bone implanted prosthesis. J Rehabil Res Dev. 2008;45(4):vii–xiv. [PMID: 18712634] [PMC free article] [PubMed] [Google Scholar]
- 100.Engineering frontline: Biomedical breakthroughs. Raleigh (NC): College of Engineering; 2006. Industrial engineer designs unique prosthesis for lame family pet; pp. 10–11. NC State Engineering Foundation Inc. Annual Report 2004–2005. [Google Scholar]
- 101.Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108–113. [PMID: 3720113] [PubMed] [Google Scholar]
- 102.Greenfield EJ. Implantation of artificial crown and bridge abutments. 1913. Int J Oral Implantol. 1991;7(2):63–68. [PMID: 1815702] [PubMed] [Google Scholar]
- 103.Ashukian ES, inventor, Ashukian ES., assignee Artificial tooth. 2721387. United States patent US. 1955 Oct 25;
- 104.Linkow LI, inventor. Oratronics, Inc, assignee. Bone adapting tissue packing post system. 3849888. United States patent US. 1974 Nov 26;
- 105.Linkow LI. The blade vent-A new dimension in endosseous implantology. Dent Concepts. 1968;11(2):3–12. [PMID: 4873733] [PubMed] [Google Scholar]
- 106.Linkow LI, Giauque F, Ghalili R, Ghalili M. Levels of osseointegration of blade-/plate-form implants. J Oral Implantol. 1995;21(1):23–34. [PMID: 7473868] [PubMed] [Google Scholar]
- 107.Cappuccilli M, Conte M, Praiss ST. Placement and postmortem retrieval of a 28-year-old implant: A clinical and histologic report. J Am Dent Assoc. 2004;135(3):324–329. doi: 10.14219/jada.archive.2004.0181. [PMID: 15058620] [DOI] [PubMed] [Google Scholar]
- 108.Noack N, Willer J, Hoffmann J. Long-term results after placement of dental implants: Longitudinal study of 1,964 implants over 16 years. Int J Oral Maxillofac Implants. 1999;14(5):748–755. [PMID: 10531748] [PubMed] [Google Scholar]
- 109.Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6(2):107–116. doi: 10.1038/nri1780. [PMID: 16491135] [DOI] [PubMed] [Google Scholar]
- 110.Pitkin M, inventor. In-bone implantable shaft for prosthetic joints or for direct skeletal attachment of external limb prostheses and method of its installation. 20090062928. United States patent US. 2009 Mar 5;
- 111.Linkow LI, Donath K, Lemons JE. Retrieval analyses of a blade implant after 231 months of clinical function. Implant Dent. 1992;1(1):37–43. [PMID: 1288796] [PubMed] [Google Scholar]
- 112.Wagner H, Wagner M. Conus hip prosthesis. Acta Chir Orthop Traumatol Cech. 2001;68(4):213–221. [PMID: 11706545] [PubMed] [Google Scholar]
- 113.Brånemark R, Ohrnell LO, Skalak R, Carlsson L, Brånemark PI. Biomechanical characterization of osseointegration: An experimental in vivo investigation in the beagle dog. J Orthop Res. 1998;16(1):61–69. doi: 10.1002/jor.1100160111. [PMID: 9565075] [DOI] [PubMed] [Google Scholar]
- 114.Klokkevold PR, Johnson P, Dadgostari S, Caputo A, Davies JE, Nishimura RD. Early endosseous integration enhanced by dual acid etching of titanium: A torque removal study in the rabbit. Clin Oral Implants Res. 2001;12(4):350–357. doi: 10.1034/j.1600-0501.2001.012004350.x. English, French, German. [PMID: 11488864] [DOI] [PubMed] [Google Scholar]
- 115.Cameron HU, Pilliar RM. Porous vitallium in implant surgery. J Biomed Mater Res. 1974;8(5):283–289. doi: 10.1002/jbm.820080510. [PMID: 4609987] [DOI] [PubMed] [Google Scholar]
- 116.Luedemann RE, Thomas KA, Cook SD. Bone remodeling associated with a flexible femoral intramedullary implant. Biomater Med Devices Artif Organs. 1986;14(3–4):181–194. doi: 10.3109/10731198609117542. [PMID: 3814713] [DOI] [PubMed] [Google Scholar]
- 117.Wilke A, Richter A, Traub F, Endres S, Orth J. [Semiquantitative analysis of bone regeneration on porous metallic wire-mesh specimens with or without HA-coating in comparison to pure titanium in animal experiments] Z Orthop Ihre Grenzgeb. 2002;140(1):95–100. doi: 10.1055/s-2002-22098. German. [PMID: 11898072] [DOI] [PubMed] [Google Scholar]
- 118.Piecuch JF. Extraskeletal implantation of a porous hydroxyapatite ceramic. J Dent Res. 1982;61(12):1458–1460. doi: 10.1177/00220345820610121801. [PMID: 6294161] [DOI] [PubMed] [Google Scholar]
- 119.Cowlard FC, Lewis JC. Vitreous carbon—A new form of carbon. J Mat Sci. 1967;2(6):507–512. [Google Scholar]
- 120.Nowicki B, Runyan RS, Smith N, Krouskop TA. Kinetics of colonization of a porous vitreous carbon percutaneous implant. Biomaterials. 1990;11(6):389–392. doi: 10.1016/0142-9612(90)90092-5. [PMID: 2207227] [DOI] [PubMed] [Google Scholar]
- 121.Zim S. Skeletal volume enhancement: Implants and osteotomies. Curr Opin Otolaryngol Head Neck Surg. 2004;12(4):349–356. doi: 10.1097/01.moo.0000130576.04818.55. [PMID: 15252260] [DOI] [PubMed] [Google Scholar]
- 122.Romo T, 3rd, Sclafani AP, Sabini P. Use of porous highdensity polyethylene in revision rhinoplasty and in the platyrrhine nose. Aesthetic Plast Surg. 1998;22(3):211–221. doi: 10.1007/s002669900193. [PMID: 9618188] [DOI] [PubMed] [Google Scholar]
- 123.Guelcher SA, Hollinger JO. An introduction to biomaterials. Boca Raton (FL): CRC/Taylor & Francis; 2006. p. 553. [Google Scholar]
- 124.Yamamoto H, Shibata Y, Tachikawa T, Miyazaki T. In vivo performance of two different hydroxyapatite coatings on titanium prepared by discharging in electrolytes. J Biomed Mater Res B Appl Biomater. 2006;78(1):211–214. doi: 10.1002/jbm.b.30477. [PMID: 16362960] [DOI] [PubMed] [Google Scholar]
- 125.Cho YJ, Heo SJ. Osseointegration enhanced by electronbeam-deposition on the Ti-implant surface. J Korean Acad Prosthodont. 2004;42(3):340–351. [Google Scholar]
- 126.Spector M, Hanlon JG, Nancollas GH. Chemistry and structure of calcium-containing coatings and modified surfaces for titanium alloy orthopedic prostheses. In: Brown PW, editor. Hydroxyapatite and related materials. Boca Raton (FL): CRC Press, Inc; 1994. pp. 117–126. [Google Scholar]
- 127.Oosterbos CJ, Vogely HCh, Nijhof MW, Fleer A, Verbout AJ, Tonino AJ, Dhert WJ. Osseointegration of hydroxyapatite-coated and noncoated Ti6Al4V implants in the presence of local infection: A comparative histomorphometrical study in rabbits. J Biomed Mater Res. 2002;60(3):339–347. doi: 10.1002/jbm.1288. [PMID: 11920656] [DOI] [PubMed] [Google Scholar]
- 128.Franke Stenport V, Johansson CB. Enamel matrix derivative and titanium implants. J Clin Periodontol. 2003;30(4):359–363. doi: 10.1034/j.1600-051x.2003.00326.x. [PMID: 12694436] [DOI] [PubMed] [Google Scholar]
- 129.Bloebaum RD, Willie BM, Mitchell BS, Hofmann AA. Relationship between bone ingrowth, mineral apposition rate, and osteoblast activity. J Biomed Mater Res A. 2007;81(2):505–514. doi: 10.1002/jbm.a.31087. [PMID: 17236212] [DOI] [PubMed] [Google Scholar]
- 130.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13(5):377–383. doi: 10.1016/s1084952102000940. [PMID: 12324220] [DOI] [PubMed] [Google Scholar]
- 131.Gorelik JV, Cherepanova OA, Voronkina IV, Diakonov IA, Blinova MI, Pinaev GP. Laminin-2/4 from human placenta is a better adhesion agent for primary keratinocytes than laminin-1 from EHS sarcoma. Cell Biol Int. 2001;25(5):395–402. doi: 10.1006/cbir.2000.0642. [PMID: 11401326] [DOI] [PubMed] [Google Scholar]
- 132.Clark RAF, editor. The molecular and cellular biology of wound repair (The language of science) 2nd ed. New York (NY): Plenum Press; 1996. [Google Scholar]
- 133.Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: Preliminary study. Clin Orthop Relat Res. 2007;461:88–95. doi: 10.1097/BLO.0b013e318073c2b2. [PMID: 17549034] [DOI] [PubMed] [Google Scholar]
- 134.Hofmann AA, Bloebaum RD, Bachus KN. Progression of human bone ingrowth into porous-coated implants. Rate of bone ingrowth in humans. Acta Orthop Scand. 1997;68(2):161–166. doi: 10.3109/17453679709004000. [PMID: 9174454] [DOI] [PubMed] [Google Scholar]
- 135.Shin Y, Akao M. Tissue reactions to various percutaneous materials with different surface properties and structures. Artif Organs. 1997;21(9):995–1001. doi: 10.1111/j.1525-1594.1997.tb00514.x. [PMID: 9288870] [DOI] [PubMed] [Google Scholar]
- 136.Merritt K, Shafer JW, Brown SA. Implant site infection rates with porous and dense materials. J Biomed Mater Res. 1979;13(1):101–108. doi: 10.1002/jbm.820130111. [PMID: 429378] [DOI] [PubMed] [Google Scholar]
- 137.Aaron RK, Herr HM, Ciombor DM, Hochberg LR, Donoghue JP, Briant CL, Morgan JR, Ehrlich MG. Horizons in prosthesis development for the restoration of limb function. J Am Acad Orthop Surg. 2006;14(10 Spec No.):S198–S204. doi: 10.5435/00124635-200600001-00043. [PMID: 17003199] [DOI] [PubMed] [Google Scholar]
- 138.Bryant RA, Nix DP. Acute and chronic wounds: Current management concepts. 3rd ed. St. Louis (MO): Mosby Elsevier; 2007. [Google Scholar]
- 139.Eming SA, Medalie DA, Tompkins RG, Yarmush ML, Morgan JR. Genetically modified human keratinocytes overexpressing PDGF-A enhance the performance of a composite skin graft. Hum Gene Ther. 1998;9(4):529–539. doi: 10.1089/hum.1998.9.4-529. [PMID: 9525314] [DOI] [PubMed] [Google Scholar]
- 140.Sclafani AP, Romo T, 3rd, Ukrainsky G, McCormick SA, Litner J, Kevy SV, Jacobson MS. Modulation of wound response and soft tissue ingrowth in synthetic and allogeneic implants with platelet concentrate. Arch Facial Plast Surg. 2005;7(3):163–169. doi: 10.1001/archfaci.7.3.163. [PMID: 15897404] [DOI] [PubMed] [Google Scholar]
- 141.Tober KL, Cannon RE, Spalding JW, Oberyszyn TM, Parrett ML, Rackoff AI, Oberyszyn AS, Tennant RW, Robertson FM. Comparative expression of novel vascular endothelial growth factor/vascular permeability factor transcripts in skin, papillomas, and carcinomas of v-Ha-ras Tg.AC transgenic mice and FVB/N mice. Biochem Biophys Res Commun. 1998;247(3):644–653. doi: 10.1006/bbrc.1998.8787. [PMID: 9647747] [DOI] [PubMed] [Google Scholar]
- 142.Shi C, Cheng T, Qu J. The effects of nerve growth factor on skin healing and blood recovery in irradiated mice. Chin Med Sci J. 2002;17(2):127–128. [PMID: 12906169] [PubMed] [Google Scholar]
- 143.Brånemark PI. Osseointegration and its experimental studies. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 144.Charnley J. Arthroplasty of the hip. A new operation. Lancet. 1961;1(7187):1129–1132. doi: 10.1016/s0140-6736(61)92063-3. [PMID: 15898154] [DOI] [PubMed] [Google Scholar]
- 145.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;(239):263–285. [PMID: 2912628] [PubMed] [Google Scholar]
- 146.Bowlby AA. Surgical pathology and morbid anatomy. London (UK): J. & A. Churchill; 1895. p. 329. Submitted for publication August 31, 2008. Accepted in revised form January 14, 2009. [Google Scholar]