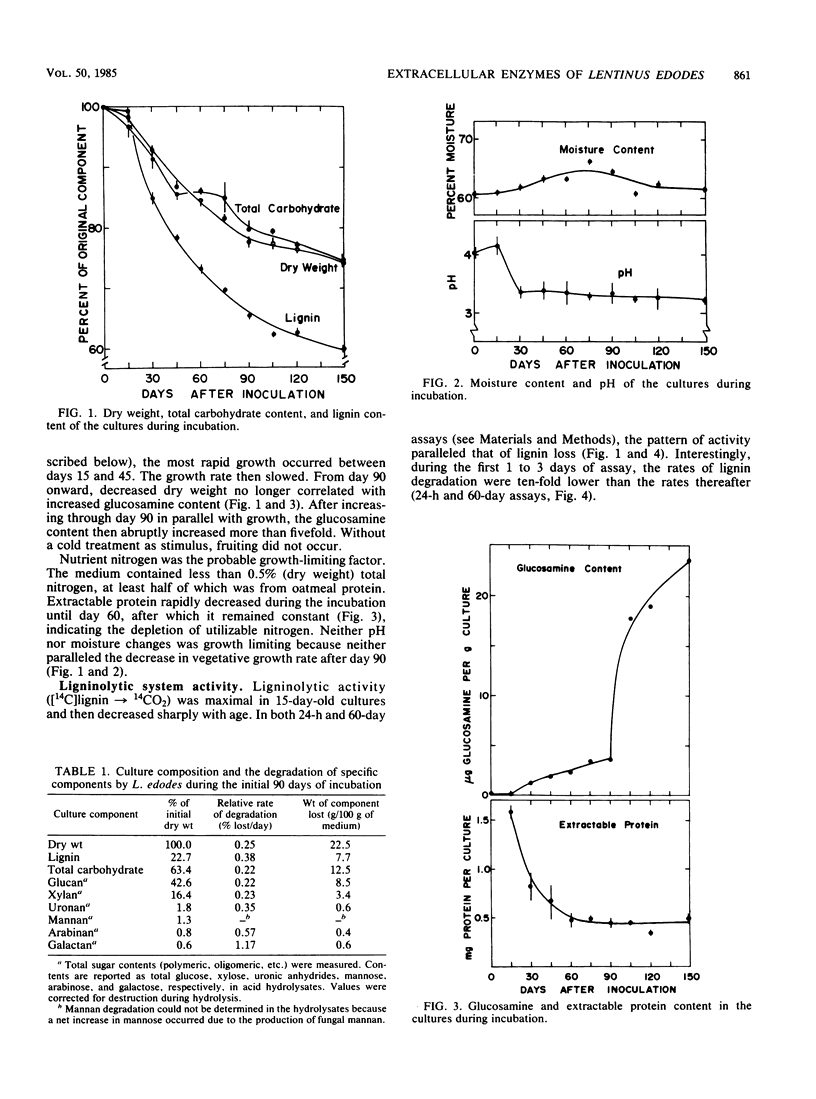
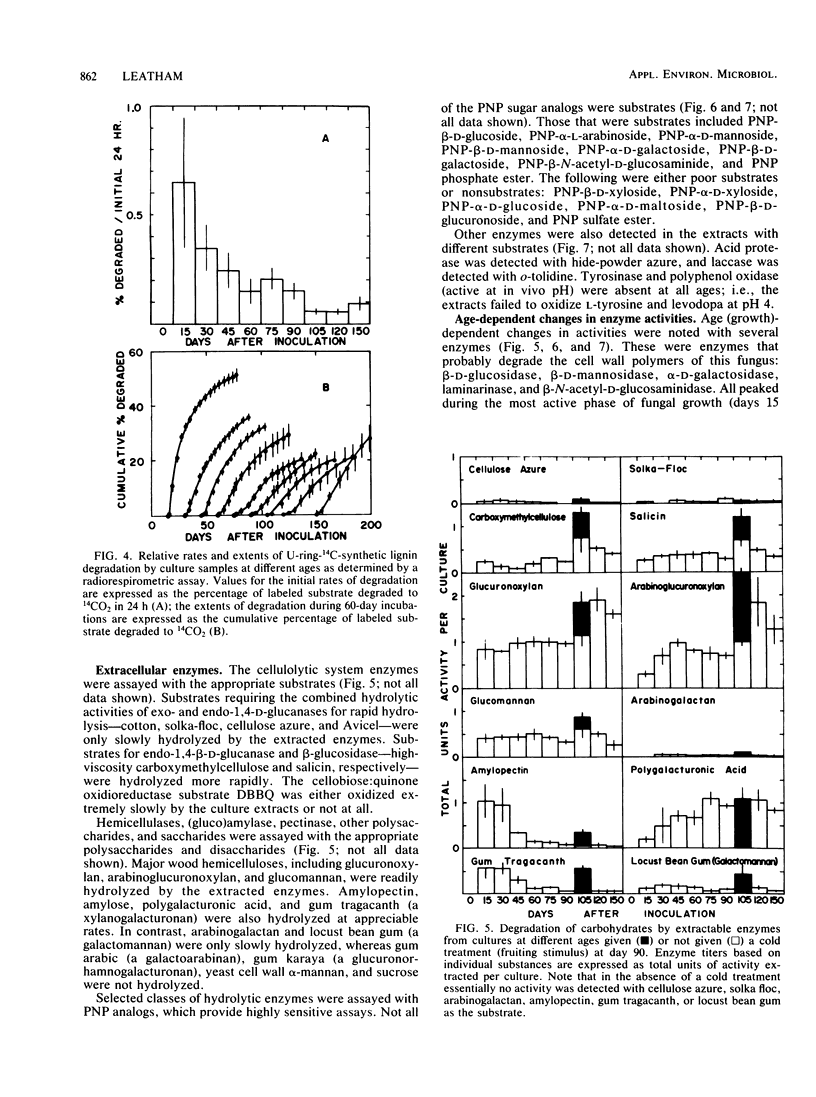
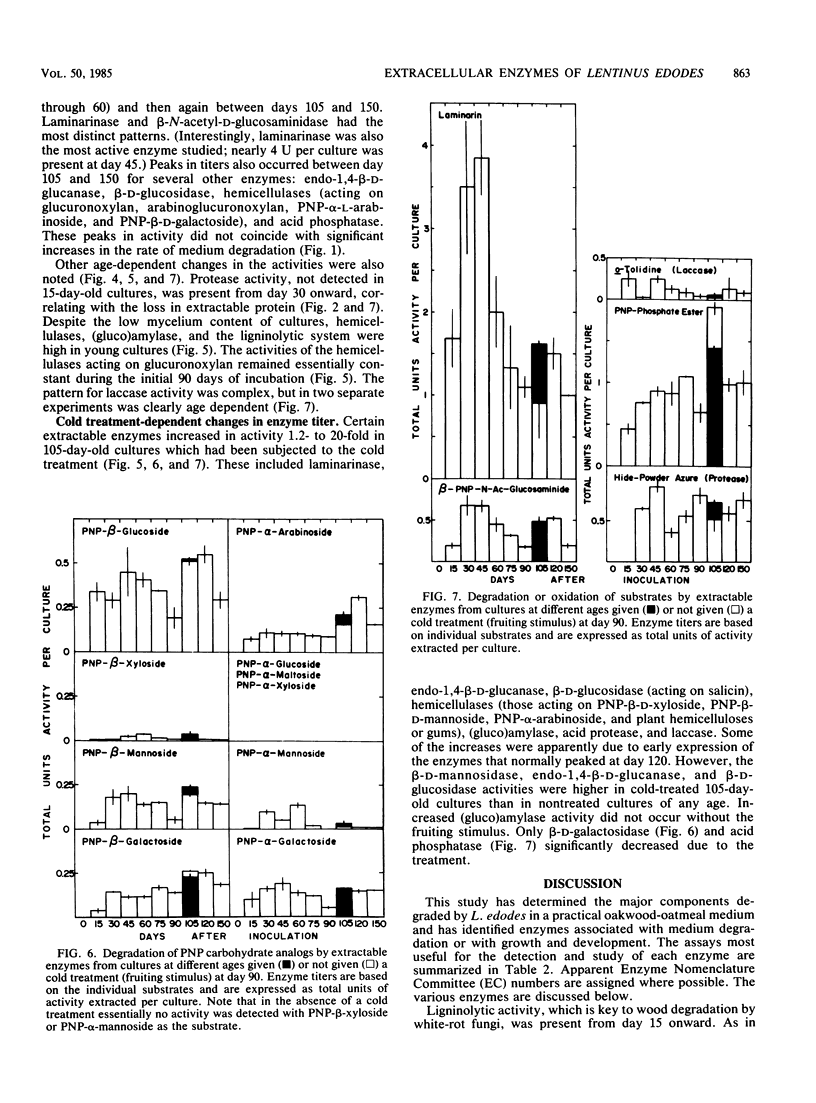
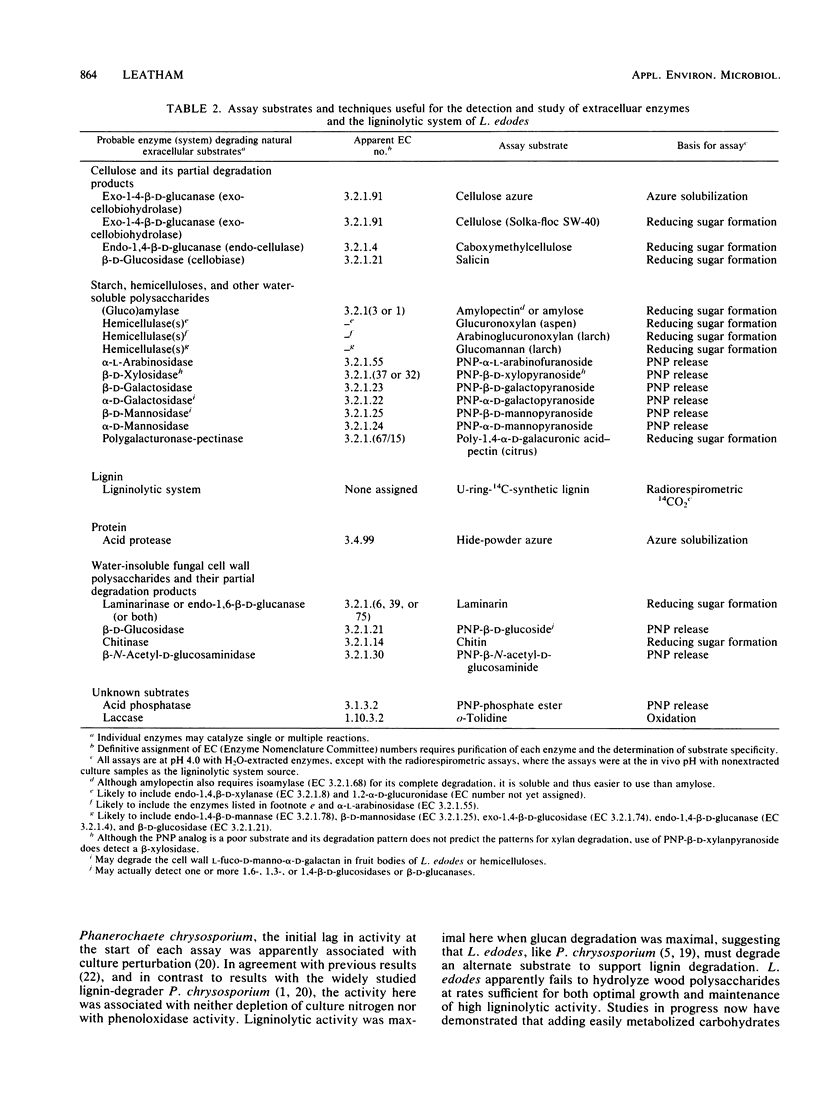
Abstract
Although the commercially important mushroom Lentinus (= Lentinula) edodes (Berk.) Sing. can be rapidly cultivated on supplemented wood particles, fruiting is not reliable. This study addressed the problem by developing more information about growth and development on a practical oakwood-oatmeal medium. The study determined (i) the components degraded during a 150-day incubation at 22°C, (ii) the apparent vegetative growth pattern, (iii) the likely growth-limiting nutrient, and (iv) assays that can be used to study key extracellular enzymes. All major components of the medium were degraded, lignin selectively so. The vegetative growth rate was most rapid during the initial 90 days, during which weight loss correlated with glucosamine accumulation (assayed after acid hydrolysis). The rate then slowed; in apparent preparation for fruiting, the cultures rapidly accumulated glucosamine (or its oligomer or polymer). Nitrogen was growth limiting. Certain enzyme activities were associated with the pattern of medium degradation, with growth, or with development. They included cellulolytic system enzymes, hemicellulases, the ligninolytic system, (gluco-)amylase, pectinase, acid protease, cell wall lytic enzymes (laminarinase, 1,4-β-d-glucosidase, β-N-acetyl-d-glucosaminidase, α-d-galactosidase, β-d-mannosidase), acid phosphatase, and laccase. Enzyme activities over the 150-day incubation period with and without a fruiting stimulus are reported. These results provide a basis for future investigations into the physiology and biochemistry of growth and fruiting.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Field C., Schekman R. Localized secretion of acid phosphatase reflects the pattern of cell surface growth in Saccharomyces cerevisiae. J Cell Biol. 1980 Jul;86(1):123–128. doi: 10.1083/jcb.86.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägerdal B., Harris H., Pye E. K. Association of beta-glucosidase with intact cells of Thermoactinomyces. Biotechnol Bioeng. 1979 Mar;21(3):345–355. doi: 10.1002/bit.260210302. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Zeikus J. G. Requirement for a growth substrate during lignin decomposition by two wood-rotting fungi. Appl Environ Microbiol. 1976 Jul;32(1):192–194. doi: 10.1128/aem.32.1.192-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard T. J., Phillips L. E. Study of phenoloxidase activity during the reproductive cycle in Schizophyllum commune. J Bacteriol. 1973 Apr;114(1):7–10. doi: 10.1128/jb.114.1.7-10.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- SUTTON D. D., LAMPEN J. O. Localization of sucrose and maltose fermenting systems in Saccharomyces cerevisiae. Biochim Biophys Acta. 1962 Jan 29;56:303–312. doi: 10.1016/0006-3002(62)90567-x. [DOI] [PubMed] [Google Scholar]
- Shida M., Haryu K., Matsuda K. On the water-soluble heterogalactan from the fruit bodies of Lentinus edodes. Carbohydr Res. 1975 May;41:211–218. doi: 10.1016/s0008-6215(00)87019-6. [DOI] [PubMed] [Google Scholar]
- Shida M., Ushioda Y., Nakajima T., Matsuda K. Structure of the alkali-insoluble skeletal glucan of Lentinus edodes. J Biochem. 1981 Oct;90(4):1093–1100. doi: 10.1093/oxfordjournals.jbchem.a133561. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Niederpruem D. J. Control of beta-glucosidases in schizophyllum commune. Can J Microbiol. 1967 Aug;13(8):1009–1020. doi: 10.1139/m67-135. [DOI] [PubMed] [Google Scholar]


