Abstract
 A conserved 2-His-1-Glu metal center, as found in natural non-heme iron-containing enzymes, was engineered into sperm whale myoglobin by replacing Leu29 and Phe43 with Glu and His, respectively (swMb L29E, F43H, H64, called FeBMb(-His)). A high resolution (1.65 Å) crystal structure of Cu(II)-CN− -FeBMb(-His) was determined, demonstrating that the unique 2-His-1-Glu metal center was successfully created within swMb. The FeBMb(-His) can bind Cu, Fe or Zn ions, with both Cu(I)-FeBMb(-His) and Fe(II)-FeBMb(-His) exhibiting nitric oxide reductase (NOR) activities. Cu dependent NOR activity was significantly higher than that of Fe in the same metal binding site. EPR studies showed that the reduction of NO to N2O catalyzed by these two enzymes resulted in different intermediates; a five-coordinate heme-NO species was observed for Cu(I)-FeBMb(-His) due to the cleavage of the proximal heme Fe-His bond, while Fe(II)-FeBMb(-His) remained six-coordinate. Therefore, both the metal ligand, Glu29, and the metal itself, Cu or Fe, play crucial roles in NOR activity. This study presents a novel protein model of NOR and provides insights into a newly discovered member of NOR family, gNOR.
A conserved 2-His-1-Glu metal center, as found in natural non-heme iron-containing enzymes, was engineered into sperm whale myoglobin by replacing Leu29 and Phe43 with Glu and His, respectively (swMb L29E, F43H, H64, called FeBMb(-His)). A high resolution (1.65 Å) crystal structure of Cu(II)-CN− -FeBMb(-His) was determined, demonstrating that the unique 2-His-1-Glu metal center was successfully created within swMb. The FeBMb(-His) can bind Cu, Fe or Zn ions, with both Cu(I)-FeBMb(-His) and Fe(II)-FeBMb(-His) exhibiting nitric oxide reductase (NOR) activities. Cu dependent NOR activity was significantly higher than that of Fe in the same metal binding site. EPR studies showed that the reduction of NO to N2O catalyzed by these two enzymes resulted in different intermediates; a five-coordinate heme-NO species was observed for Cu(I)-FeBMb(-His) due to the cleavage of the proximal heme Fe-His bond, while Fe(II)-FeBMb(-His) remained six-coordinate. Therefore, both the metal ligand, Glu29, and the metal itself, Cu or Fe, play crucial roles in NOR activity. This study presents a novel protein model of NOR and provides insights into a newly discovered member of NOR family, gNOR.
Rational design of functional enzymes is a powerful strategy to gain deep insights into more complex native enzymes, as it is an ultimate test of our knowledge about the enzyme structure and function.1 By conferring new activities, such an approach can reveal not only necessary but also enough structural features for the function of the native enzymes. One such example is bacterial nitric oxide reductase (NOR),2 which catalyzes two-electron reduction of NO to N2O, a crucial step in denitrification pathway.3 Since no 3D structure is available, bioinformatic studies suggest that NOR share similar structural features as heme-copper oxidases (HCOs) whose structures are available.4 Both NOR and HCO contain a heme b3 and a 3-histidine (His) metal center, with the metal site occupied by a non-heme iron in NOR (called FeB) and a copper in HCOs (called CuB). Computational modeling also indicates one or two glutamates (Glu) are close to the FeB site,5 which are essential for NOR activity.6 However, whether the Glu serves as a ligand to FeB remains uncertain. Recently, we have rationally designed a structural and functional NOR by construction of a 3-His-1-Glu FeB center in sperm whale myoglobin (swMb), called FeBMb (swMb L29H, F43H, H64, V68E), and demonstrated that both His and Glu liagnds are essential for iron binding and NO reduction activity.2a
While it is exciting to find that the 3-His-1-Glu FeB center can bind iron and confer NOR activity, a recent sequence alignment has predicted that a unique quinone-oxidizing NOR, gNOR, may exhibit a novel FeB site, with one of 3-His ligands replaced by an Asp residue, although the exact structure is not available.7 This finding is quite interesting, as an inspection of the active site of non-heme iron containing enzymes in nature indicates that the majority use a similar conserved 2-His-1-carboxylate (Asp/Glu) facial triad for iron binding and substrate oxidation.8 To test whether the unique 2-His-1-Asp/Glu non-heme metal center could be utilized to mimic NOR for NO reduction, we herein report introduction of a 2-His-1-Glu metal center in swMb by replacing His29 in FeBMb with a Glu residue while keeping Val68 intact (FeBMb H29E, E68V, or swMb L29E, F43H, H64, called FeBMb(-His)). As designed and demonstrated, FeBMb(-His) can bind iron and copper, with both proteins displaying NOR activity.
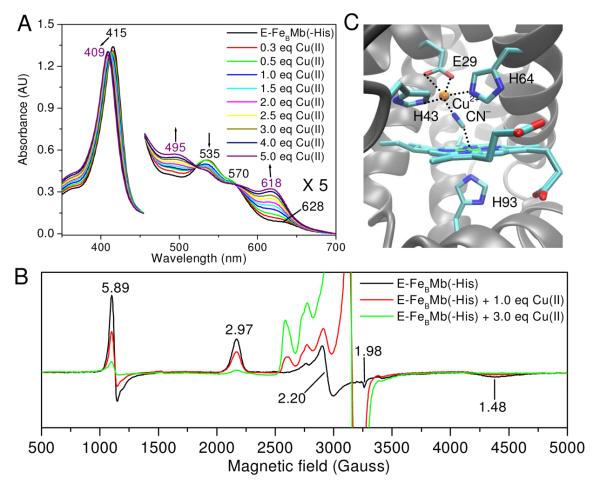
The FeBMb(-His) was prepared using a protocol described previously.2, 9 As isolated, it displays a red color, with a Soret band at 415 nm and visible absorptions at 535 and 570 nm (Figure 1A), resembling those of metMb in the presence of imidazole.10 The EPR spectrum exhibits signals at g = 2.97, 2.20, and 1.48 (Figure 1B), typical of a low-spin ferric heme and similar to those of the heme b with bis-histidine ligation in NOR (g = 2.96, 2.26, and 1.46).11 In addition, minor high-spin heme signals at g = 5.89 and 1.98 were observed, in agreement with the UV-vis spectrum that shows a small charge-transfer band at 628 nm. Addition of Cu2+ to oxidized protein that contains ferric heme, but no metal ion in the FeB site (called ferric E-FeBMb(-His)) resulted in a shift of the Soret band from 415 to 409 nm, decrease of 535 nm absorption and concomitant increase of 495 and 618 nm bands. The resultant new spectrum (409, 495 and 618 nm) is characteristic of a high-spin ferric heme, suggesting that one of the ligands dissociates from the heme iron and coordinates to copper, causing a low-spin to high-spin heme transition. This conclusion was further supported by EPR spectroscopy (Figure 1B); the low-spin signal at g = 2.97 decreased upon Cu2+ addition. Instead of increase of the high-spin signal at g = 5.89, as predicted by UV-vis spectra, the high-spin signal decreased, most likely due to spin-coupling between heme-Fe(III) and Cu(II).12 To support this interpretation, addition of Zn2+, a metal ion with no unpaired electrons, produced an increase in the high-spin signals and a decrease in the low-spin signals (Figure S1). Furthermore, titration of ferric E-FeBMb(-His) with Cu(II) in presence of cyanide results in a shift in the Soret band to 421 nm (Figure S2) due to the formation of a heme-Fe(III)-CN− -Cu(II) bridge, as evidenced by a high resolution crystal structure (Figure 1C, Table S1). In the structure, two His and one Glu coordinate to Cu(II) (2.27 Å to H43, 2.30 Å to H64, and 2.25Å to E29) with the N atom of CN (1.83 Å) making up the fourth coordination. A weak interaction with the other O atom of E29 (3.01 Å) also exists. These results demonstrate that a unique 2-His-1-Glu metal center was successfully created in the swMb.
Figure 1.
(A) UV-vis titration of ferric E-FeBMb(-His) with Cu(II) in 25 mM KH2PO4, 75 mM KCl, pH 7.0, 25 °C; (B) X-band EPR spectra of ferric EFeBMb(-His) (0.5 mM) in the same buffer as in (A) and that in the presence of 1.0 or 3.0 eq Cu(II). The spectra were collected at 20 K, 5 mW power, and 9.05 GHz; (C) Crystal structure of Cu(II)-CN− -FeBMb(-His) at 1.65 Å resolution (PDB entry 3MN0).
The UV-vis spectrum of deoxy E-FeBMb(-His) (Soret, 432 nm; visible, 556 nm, Figure S3) is characteristic of a five-coordinate ferrous heme, indicating a conformation change upon heme reduction. In the presence of Cu(I), Fe(II) or Zn(II), the Soret and visible bands decrease slightly in intensity, providing evidence of metal binding (Figure S3). Also, nitric oxide readily binds to deoxy E-FeBMb(-His), resulting in new peaks at 419, 545 and 578 nm (Figure S4). The complex of deoxy E-FeBMb(-His)-NO is stable without metal or with Zn(II) in the FeB site. However, the Soret band of Cu(I)-FeBMb(-His)-NO was found to be 400 nm, which becomes more intense and is red-shifted to 416 nm after about 1 hr. This observation is in contrast to Fe(II)-FeBMb(-His)-NO, in which the Soret band decreases and is blue-shifted from 419 nm to 416 nm (Figure S5). Note that the resultant spectrum in both cases is similar to that of ferric E-FeBMb(-His) in presence of NO (Figure S4B). These observations provide valuable evidence of NO reduction activity for both Cu(I)-FeBMb(-His) and Fe(II)-FeBMb(-His). The two electrons required for the reduction of NO to N2O come from the reduced heme iron and from either Cu(I) or Fe(II). After NO reduction, the heme returns to a ferric state, mimicking one turnover conditions. In addition, the contrasting spectral changes observed suggest that different intermediates occur during NO reduction, depending on which metal is in the non-heme FeB site (Cu(I) or Fe(II)).
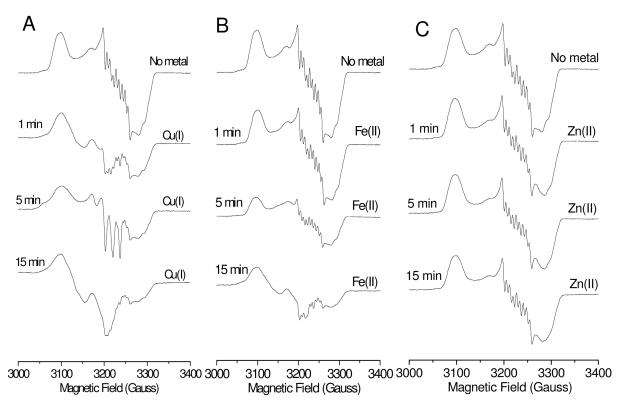
To provide more information about the intermediates, the NO reduction process was monitored by EPR. As shown in Figure 2, the spectrum of E-FeBMb(-His)-NO is typical of a six-coordinate low-spin heme-NO species with 9-line hyperfine splitting resulting from both the bound NO and the proximal histidine.13 In the presence of Cu(I), the hyperfine splitting decreases after 1 min of incubation with NO, which is likely due to a weakening of the proximal histidine bond to the heme iron.14 After 5 min, a 3-line hyperfine structure appears that is characteristic of a five-coordinate heme-NO species, indicating that the proximal heme Fe-His bond is broken.13 In contrast, this spectral change was not observed for Cu(I)-CuBMb-NO that has a 3-His metal center (H29, H43 and H64),14 indicating that Glu29 plays a crucial role in forming a five-coordinate heme intermediate. At longer times, the hyperfine splitting evolved into a broad peak, likely due to the reduction of NO and oxidation of FeBMb(-His). Note that both Cu(II)-NO15 and six-coordinate ferric heme-NO1b complexes are EPR silent, probably due to antiferromagnetic coupling between the unpaired electron on NO and that in Cu(II) or ferric heme. On the other hand, for Fe(II)-FeBMb(-His)-NO, only slight signal decreases were observed up to 5 min. At 15 min, similar to that of Cu(I)-FeBMb(-His)-NO at 1 min, the hyperfine splitting decreased, suggesting the heme Fe-His bond was weakened, but not cleaved. In case of redox-inactive Zn(II), no spectral changes were observed for Zn(II)-FeBMb(-His)-NO. Interestingly, a five-coordinate heme-NO species has been observed for both NOR11,16 and the member of the HCO family with the highest NO reduction activity, cytochrome cbb3 oxidases.17 However, in our cases, this five-coordinate species was detected only for Cu(I)-FeBMb(-His), not for Fe(II)-FeBMb(-His) or Zn(II)-FeBMb(-His). These observations suggest that not only the ligand, as in Glu29, but also the identity of the metal in the non-heme center play crucial roles in determining the nature of the intermediate.
Figure 2.
EPR spectra of deoxy E-FeBMb(-His) (0.5 mM) in the presence of NO after 5 min (top line), with 2 eq Cu(I) (A), Fe(II) (B) or Zn(II) (C) incubated with NO for 1 min, 5 min, and 15 min. Spectra were collected in 50 mM Bis-Tris pH 7.0 at 30 K, 0.2 mW power, and 9.05 GHz.
To confirm the product of NO reduction, GC/MS was carried out under single turnover conditions by monitoring N2O formation in the head space of the solution (Figure S6). When Cu(I)-FeBMb(-His) was exposed to excess NO, N2O could be observed to form with increased yield over time as estimated from the ratio of N2O:NO peaks (~32% at 20 hr). N2O production was also observed for Fe(II)-FeBMb(-His) (~6% at 20 hr). In contrast, no N2O formation was observed for Zn(II)-FeBMb(-His), metal alone, and wtMb with Cu(I)14 or with Fe(II)2a. These results demonstrate that Cu(I) or Fe(II) in the FeB site plays a vital role in NO reduction. Note that because of the high solubility of N2O (~25 mM in water at room temperature), GC/MS cannot be used to quantify the rates of NO reduction in the head space under these conditions. The relative activities of Cu(I)-FeBMb(-His) and Fe(II)-FeBMb(-His) are likely closely associated with the intermediates formed during NO reduction, with a five-coordinate heme intermediate leading to high NOR activity.2b, 10, 16, 17
In summary, the 2-His-1-Glu metal center commonly found in natural non-heme iron enzymes was engineered into myoglobin, and this new protein is capable of binding both Cu(I) or Fe(II) in the designed non-heme FeB site and of reducing NO to N2O via different intermediates. This study thus presents a novel structural and functional protein model of NOR and provides insights into the newly discovered member of NOR family, gNOR.
Supplementary Material
Acknowledgements
We thank Dr. Mark J. Nilges for help with EPR analysis, Furong Sun and Beth D. Eves for aiding in GC/MS data collection, and Dr. James Hemp for discussions regarding gNOR. This work was supported by the NIH Grant GM062211.
Footnotes
Supporting Information Available: Complete ref.7, experimental details, EPR for Zn(II) binding, UV-vis and GC/MS for NO reduction, and X-ray crystallographic data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Lu Y, Yeung N, Sieracki N, Marshall NM. Nature. 2009;460:855–862. doi: 10.1038/nature08304. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Collman JP, Yang Y, Dey A, Decréau RA, Ghosh S, Ohta T, Solomon EI. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15660–15665. doi: 10.1073/pnas.0808606105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kaplan J, DeGrado WF. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11566–11570. doi: 10.1073/pnas.0404387101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wasser IM, de Vries S, Moёnne-Loccoz P, Schröder I, Karlin KD. Chem. Rev. 2002;102:1201–1234. doi: 10.1021/cr0006627. [DOI] [PubMed] [Google Scholar]
- 2.(a) Yeung N, Lin Y-W, Gao Y-G, Zhao X, Russell BS, Lei L, Miner KD, Robinson H, Lu Y. Nature. 2009;462:1079–1082. doi: 10.1038/nature08620. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lin Y-W, Yeung N, Gao Y-G, Miner KD, Tian S, Robinson H, Lu Y. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8581–8586. doi: 10.1073/pnas.1000526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watmough NJ, Field SJ, Hughes RJL, Richardson DJ. Biochem. Soc. Trans. 2009;37:392–399. doi: 10.1042/BST0370392. [DOI] [PubMed] [Google Scholar]
- 4.Ji H, Das TK, Puustinen A, Wikström M, Yeh S, Rousseau DL. J. Inorg. Biochem. 2010;104:318–323. doi: 10.1016/j.jinorgbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimann J, Flock U, Lepp H, Honigmann A, Ädelroth P. Biochim. Biophys. Acta. 2007;1767:362–373. doi: 10.1016/j.bbabio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Butland G, Spiro S, Watmough NJ, Richardson DJ. J. Bacteriol. 2001;183:189–199. doi: 10.1128/JB.183.1.189-199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sievert SM, et al. Appl. Environ. Microbiol. 2008;74:1145–1156. doi: 10.1128/AEM.01844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Kovaleva EG, Lipscomb JD. Nat. Chem. Biol. 2008;4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tshuva EY, Lippard SJ. Chem. Rev. 2004;104:987–1011. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]
- 9.(a) Sigman JA, Kwok BC, Lu Y. J. Am. Chem. Soc. 2000;122:8192–8196. [Google Scholar]; (b) Sigman JA, Kim HK, Zhao X, Carey JR, Lu Y. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3629–3634. doi: 10.1073/pnas.0737308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diven WF, Goldsack DE, Alberty RA. J. Biol. Chem. 1965;240:2437–2441. [PubMed] [Google Scholar]
- 11.Sakurai T, Sakurai N, Matsumoto H, Hirota S, Yamauchi O. Biochem. Biophys. Res. Commun. 1998;251:248–251. doi: 10.1006/bbrc.1998.9451. [DOI] [PubMed] [Google Scholar]
- 12.Cheesman MR, Oganesyan VS, Watmough NJ, Butler CS, Thomson AJ. J. Am. Chem. Soc. 2004;126:4157–4166. doi: 10.1021/ja038858m. [DOI] [PubMed] [Google Scholar]
- 13.Decatur SM, Franzen S, DePillis GD, Dyer RB, Woodruff WH, Boxer SG. Biochemistry. 1996;35:4939–4944. doi: 10.1021/bi951661p. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Yeung N, Russell BS, Garner DK, Lu Y. J. Am. Chem. Soc. 2006;128:6766–6767. doi: 10.1021/ja058822p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarma M, Kalita A, Kumar P, Singh A, Mondal B. J. Am. Chem. Soc. 2010;132:7846–7847. doi: 10.1021/ja102279c. [DOI] [PubMed] [Google Scholar]
- 16.Kumita H, Matsuura K, Hino T, Takahashi S, Hori H, Fukumori Y, Morishima I, Shiro Y. J. Biol. Chem. 2004;279:55247–55254. doi: 10.1074/jbc.M409996200. [DOI] [PubMed] [Google Scholar]
- 17.Pinakoulaki E, Stavrakis S, Urbani A, Varotsis C. J. Am. Chem. Soc. 2002;124:9378–9379. doi: 10.1021/ja0271633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.