Abstract
Purpose
Females are often reported to be generally more resistant to fatigue than males for relative intensity tasks. This has been observed repeatedly for elbow flexors, whereas at the ankle sex differences appear less robust, suggesting localized rather than systemic influences. Thus, the purpose of this study was to examine sex differences in fatigue resistance at muscle groups in a single cohort and which factors, if any, predict endurance time.
Methods
Thirty-two (16 female) young adults (19 to 44 yrs) performed sustained isometric contractions at 50% maximum voluntary isometric contraction to failure for elbow flexion and ankle dorsiflexion. Pain, exertion, and muscle electromyography (EMG) were assessed throughout. Self-reported baseline activity was measured using the International Physical Activity Questionnaire.
Results
Females (112.3 ± 6.2 sec) were significantly more resistant to fatigue than males (80.3 ± 5.8 sec) at the elbow (p=0.001); but not at the ankle (p=0.45; 140.6 ± 10.7 vs. 129.2 ± 10.5 sec). Peak torque was greater in males than females (p < 0.0001) at the ankle (45.0 ± 1.7 vs. 30.1 ± 1.0 Nm) and the elbow (75.7 ± 3.1 vs. 34.4 ± 2.2 Nm). Peak torque was significantly related to endurance time at the elbow (R2 = 0.30), but not at the ankle (R2 = 0.03). Peak pain, rate of pain increase, peak exertion, EMG, and baseline physical activity did not differ between sexes.
Conclusion
Sex differences in fatigue resistance are muscle group specific. Women were more fatigue-resistant at the elbow but not the ankle during a sustained isometric contraction. Further, factors which may contribute to fatigue-resistance for one muscle group (e.g. sex, peak torque) may not be critical at another.
Keywords: Isometric contraction, elbow, ankle, maximum endurance time
INTRODUCTION
Females have demonstrated greater fatigue resistance for a variety of tasks, including: isometric force-matching (19, 22), isometric position-matching (22), and isometric contractions with intermittent rests (37). Several factors have been considered to explain observed differences in fatigue resistance, ranging from muscle mass differences to hormonal influences (12). However others have reported no sex differences in fatigue resistance with isometric force-matching tasks (9, 41). In a recent review, Hunter (2009) suggests sex differences in muscle fatigue can be influenced by task specificity; encompassing contraction mode, contraction intensity, position- versus load-matching tasks, cognitive load, age, and muscle group (16). Thus, the statement that females are more fatigue resistant than males for relative intensity tasks is clearly an over simplification.
In particular, a review of several fatigue studies suggests there are differences in the sex effect between elbow flexor and ankle dorsiflexor muscles (16). However, this comparison has never been directly assessed in the same cohort of individuals. Thus, these apparent sex differences between muscle groups could simply be due to cohort or methodological variance. Other potential mediating factors may include: peak torque (18, 19), muscle activation strategies (18, 34), and/or noxious afferent feedback (16). Whether these factors contribute to sex differences or vary between muscle groups is not clear.
Thus, the primary purpose of this study was to examine sex differences in fatigue resistance at two distinct muscle groups, the elbow flexors and the ankle dorsiflexors; considering several potential contributors to fatigue. These factors included: peak torque, self-reported activity level, muscle activation strategy, perceived pain, perceived exertion, and rate of increase for pain and exertion. This finding may help to better elucidate the underlying mechanisms contributing to sex differences in fatigue resistance. For example, if fatigue differences are indeed muscle group specific, local (e.g. muscle mass) rather than systemic (e.g. hormonal milieu) mechanisms are likely to be responsible for this phenomenon. This information may be used to better discern whether sex differences are widespread or localized phenomena. An improved understanding of factors influencing fatigue may be useful for mathematical fatigue modeling applications as well as impact optimal training and performance in sport, exercise, and rehabilitation applications.
METHODS
Subjects
Thirty-two (16 male, 16 female) recreationally-active young adults participated in this study (see Table 1 for subject demographics). All subjects were classified as healthy with no major medical history, including: cardiovascular disease, asthma, diabetes, elbow or ankle major joint trauma, neuromuscular disease or chronic pain conditions. Subjects who were college- or professional-level athletes were excluded due to their unique level of training. Prior to participation all subjects provided written, informed consent. The study procedures were approved by the University of Iowa Institutional Review Board. All participants were reimbursed for their time.
Table 1.
Mean (SEM) subject demographic information by sex
| N | Height (cm) |
Weight (kg) |
Age (yr) |
IPAQ (MET*hr) |
|
|---|---|---|---|---|---|
| Male | 16 | 177.8* (1.8) |
78.5* (2.1) |
24.4 (1.4) |
57.7 (11.1) |
| Female | 16 | 164.6* (1.8) |
59.4* (1.9) |
23.0 (0.5) |
38.1 (10.9) |
IPAQ = International Physical Activity Questionnaire, total score
Significant differences between sexes (p < 0.05).
Experimental Procedures
Subjects performed isometric force-matching fatigue tasks at 50% maximal voluntary isometric contraction (MVIC) for both elbow flexion and ankle dorsiflexion during one visit. The order of testing was block-randomized by sex to minimize any fatigue effects. A 20 min rest was provided between the two fatigue tasks, during which time participants completed the activity questionnaire.
Fatigue Task
The fatigue task for each muscle group started with a 5-minute warm-up on a stationary bike, followed by assessing maximum torque generating capability using a Biodex Isokinetic Dynamometer System 3 (Biodex Medical Systems, New York). Maximum torque was operationally defined as the maximum of three MVIC trials separated by 1 minute rests. The fatigue task was then performed using the isokinetic dynamometer at 50% MVIC until volitional failure with visual and verbal feedback. Failure was operationally defined as the inability to maintain torque within 10% of the target level for five seconds or falling below the target level three times within a 30 second window. For the elbow flexion fatigue task, subjects were seated, with the forearm positioned at 60° of elbow flexion, and the shoulder flexed approximately 30°. For the ankle dorsiflexion fatigue task, subjects were tested in a seated position with the knee flexed approximately 90° and the ankle positioned at 20°of plantarflexion.
Perceptual ratings
Participants were asked to verbally rate their pain and exertion throughout and immediately following the fatigue tasks using the Borg Category Ratio 0-10 numeric rating scale (Borg CR10)(1). A written script, modified from Borg (1998), was read to each participant to ensure consistent instructions for scale use. The peak values were extracted for further analysis. The mean rate of change for pain and exertion were calculated using linear regression techniques for each individual subject (i.e., slope of each rating vs. time relationship). Immediately following the fatigue protocol for both the ankle and elbow, participants completed the McGill Pain Questionnaire – short form (SF MPQ). The SF MPQ is comprised of 3 subscales, providing both qualitative and quantitative pain assessments. Participants rated their pain using 15-adjective verbal descriptors, a 10 cm horizontal visual analog scale (VAS), and a 6-point evaluative scale.
Demographic data
Height (cm) and weight (kg) were measured using calibrated scales to document subject demographics. Activity levels were assessed using the International Physical Activity Questionnaire (IPAQ), a self-report measure of activities performed in the past 7 days (3). Assessments include estimates of leisure; domestic and gardening; work; and transportation related physical activities. Calculated algorithms based upon activity levels classify the participant as low, moderate or high physical activity (3).
Muscle activity
Muscle activity was measured using 4 channels of surface muscle electromography (EMG, Delsys Bagnoli, Boston, MA), band pass filtered from 20 – 450 Hz. For the elbow, EMG electrodes were attached to the skin over the biceps brachii, brachioradialis, triceps brachii, and upper trapezius muscles. For the ankle, EMG electrodes were attached to the skin over the tibialis anterior, medial and lateral gastrocnemius and soleus muscles. The skin was prepared with 70% alcohol wipes and electrodes were adhered using medical-grade adhesive tapes. The EMG signals are pre-amplified peripherally at the electrode (10 times) and again prior to analog-to-digital conversion (1000 times) to maximize signal quality.
Data Analysis
All torque and EMG signals were collected using custom Labview (National Instruments) software at 1000 Hz. Torque signals were low-pass filtered at 10 Hz. Absolute and normalized (by body mass) peak torque values were used in the analyses. Times to fatigue were determined from the torque tracings offline, and corroborated with stop-watch results collected during each fatigue trial. The EMG data were rectified, filtered using a moving average with a 200ms window and standardized by their respective maximum EMG values. EMG data were averaged across each successive 5% time interval from 0 to 100% endurance time for each muscle.
Statistical Analysis
Summary statistics were calculated for endurance time, peak torque, peak torque normalized by body mass, peak pain and exertion, mean rate of change for pain and exertion, surface EMG, and demographic data. Data are reported as mean ± standard error of the mean (SEM) within the text and figures. Independent and paired t-tests were used to compare endurance time and peak torque between sexes for each muscle group, and secondarily between the two muscle groups. Repeated measures analysis of variance (ANOVA) was used to compare EMG amplitude between sexes and between muscle groups at 25, 50, and 75% of task duration, with follow-up paired t-tests as appropriate. Effect sizes (Cohen’s d) were calculated for between-joint and between-sex differences. Large effect sizes were operationally defined as d values of 1.0 or greater. Correlation analyses assessed the relationships between endurance time and the following variables: peak torque, normalized peak torque (peak torque/body mass), peak pain, peak exertion, rate of pain increase, rate of exertion increase for each muscle group separated by sex. Stepwise, linear regression techniques were utilized to model endurance time for each muscle group as a function of sex, peak torque, rate of pain or exertion, and activity level. All statistical analyses were performed using SPSS (v16.0, Chicago, IL), with alpha set at 0.05.
RESULTS
Subjects
Subject demographics are provided in Table 1. Men were heavier and taller than women but age and activity levels were not significantly different. Self-reported activity levels were predominantly in the moderate (n = 15) and high activity (n=15) levels, with only 1 in the low range, based on normative data for the IPAQ (3). One subject’s IPAQ data was excluded as the survey was not completed correctly.
Endurance Time
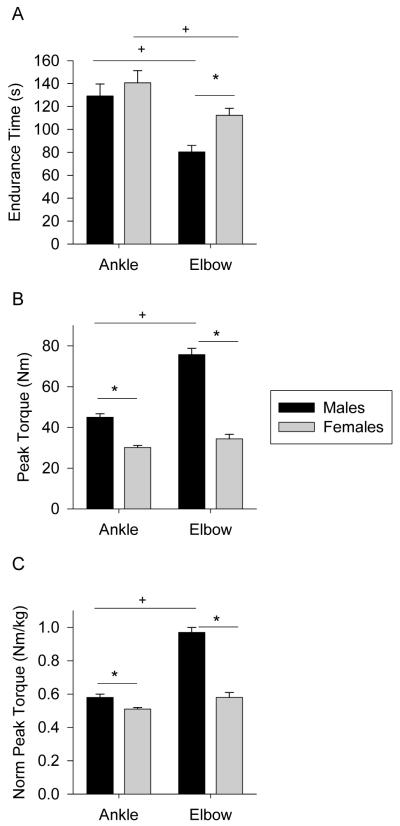
Females (112.3 ± 6.2 sec) were significantly more resistant to fatigue than males (80.3 ± 5.8 sec) for elbow flexion (p=0.001; see Figure 1). Conversely, no significant difference (p=0.45) in endurance time occurred between females (140.6 ± 10.7 sec) and males (129.2 ± 10.5 sec) for the ankle dorsiflexion task. The corresponding effect sizes (Cohen’s d) for the observed sex-difference were large for the elbow flexors but small for the ankle dorsiflexors (Table 2). Comparing endurance times between muscle groups revealed that ankle dorsiflexion was more resistant to fatigue than elbow flexion for both females (Δ 28.3 s; p= 0.021) and males (Δ 48.9 s; p< 0.001). The corresponding effect sizes for the ankle to elbow muscle group differences were 0.85 and 1.49 for females and males, respectively.
Figure 1.
Mean (SEM) endurance time (A), peak torque (B), and peak torque normalized by body mass (C) for the ankle and elbow muscle groups by sex. * Significant difference between sexes (p < 0.05). + Significant difference between muscle groups (p < 0.05).
Table 2.
Effect sizes (Cohen’s d) for endurance time and peak torque
| Muscle Group | Endurance Time |
Peak Torque |
Peak Torque/ Body Mass |
|---|---|---|---|
| Elbow Flexors | 1.38 | − 3.95 | − 2.90 |
| Ankle Dorsiflexors | 0.28 | − 2.75 | − 1.01 |
Note: Effect sizes represent female to male (F:M) ratios.
Peak Torque
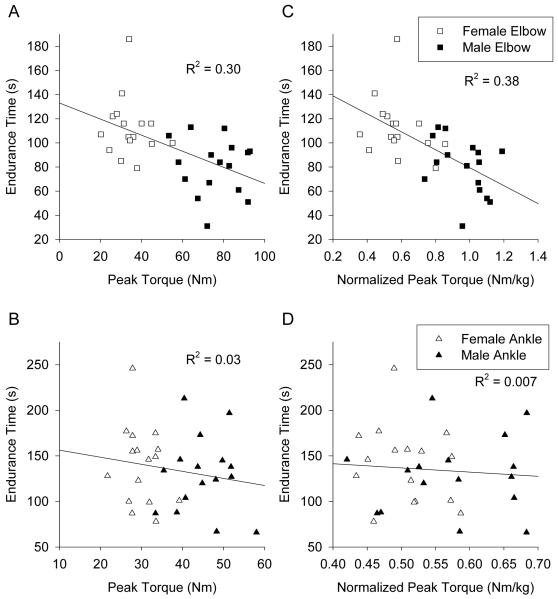
Males exhibited greater peak torque (p < 0.0001) than females (Figure 1B) at both the ankle (45.0 ± 1.7 Nm vs. 30.1 ± 1.0 Nm, respectively) and the elbow (75.7 ± 3.1 Nm vs. 34.4 ± 2.2 Nm, respectively). Similar differences were observed for normalized peak torque (Figure 1C) for males and females, respectively at the ankle (0.58 ± 0.02 Nm/kg vs. 0.51 ± 0.01 Nm/kg) and the elbow (0.97 ± 0.03 Nm/kg vs. 0.58 ± 0.03 Nm/kg). The corresponding effect sizes for peak torque sex differences were large for both muscle groups (Table 2). Peak torque and normalized peak torque were significantly related to endurance time at the elbow (Figure 2A and C), but not at the ankle (Figure 2B and D).
Figure 2.
Linear regressions of endurance time versus absolute (A, B) and normalized (C, D) peak torque are shown for the elbow flexors (A, C) and ankle dorsiflexors (B, D).
Peak torque and normalized peak torque were significantly greater for the elbow flexors than the ankle dorsiflexors for males (p< 0.0001) but did not reach significance (p > 0.06) in females. Accordingly, the effect sizes for the between-joint peak torque differences were large for males (3.16) but only moderate for females (0.65).
Muscle Activity
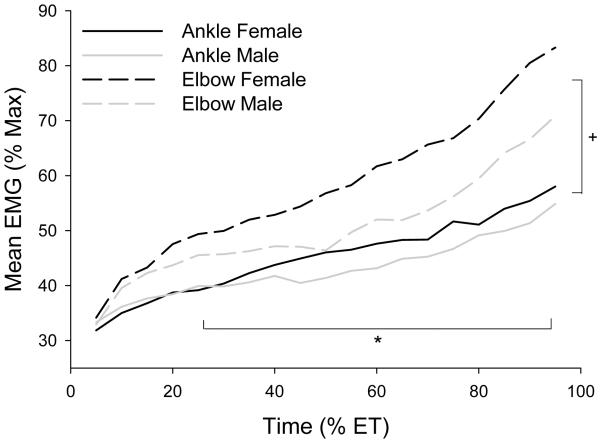
EMG data was incomplete due to data collection complications during the ankle (n=4) and elbow (n=6) fatigue tasks, thus sample sizes were reduced for the muscle activity analyses. Using the ANOVA, mean EMG increased significantly over time during the 50% fatigue tasks for both muscle groups and sexes (p < 0.0001; Figure 3). Muscle activity did not vary between males and females (p = 0.13). However, EMG was significantly higher at the elbow than the ankle overall (p = 0.001), and increased at a greater rate than the ankle (p = 0.04). Follow-up paired t-tests at 25, 50 and 75% of total endurance time were further analyzed to determine if one muscle group was consistently greater. These tests revealed elbow muscle activity was significantly greater than ankle muscle activity at each relative time point assessed: 25% (p = 0.012), 50% (p = 0.003), and 75% (p = 0.001) of endurance time.
Figure 3.
Mean EMG for ankle and elbow muscle groups by sex. * Significant difference over time for both muscle groups (p < 0.0001). + Significant difference between muscle groups (p < 0.05)
Perceived Pain and Exertion
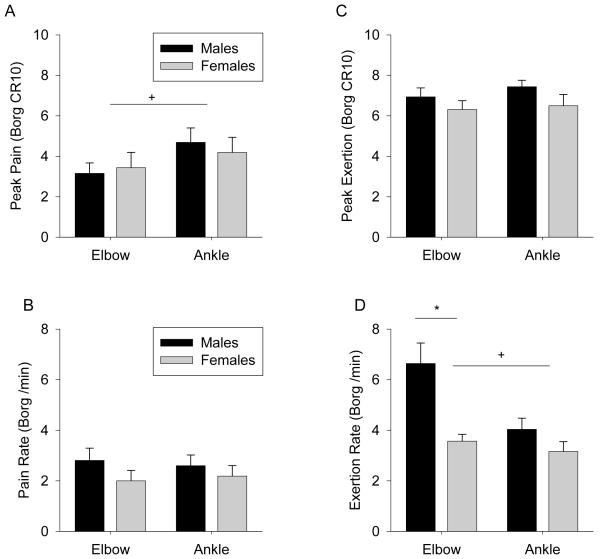
Both males and females reported similar peak pain and exertion ratings across both muscle groups (Figure 4A and C, p > 0.15). The absolute time of each fatigue task did not appear to influence peak perceptual ratings, as females were able to sustain the elbow task longer than males with no significant difference in peak ratings. The ankle fatigue task was reported to be significantly more painful than the elbow task across all subjects (Figure 4A, p = 0.016), but did not achieve significance when considering only male (p = 0.08) or female (p = 0.09) subjects separately. Peak exertion did not vary between muscle groups (Figure 4B, p = 0.24). The mean pain increase per minute did not vary between sexes or muscle groups (Figure 4B and D, p > 0.65). However men reported significantly faster increases in perceived exertion at the elbow (p = 0.002) than females; resulting in a significant overall difference between muscle groups (p = 0.003). The most frequently identified qualitative pain descriptors on the SF MPQ immediately post-fatigue for both tasks were “cramping,” “aching,” and “tiring/exhausting”.
Figure 4.
Mean (SEM) peak pain (A), rate of pain increase per minute (B), peak exertion (C), and rate of exertion increase per minute (D) for ankle and elbow muscle groups and sex. * Significant difference between sexes (p < 0.05). + Significant difference between muscle groups (p < 0.05).
No pain measures (peak or rate of change) resulted in significant correlations with endurance time.. In males, both ankle and elbow endurance times were related to exertion rate of change (r = −0.81 and −0.82, respectively). While in females, only ankle endurance time was correlated to exertion rate of change (r = −0.67). Peak exertion did not significantly correlate with any endurance time variables.
Predicting Endurance Time
For the elbow, only sex was a significant predictor in the model to predict endurance time (considering peak torque, sex, and self-reported activity) using stepwise linear regression techniques (R2 = 0.30). Once sex was accounted for, peak torque did not add any additional predictive information, but these two variables were collinear (r = 0.89). Thus, either variable provided essentially equivalent information (i.e., see Figure 2A). For the ankle, no linear regression model achieved significance, using peak torque, sex, and self-reported activity levels as possible predictors.
DISCUSSION
The most notable findings of this study are: 1) the large sex differences in fatigue associated with sustained isometric contractions at the elbow were not observed at the ankle; 2) peak torque was a good predictor of fatigue-resistance only at the elbow; 3) muscle activation strategies differed between muscle groups, but not between sexes; 4) no sex differences were exhibited for peak pain or exertion ratings across both muscle groups; and 5) the ankle fatigue task was reported to be significantly more painful than the elbow task across all subjects.
The observed endurance times for both ankle dorsiflexion and elbow flexion are consistent with other published values (10, 29, 34), suggesting our study population was not substantially different from other populations investigated. The observed sex differences for the elbow flexors (effect size, d = 1.4) are largely in accordance with previous findings at the elbow, with a median effect size of 0.8 from previous studies (range −0.7 to 3.9) (2, 4, 9, 18, 19, 21, 22, 44). At the ankle, the median effect size was 0.1, (range −1.1 to 3.1) (14, 15, 24, 31, 33, 39), similar to that observed here (d=0.3). Thus, females consistently are significantly more resistant to fatigue than males for elbow flexors, but not for ankle dorsiflexors. Few other muscle groups have been systematically studied for sex differences, but the limited evidence available suggests sex differences may not be readily predictable, as shoulder abduction was not different between men and women, whereas trunk flexion was more fatigue-resistant in females (43).
Historically, assessment of isometric endurance between men and women has been performed at single, not multiple muscle groups. Underlying differences in protocols and/or laboratory settings confounds the ability to conclusively evaluate regional versus systemic sex differences in fatigue-resistance. A limited number of studies include two-muscle group protocols, but typically had very small sample sizes and/or no representation of women. The current study demonstrates that sex differences in fatigue development during a sustained isometric force task are regional and muscle group dependent. Thus, the regional differences suggested in a recent review (16) are further substantiated by our findings.
In studies that have observed fatigue sex differences, the most commonly postulated explanatory mechanisms can be parceled into: muscle mass / perfusion, neuromuscular activation, and substrate utilization. Hicks, et al (2001) suggests that larger massed muscles may result in greater intramuscular pressure and blood flow occlusion at a given contraction intensity, resulting in more rapid fatigue when compared to smaller muscles (12). As peak torque is roughly proportional to muscle mass (via cross-sectional area), endurance time has been noted to decrease linearly (23) or exponentially (40) with increasing peak torque. However, in the current study, females were weaker than males for both muscle groups; yet only the elbow yielded a significant sex-difference in endurance time. In addition, peak torque explained 30% of the variance in endurance time at the elbow, but only 3% at the ankle. These findings suggests muscle mass may be one contribution to fatigue differences, but cannot fully account for variations in endurance time between men and women, particularly across bodily regions.
Additional vascular mechanisms, such as vascular reactivity and vasoconstriction, are not uniform throughout the body (36); thus could influence muscle perfusion during a sustained fatiguing contraction. Clearly, the lower extremities are chronically exposed to elevated hydrostatic pressures in upright postures, suggesting upper and lower limbs may differentially respond to changes in intramuscular pressure. Sex differences have been documented in vasodilatation (35); capillary fluid filtration (28); and blood flow during sustained (40) and brief maximal (20) contractions. However, blood flow and vascular conductance were not able to explain sex differences in fatigue using an intermittent contraction endurance task (20). Thus, it is not yet clear whether differential limb vascular response can potentially explain a portion of the regional fatigue sex differences observed between the elbow and the ankle.
Neuromuscular activation strategies, assessed via comparison of EMG amplitudes, were similar to previous studies with a gradual increase in activation over time during a submaximal isometric task (8, 24). Unfortunately few, if any, studies have compared EMG activation between men and women at more than one muscle group. Consistent with previous sustained isometric tasks, that did not strength-match women, no sex differences in activation strategy were evident at the elbow (19, 23). However, when matched for strength, conflicting results have been observed. For example, at the elbow women display a reduced rate of activation despite similar endurance times as men (18); whereas at the ankle, no sex differences in activation strategy or endurance time were observed (11). Although not strength-matched, we also observed no difference in ankle activation strategy between men and women. However, both males and females consistently displayed greater EMG activity at the elbow compared to the ankle, suggesting activation strategies may vary more between muscle groups than between sexes. Thus, muscle activation does not appear to play a key mechanistic role in explaining the fatigue sex differences observed only at the elbow.
Another possible neuromuscular activation component which could result in apparent sex differences in fatigue is central activation. If women do not maximally activate during the MVIC testing, i.e. interpolated twitch techniques, then their relative-intensity target workload will be less than expected. A recent meta-analysis modeled endurance times as a function of task intensity for sustained isometric contractions at several joints (7). Using these models, the current 30 sec difference in endurance time observed between men and women at the elbow would require a difference in central activation of 14-16% between the sexes (e.g. 100% vs. 84 – 86%). It has been suggested that women are less able to achieve full central activation, similar to that seen in children (5), although the available data is inconsistent. Central activation ratios did not significantly differ between men and women for the elbow flexors (≤ 4%) (17) or the ankle dorsiflexors (≤ 1%) (26) prior to or following a fatiguing task. Thus it is unlikely voluntary activation sufficiently explains the difference in endurance time observed for the elbow flexors.
Substrate utilization has been suggested as another mechanism that may contribute to differences in fatigue between men and women (12). Men may preferentially rely on glycolytic pathways (38), whereas women may preferentially use oxidative processes for energy metabolism (42). While this may contribute to sex differences in fatigue, particularly when evaluating fatigue-resistance across a range of relative intensities, it is not clear how differences in substrate utilization may help explain the sex differences observed at a single intensity at the elbow but not the ankle. It may reflect differential motor-unit activation between men and women or possibly differences in daily functional use of these two muscle groups (e.g. training) between the sexes (e.g. walking versus lifting and carrying). Future studies are warranted to specifically assess the effect of daily use patterns on fatigue sex differences.
Variations in endurance time across bodily regions may be readily explained by differences in muscle composition. Elbow flexors (biceps brachii) are predominantly composed of type II fibers (~61 ± 5% type II) (30) whereas the ankle dorsiflexors (tibialis anterior) are composed of primarily type I fibers (~77 ± 7% type I) (25). These reported compositions mirror our observed endurance times, with the ankle dorsiflexors being more fatigue resistant than the elbow flexors overall. However, muscle composition has not been shown to significantly differ between men and women (32). Thus, while muscle fiber composition may be the leading explanation for the fatigue differences between muscle-groups, it is less clear how muscle composition contributes to the sex differences observed predominantly for one muscle group. As previously mentioned, it may be that in muscle groups with greater type II fibers, women are better able to sustain contractions due to their preferential use of oxidative metabolism and activation of type I fibers. However, the relationship between muscle composition and fatigue resistance is complex and may vary by task intensity. Endurance times of the elbow flexor and extensor groups did not differ at 40% MVIC, but differed by more than 600% at 10% MVIC, despite similar compositions (6).
Differential excitation between men and women of group III and IV afferents during fatigue may lead to differences in muscle activation and/or endurance time (16). However, in this study neither rate of pain increase or peak pain varied between sexes. This differs somewhat from studies demonstrating sex differences in elicited pain response following isometric exercise (13, 27). Only the rate of exertion was significantly related to endurance time suggesting that nociceptive input was not a primary mechanism explaining the observed localized sex differences. Group III and IV afferents include a wide variety of afferent input, including nociceptive signals in response to changes in metabolite concentration. Thus afferent signals uniquely contributing to perceived exertion may be important.
Several study limitations warrant discussion as they may impact further interpretation. The lack of significant sex-difference in endurance time at the ankle may be a result that our study was underpowered to detect that level of effect size (0.3). Power analysis estimates indicate 178 subjects per group would be needed to detect this small effect size as significant (p ≤ 0.05, β = 0.2). A small effect size would put into question the clinical relevance even if statistical significance was present with a sufficient sample size. A second potential limitation is that no methods such as interpolated twitch were employed to measure the degree of muscle activation during the MVIC, therefore we could not quantify if men and women were able to similarly fully activate their elbow flexor or ankle dorsiflexor muscle groups. Third, we caution against the extension of these results to additional muscle groups or tasks. Future studies are needed to better define whether sex differences in fatigue can be generalized across neighboring joints and/or extremities and to examine whether these muscle-dependent sex differences also occur with position-matching tasks in addition to force-matching tasks.
These results may have implications in rehabilitation and sport, as well as ergonomics. Regardless of whether the goal is to restore function or improve performance, exercise prescription may be erroneously based on inappropriate dose-response relationship assumptions that the body fatigues uniformly between sexes and muscle groups. This information may be most directly applicable to the post-surgical patient where isometric contractions are a frequent intervention. In addition, it may be valuable for the advancement of mathematical fatigue models, used increasingly in ergonomic applications. To this end, accurate models will require more information highlighting the multifaceted influences on fatigue.
In summary, this study demonstrated that sex differences in fatigue resistance are not necessarily uniform and systemic, but can vary by region suggesting strong localized influences. Women were significantly more fatigue-resistant for the elbow flexors but not the ankle dorsiflexors during sustained isometric contractions. Further, peak torque was associated with endurance time at the elbow but not the ankle. Thus, factors which may contribute to fatigue-resistance for one muscle group (e.g. sex, peak torque) may not be critical for another. Future studies are needed to better delineate additional underlying mechanisms that may contribute to this phenomenon.
ACKNOWLEDGMENTS
This study was sponsored in part from funding from the NIH: K12 HD055931 (LFL), 1K01AR056134 (LFL), and NRSA F31 AR056175 (KGA); and the Foundation for Physical Therapy (KGA). The authors have no conflicts of interest to report. We would like to acknowledge Carol Leigh for her assistance with manuscript preparation and Grant Norland with his assistance with data collection. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Disclosure of funding from NIH, etc. This study was sponsored in part from funding from the National Institutes of Health: K12 HD055931 (LFL), 1K01AR056134 (LFL), and NRSA F31 AR056175 (KGA); and the Foundation for Physical Therapy (KGA).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1998. pp. 63–7. [Google Scholar]
- 2.Calder KM, Stashuk DW, McLean L. Physiological characteristics of motor units in the brachioradialis muscle across fatiguing low-level isometric contractions. J Electromyogr Kinesiol. 2008;18(1):2–15. doi: 10.1016/j.jelekin.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrova NA, Arabadzhiev TI, Hogrel JY, Dimitrov GV. Fatigue analysis of interference EMG signals obtained from biceps brachii during isometric voluntary contraction at various force levels. J Electromyogr Kinesiol. 2009;19(2):252–8. doi: 10.1016/j.jelekin.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Dotan R, Falk B. Task-specific sex differences in muscle fatigue: is there a common underlying cause? Exerc Sport Sci Rev. 2010;38(1):36. doi: 10.1097/JES.0b013e3181c5ce0c. [DOI] [PubMed] [Google Scholar]
- 6.Fallentin N, Jorgensen K. Blood pressure response to low level static contractions. Eur J Appl Physiol Occup Physiol. 1992;64(5):455–9. doi: 10.1007/BF00625067. [DOI] [PubMed] [Google Scholar]
- 7.Frey Law LA, Avin KG. Endurance time is joint-specific: A modeling and meta-analysis investigation. Ergonomics. 2010 doi: 10.1080/00140130903389068. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol. 1993;460:549–72. doi: 10.1113/jphysiol.1993.sp019486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamet D, Maton B. The fatigability of two agonistic muscles in human isometric voluntary submaximal contraction: an EMG study. I. Assessment of muscular fatigue by means of surface EMG. Eur J Appl Physiol Occup Physiol. 1989;58(4):361–8. doi: 10.1007/BF00643510. [DOI] [PubMed] [Google Scholar]
- 10.Hansen JW. Effect of dynamic training on the isometric endurance of the elbow flexors. Int Z Angew Physiol. 1967;23(4):367–70. doi: 10.1007/BF00698046. [DOI] [PubMed] [Google Scholar]
- 11.Hatzikotoulas K, Siatras T, Spyropoulou E, Paraschos I, Patikas D. Muscle fatigue and electromyographic changes are not different in women and men matched for strength. Eur J Appl Physiol. 2004;92(3):298–304. doi: 10.1007/s00421-004-1095-4. [DOI] [PubMed] [Google Scholar]
- 12.Hicks AL, Kent-Braun J, Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29(3):109–12. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40(11):1880–9. doi: 10.1249/MSS.0b013e31817eeecc. [DOI] [PubMed] [Google Scholar]
- 14.Houtman CJ, Heerschap A, Zwarts MJ, Stegeman DF. pH heterogeneity in tibial anterior muscle during isometric activity studied by (31)P-NMR spectroscopy. J Appl Physiol. 2001;91(1):191–200. doi: 10.1152/jappl.2001.91.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Houtman CJ, Stegeman DF, Van Dijk JP, Zwarts MJ. Changes in muscle fiber conduction velocity indicate recruitment of distinct motor unit populations. J Appl Physiol. 2003;95(3):1045–54. doi: 10.1152/japplphysiol.00665.2002. [DOI] [PubMed] [Google Scholar]
- 16.Hunter SK. Sex differences and mechanisms of task-specific muscle fatigue. Exerc Sport Sci Rev. 2009;37(3):113–22. doi: 10.1097/JES.0b013e3181aa63e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol. 2006;101(4):1036–44. doi: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hunter SK, Critchlow A, Shin IS, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol. 2004;96(1):195–202. doi: 10.1152/japplphysiol.00893.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol. 2001;91(6):2686–94. doi: 10.1152/jappl.2001.91.6.2686. [DOI] [PubMed] [Google Scholar]
- 20.Hunter SK, Griffith EE, Schlachter KM, Kufahl TD. Sex differences in time to task failure and blood flow for an intermittent isometric fatiguing contraction. Muscle Nerve. 2009;39(1):42–53. doi: 10.1002/mus.21203. [DOI] [PubMed] [Google Scholar]
- 21.Hunter SK, Lepers R, MacGillis CJ, Enoka RM. Activation among the elbow flexor muscles differs when maintaining arm position during a fatiguing contraction. J Appl Physiol. 2003;94(6):2439–47. doi: 10.1152/japplphysiol.01038.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hunter SK, Ryan DL, Ortega JD, Enoka RM. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol. 2002;88(6):3087–96. doi: 10.1152/jn.00232.2002. [DOI] [PubMed] [Google Scholar]
- 23.Hunter SK, Schletty JM, Schlachter KM, Griffith EE, Polichnowski AJ, Ng AV. Active hyperemia and vascular conductance differ between men and women for an isometric fatiguing contraction. J Appl Physiol. 2006;101(1):140–50. doi: 10.1152/japplphysiol.01567.2005. [DOI] [PubMed] [Google Scholar]
- 24.Hunter SK, Yoon T, Farinella J, Griffith EE, Ng AV. Time to task failure and muscle activation vary with load type for a submaximal fatiguing contraction with the lower leg. J Appl Physiol. 2008;105(2):463–72. doi: 10.1152/japplphysiol.90398.2008. [DOI] [PubMed] [Google Scholar]
- 25.Jaworowski A, Porter MM, Holmback AM, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand. 2002;176(3):215–25. doi: 10.1046/j.1365-201X.2002.t01-2-01004.x. [DOI] [PubMed] [Google Scholar]
- 26.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93(5):1813–23. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- 27.Koltyn KF, Trine MR, Stegner AJ, Tobar DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sports Exerc. 2001;33(2):282–90. doi: 10.1097/00005768-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Lindenberger M, Lanne T. Decreased capillary filtration but maintained venous compliance in the lower limb of aging women. Am J Physiol Heart Circ Physiol. 2007;293(6):H3568–74. doi: 10.1152/ajpheart.00725.2007. [DOI] [PubMed] [Google Scholar]
- 29.Lowery M, Nolan P, O’Malley M. Electromyogram median frequency, spectral compression and muscle fibre conduction velocity during sustained sub-maximal contraction of the brachioradialis muscle. J Electromyogr Kinesiol. 2002;12(2):111–8. doi: 10.1016/s1050-6411(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 30.MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol. 1984;57(5):1399–403. doi: 10.1152/jappl.1984.57.5.1399. [DOI] [PubMed] [Google Scholar]
- 31.Melbech S, Johansen SH. Endurance time in slow and fast contracting muscle groups. Work Environ Health. 1973:1062–4. [Google Scholar]
- 32.Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol. 1993;66(3):254–62. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- 33.Ng AV, Dao HT, Miller RG, Gelinas DF, Kent-Braun JA. Blunted pressor and intramuscular metabolic responses to voluntary isometric exercise in multiple sclerosis. J Appl Physiol. 2000;88(3):871–80. doi: 10.1152/jappl.2000.88.3.871. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi J. Effects of contraction level on the changes of surface electromyogram during fatiguing static contractions. Ann Physiol Anthropol. 1993;12(4):229–41. doi: 10.2114/ahs1983.12.229. [DOI] [PubMed] [Google Scholar]
- 35.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol. 2008;104(3):655–64. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- 36.Proctor DN, Newcomer SC. Is there a difference in vascular reactivity of the arms and legs? Med Sci Sports Exerc. 2006;38(10):1819–28. doi: 10.1249/01.mss.0000230340.79247.52. [DOI] [PubMed] [Google Scholar]
- 37.Russ DW, Elliott MA, Vandenborne K, Walter GA, Binder-Macleod SA. Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;282(2):E448–57. doi: 10.1152/ajpendo.00285.2001. [DOI] [PubMed] [Google Scholar]
- 38.Russ DW, Lanza IR, Rothman D, Kent-Braun JA. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve. 2005;32(5):647–55. doi: 10.1002/mus.20396. [DOI] [PubMed] [Google Scholar]
- 39.Shahidi AV, Mathieu PA. Endurance time characteristics of human ankle dorsiflexors and plantarflexors. Eur J Appl Physiol Occup Physiol. 1995;71(2-3):124–30. doi: 10.1007/BF00854968. [DOI] [PubMed] [Google Scholar]
- 40.Thompson BC, Fadia T, Pincivero DM, Scheuermann BW. Forearm blood flow responses to fatiguing isometric contractions in women and men. Am J Physiol Heart Circ Physiol. 2007;293(1):H805–12. doi: 10.1152/ajpheart.01136.2006. [DOI] [PubMed] [Google Scholar]
- 41.Ulmer HV, Knieriemen W, Warlo T, Zech B. Interindividual variability of isometric endurance with regard to the endurance performance limit for static work. Biomed Biochim Acta. 1989;48(5-6):S504–8. [PubMed] [Google Scholar]
- 42.Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol. 2005;98(1):160–7. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- 43.Yassierli, Nussbaum MA, Iridiastadi H, Wojcik LA. The influence of age on isometric endurance and fatigue is muscle dependent: a study of shoulder abduction and torso extension. Ergonomics. 2007;50(1):26–45. doi: 10.1080/00140130600967323. [DOI] [PubMed] [Google Scholar]
- 44.Yoon T, Schlinder Delap B, Griffith EE, Hunter SK. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve. 2007;36(4):515–24. doi: 10.1002/mus.20844. [DOI] [PubMed] [Google Scholar]