Abstract
The Dorsomedial Nucleus of the Hypothalamus (DMH) is known to play important roles in ingestive behavior and body weight homeostasis. The DMH contains neurons expressing Neuropeptide Y (NPY) during specific physiological conditions of hyperphagia and obesity, however, the role of DMH-NPY neurons has yet to be characterized. In contrast to the DMH-NPY neurons, NPY expressing neurons have been best characterized in the Arcuate Nucleus of the Hypothalamus (ARH). The purpose of this study is to characterize the chemical phenotype of DMH-NPY neurons by comparing the gene expression profiles of NPY neurons in the DMH and ARH isolated from postnatal NPY-hrGFP mice by microarray analysis.
Twenty genes were differentially expressed in the DMH-NPY neurons compared to the ARH. Among them, there were several transcriptional factors that play important roles in the regulation of energy balance. DMH-NPY neurons expressed Glutamic Acid Decarboxylase (GAD) 65 and 67, suggesting that they may be GABAergic, similar to ARH-NPY neurons. While ARH-NPY neurons expressed leptin receptor (ObRb) and displayed the activation of STAT3 in response to leptin administration, DMH-NPY neurons showed neither. These findings strongly suggest that DMH-NPY neurons could play a distinct role in the control of energy homeostasis and are differentially regulated from ARH-NPY neurons through afferent inputs and transcriptional regulators.
1. Introduction
The Dorsomedial Nucleus of the Hypothalamus (DMH) is one of several hypothalamic nuclei involved in the control of ingestive behavior and body weight homeostasis (Bellinger and Bernardis, 2002; Bernardis and Bellinger, 1998). A seminal study by Larsson demonstrated that an electrical stimulation of the DMH caused a voracious drive to eat in sheep, suggesting that the primary output of the DMH is an orexigenic drive (Larsson, 1954). In 1970, Bernardis et al. (Bernardis, 1970) supported this conclusion with the demonstration that DMH lesioned rats were hypophagic, hypodipsic, and growth retarded. They have subsequently shown that DMH lesioned rats provide some protection against the development of diet induced obesity (DIO) when fed a high fat diet (Bernardis and Bellinger, 1986). The importance of the DMH in body weight homeostasis is further supported by the finding that the DMH receives major projections from the Arcuate Nucleus of Hypothalamus (ARH), a key hypothalamic site for sensing peripheral signals, and has a major efferent projection to the Paraventricular Nucleus of Hypothalamus (PVH) (Bouret et al., 2004; Cone et al., 2001; Gao and Horvath, 2008; Grove and Smith, 2003; Schwartz et al., 2000; Thompson et al., 1996; Thompson and Swanson, 1998). The DMH has been also shown to play an important role in thermoregulation (Dimicco and Zaretsky, 2007). The electrical and chemical stimulation of the DMH increases brown adipose tissue (BAT) temperature (Freeman and Wellman, 1987; Zaretskaia et al., 2002). The DMH projects to the brainstem, the site of sympathetic neurons that innervate BAT (Cao et al., 2004). Thus, the DMH provides inputs to both neuroendocrine and autonomic circuits involved in the maintenance of energy homeostasis.
While the most characterized population of Neuropeptide Y (NPY) neurons in the hypothalamus is the ARH, the DMH also contains neurons that transiently express NPY during specific physiological states. The role of the DMH-NPY neurons is unknown, but the level of NPY expression in the DMH appears to reflect the changes in energy status (Li et al., 1998a; Li et al., 1998b). NPY expression is high in the DMH during the postnatal period when the animals require high energy intake for the rapid growth (Grove et al., 2001; Grove et al., 2003; Grove and Smith, 2003). However, NPY expression in the DMH gradually declines after the second postnatal week and levels remain low in the normal adult rodents. Interestingly, several studies have shown that a high level of NPY expression is induced in specific regions of the DMH during lactation and in obesities (Guan et al., 1998a; Guan et al., 1998b; Kesterson et al., 1997; Li et al., 1998a; Li et al., 1998b), suggesting that DMH-NPY induction may contribute to the hyperphagic behavior. Our group has shown that a decrease in NPY expression in the DMH is correlated with a decrease in food intake and increase in BAT thermogenesis in lactating rats (Chen et al., 2004). Furthermore, Yang el al. recently reported that AAV mediated knockdown of NPY expression in the DMH ameliorated the hyperphagia and obesity of OLEFT rats (Yang et al., 2009). It is important to note that there are subdivisions of the DMH where NPY expression is differentially regulated, and that there are species differences in the expression of NPY within the DMH. In rats, NPY is constitutively expressed in the compact region of the DMH while NPY expression in the non-compact zone is only induced during chronic hyperphagic conditions (Grove et al., 2001; Li et al., 1998b). In normal adult mice, NPY is only expressed in the non-compact region of the DMH at a low level (unpublished observation). During hyperphagic states such as DIO, NPY is highly upregulated in the non-compact zone compared to the mice fed with normal diet (Guan et al., 1998a). While it is suggested that NPY expression in both subdivisions may play a role in modulating food intake and energy balance, the exact physiological role of DMH-NPY neurons is still unknown.
Unlike the DMH, the anatomy and functions of the NPY system in the ARH in relation to energy homeostasis are well characterized (Cone et al., 2001; Schwartz et al., 2000). The ARH contains two major neuronal populations that play opposite roles in the control of food intake. Neurons co-expressing NPY and agouti-related peptide (AgRP) are known to stimulate feeding, whereas neurons expressing proopiomelanocortin (Pomc) inhibit feeding. NPY and POMC neurons express the long form of the leptin receptor (ObRb) and are regulated by leptin (Cowley et al., 2001; Elias et al., 1999; Elmquist et al., 1999). ARH-NPY neurons also produce the inhibitory neurotransmitter Gamma-Aminobutyric acid(GABA), which plays a significant role in the function of these neurons (Tong et al., 2008).
At the present time, there is no information available about the phenotype of DMH-NPY neurons during the period when NPY expression is high and how these neurons are involved in regulating energy homeostasis. The goal of this study is to characterize the chemical phenotype of DMH-NPY neurons to better understand the role of these neurons during the postnatal period when NPY is maximal. We compared the microarray gene expression profile of purified DMH-NPY neurons to ARH-NPY neurons from postnatal NPY-hrGFP mice to find the different transcriptional factors and receptors that might account for a transient NPY expression in the DMH.
2. Materials and Methods
2.1 Animals
NPY hrGFP mice on a C57BL6 background were purchased from Jackson Laboratory (Bar Harbor, Maine). These mice express humanized renilla reniformis Green Fluorescent Protein (hrGFP) under the control of the mouse NPY promoter. Animals were housed under 12hr of light/12hr of dark cycle per day. They were fed standard chow diet (Purina lab chow #5001) and had free access to water. All animal procedures were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
2.2 Microdissection and cell dissociation of ARH and DMH for FACS (Fluorescent activated cell sorting)
Hemizygous NPY-hrGFP male mice were bred to C57BL/6 female mice (purchased from Jackson laboratory). The litters were normally born and the litter size was adjusted to 10. The pups were toe clipped for identification and genotyping. Postnatal day 16(P16) was chosen since the DMH express high levels of NPY-hrGFP positive cells. At P16, 18 GFP positive mice pups were killed by decapitation and the brains were quickly removed for microdissection in a petri dish containing ice cold Krebs buffer pH 7.4(126mM NaCl, 2.5mM KCl, 1.2mM MgCl2-6H20, 1.2mM NaH2PO4- H20, 2.4mM CaCl2-2 H20, 21.4mM NaHCO3, 11.1mM glucose). Coronal hypothalamic sections (300μm) were cut in a vibrating microtome (Leica VS 1000, Leica Microsystems, Inc., Bannockburn, IL). The sections containing ARH and DMH were identified and dissected under an inverted microscope.
For the microarray, three independent ARH and DMH pools were obtained, with each pool containing the tissue from 6 animals. The tissues were dissociated using the Papain Dissociation System (Worthington Biochemical Corporation, Lakewood, NJ). Briefly, the samples were placed into Papain/DNase for 1hour at 37°C and dissociated by a gentle trituration using a pipette. The NP-GFP+ cells were collected by FACS. The sorting was done on BD Aria II cell sorter (Becton Dickinson, NJ). A 488 nm laser was used to excite the GFP and the emission was measured in the FITC channel (530 nm). Non-fluorescent cells obtained from wild-type mice were used to gate on forward scatter (FSC) versus side scatter (SSC) and set the threshold for fluorescence. We collected approximately 8000 GFP positive cells from the ARH and 10000 cells from the DMH by FACS, for each pooled sample.
2.3 RNA isolation
After FACS sorting, the cells were collected in RNA extraction buffer (Arcturus Bioscience, Mountain View, CA) and stored at -80°C until RNA extraction. RNA was extracted using the PicoPure RNA isolation kit (Arcturus Bioscience) according to the manufacturer's protocol. An average of 11ng of total RNA was isolated from each pooled sample. The RNA quality and relative concentration were confirmed on the Picochip using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA)
2.4 Microarray procedures
Microarray assays were performed in the Affymetrix Microarray Core (Affymetrix, Inc., Santa Clara, Ca), a unit of the OHSU Gene Microarray Shared Resource. Briefly, total RNA was reverse-transcribed with an oligo-dT primer and double stranded cDNA is generated. The cDNA served as a template for the in vitro transcription (IVT) reaction that produced amplified amounts of biotin-labeled antisense mRNA. This biotinylated RNA is referred to as labeled cRNA. The samples were prepared using NuGen Ovation Biotin RNA Amplication and Labeling System_V2 (NuGEN Technologies, Inc. San Carlos, CA). Each sample target was hybridized to a Mouse Genome 430 2.0 GeneChip array. Imaging processing and expression analysis were performed using Affymetrix GCOS v. 1.4.036 software.
2.5 Microarray Analysis
A GCOS absolute expression analysis was performed for each GeneChip genome array hybridization. Following the initial analysis, the absolute analyses were rerun using global scaling to an average target intensity of 350. The scaling allows for the direct comparison of hybridization values from the different targets analyzed in this project (and with any additional GeneChip sample assays that are run using the same array type). For each analysis, scaled or unscaled, the AMC Project Report Ver. 12 (06/27/07) – GCOS parameters α1 and α2 were set to 0.05 and 0.065, respectively. These parameters set the point at which a probe set is called present (P), marginal (M), or undetectable (A). This call is based on the Detection p-value of the probe set as determined by the software.
2.6 Analysis of gene expression
For the microarray data analysis, normalized expression values by their Affymetrix identifier were imported into the online software server Genesifter (Seattle, WA). For the microarray comparisons multiple t-tests were used to identify genes with at least a 2-fold difference in gene expression (with Benjamini and Hockberg correction; p < 0.05) and at least an expression level of 200 and samples from at least one of the groups had to have a 100% present call (3 out of 3) according to Affymetric MAS 5.0.
2.7 RT-PCR
A new set of isolated DMH and ARH-NPY samples were collected for confirming gene expression. The samples were collected and RNA obtained as described above and complementary DNA was synthesized from 80-150ng total RNA using M-MLV Reverse Transcriptase (Fisher Scientific, Pittsburgh, PA). The following primer pairs were designed to amplify Npy: Forward 5′-GCTAGGTAACAAGCGAATGGGG-3′; Reverse 5′-CACATGGAAGGGTCTTCAAGC-3′, AgRP: Forward 5′-GGCCTCAAGAAGACAACTGC-3′; Reverse 5′-TGCGACTACAGAGGTTCGTG-3′, Pomc: Forward 5′-GAAGATGCCGAGATTCTGCT-3′; Reverse 5′-GTACTTCCGGGGGTTTTCAG, Cyclophlin B: Forward 5′-CAAGACTGAGTGGCTGGATGG; Reverse 5′-ACTTGAAGGGGAATGAGGAAAATA-3′, Bahl2: Forward 5′-ACCCATCCACCCACACATAC; Reverse 5′-ATCACCCTCCTCTGCTCTGA, Foxa1: Forward 5′-AAACCGGTTATGCACATTGG; Reverse 5′-GCAAGAACTAAAATGGCCACA, Pgc -1α: Forward 5′-GGAGCCGTGACCACTGACA; Reverse 5′-TGGTTTGCTGCATGGTTCTG. PCR was performed using the platinum PCR supermix (InVitrogen, Carlsbad, CA) according to the manufacturer's instruction.
2.8 In situ hybridization
PCR primers to amplify riboprobes for Pgc-1α and Foxa1 were designed using NIH BLAST. PCR products were ligated in pGEMT vector and amplified using JM109 competent cells. Plasmids were harvested and a sample sequenced to verify identity. Plasmids were linearized using appropriate enzymes. cDNA in which 50% of the UTP is radioactive was 33P labeled. After sectioning in a cryostat, brain sections were fixed in 4% paraformaldehyde and then subjected to a series of washes resulting in dehydration, delipidation, and rehydration. The sections were exposed to the labeled probes overnight in a moist chamber at 55°C. After hybridization, the slides were washed in 4× SSC, then incubated in RNase A at 37°C, and in 0.1 × SSC at 60°C. Slides were then dehydrated through a graded ethanol series and dried. For visualization of the probe, labeled sections were exposed to film (Biomax MR, Kodak) overnight.
2.9 Fluorescent in situ hybridization (FISH)
P15 NPY-hrGFP mice were anesthetized and perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde in phosphate buffer. Brains were post-fixed at 4°C overnight and cryoprotected with 30% sucrose for 48 hours. Tissue was embedded in O.C.T (Tissue Tek) and frozen at -80°C. 10μm-thick coronal sections were collected on Superfrost Plus slides (Fisher). GFP fluorescence is lost during the high temperature in situ hybridization step; thus, we pre-imaged direct GFP fluorescence in conjunction with DRAQ5 nuclear stain (Cell Signaling Technology, Beverly MA). Following FISH, the tissue was re-stained with DRAQ5 and imaged. Using Photoshop, pre- and post-FISH images were aligned using the common DRAQ5 stain as a reference to generate a composite image (Padilla, et al submitted). Frozen sections were processed as described in the TSA Plus Cy3 System manual (Perkin Elmer), using Foxa1 riboprobes labeled with digoxigenin (1:100 dilution) (Roche, Indianapolis, IN). Following the hybridization step, slides were washed and incubated overnight at 4°C with HRP-conjugated anti-digoxigenin (1:500) as described in the Perkin Elmer manual. The subsequent steps were modified as described in (Breininger and Baskin, 2000). Briefly, the slides were washed with TNT buffer for 3 times for 5 minutes each at a room temperature. Biotinyl tyramide diluted 1:50 in the amplification diluent was applied to the slides (renaissance TSA Biotin System; Perkin Elmer NEL 700A). After incubation for 10 min at RT, the slides were washed 3 times in TNT buffer for 5 min each at RT. The biotin deposited after TSA amplification was further amplified by ELF using the ELF 97 mRNA In Situ Hybridization Kit #2 (E-6604 Molecular Probes). Tissue sections were incubated in blocking reagent provided with the ELF kit for 30 minutes in a humidifying chamber. Alkaline phosphatase conjugate (1:50 in block reagent) and DRAQ5 (1:1000) was then applied to the sample for 1 hr. Slides were then washed 3 times, for 5minutes each time, with 1× wash buffer. Substrate working solution was then added to the samples for 10 min. The ELF precipitate deposited as a result of the enzyme activity emits a fluorescent product at 530nm. The post-FISH images of Foxa1 expression were then aligned with the pre-images of NPY-GFP using DRAQ5 stain as a reference to create the composite image of foxa1, NPY-GFP, and DRAQ5 stain.
2.10 Immunohistochemistry
P 15 NPY-hrGFP mice were overdosed with pentobarbital (125 mg/kg body weight, i.p) and perfused with 0.9% ice cold saline solution and then 10% neutral buffered formalin (Fisher Scientific) for FOXA1 antibody staining or 4% paraformaldehyde containing borate buffer (pH 9.5) for PGC-1α antibody. The brains were post-fixed in the same fixatives overnight and transferred to 25% sucrose buffer solution. The brains were then frozen and sectioned at 25 μm on a microtome. The tissue sections were washed in 0.05M potassium phosphate-buffered saline (KPBS) several times and pre-incubated in blocking buffer (KPBS+0.4% triton X + 2% normal donkey serum) for 30 min before incubating in rabbit anti-FOXA 1, 1:400 (sc-22841, Santa Cruz Biotechnology, Santa Cruz, CA) or anti-PGC-1α, 1:24000 (sc-13067, Santa Cruz Biotechnology) containing blocking buffer for 48 hrs at 4°C (Puigserver et al., 1999; Wolf et al., 2007). Following washes in KPBS, tissue sections were incubated for 1 hr in biotinylated donkey anti-rabbit antibody (1:600, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), then subsequently washed and incubated in avidin-biotin (A/B) solution (Vectastain elite ABC, Vector laboratories, Burlingame, CA) for 30 min. The signals were further amplified using tyramide signal amplification-indirect kit (NEN Life Sciences products, Boston, MA). The signals were visualized using Alexa 568 conjugated to streptavidin (Molecular Probes, Eugene, OR)
For p-STAT 3 immunohistochemistry (Enriori et al., 2007), NPY-hrGFP pups were sham injected for 3 days with saline, then on P16 injected with either saline or 3mg/kg leptin (PeproTech, Rocky Hill, NJ) in the morning. They were kept away from the dams on a heating pad for 45 min, then perfused with 4% paraformaldehyde in borate buffer and processed as above. After sectioning, the tissue sections were incubated in 1:250 rabbit anti-p-STAT 3 (Cell Signaling, Danvers, MA) for 60 min at room temperature and then overnight at 4°C. After adding Biotinylated donkey anti- rabbit antibody and A/B solution, tissue sections were incubated with nickle DAB solution (1.25g nickle sulfate, 0.04g DAB, 50 ml of 150 mM Sodium Acetate, 41.5 ul of 3% hydrogen peroxide) for 5-30 min. Following the DAB reaction, the sections were incubated in rabbit anti hrGFP, 1:5000 (Stratagene, La Jolla, CA) overnight and Donkey anti rabbit Alexa 499, 1:200 for visualization.
2.11 Analysis of data
For the analysis of NPY and PGC-1α colocalization, 3 sections per animal from 3 different animals were selected for imaging using a Leica SP5 AOBS confocal microscope. 40 X images of NPY-GFP (488 nm Ar laser) and PGC-1α-immunoreactivity (IR) (561 nm DPSS laser) were taken at 1μm intervals through the ARH and DMH of each section. The image stacks were analyzed using Metamorph software by compiling a maximum projection of 10μm thickness, and manually counting how many total NPY-GFP neurons contained PGC-1α-IR. T-test (p<0.05) was used to determine significant difference between groups.
For the leptin-induced pSTAT3-IR in NPY neurons, 3 sections per animal from 3 different animals were selected for the analysis. Sections were visualized with a 20× objective using a Nikon Eclipse E800 microscope. The area of interest was simultaneously illuminated with fluorescence to visualize the hrGFP and brightfield light to visualize the p-STAT3-IR. By quickly flipping back and forth between the fluorescence and the brightfield illumination, the total number of NPY-GFP neurons as well as the number of NPY neurons containing pSTAT3-IR was counted for each section. T-test (p<0.05) was used to determine significant difference between groups.
3. Results
The location of the cuts used to microdissect the tissue containing ARH and DMH-NPY neurons in the mouse hypothalamus is shown in Figure 1.A. Since the DMH and ARH are in close proximity, the purity of the DMH versus ARH isolations was assessed by performing RT-PCR for the ARH-NPY neuron specific marker AgRP expression (Figure 1.B). As expected, GFP positive cells from ARH samples expressed AgRP mRNA, while GFP positive cells sorted from the DMH did not. Furthermore, the absence of Pomc transcripts in both ARH and DMH-NPY samples confirmed that the FACS samples contain a majority of GFP positive cells. However, Melanin-concentrating hormone (Mch) mRNAs were occasionally detected in the DMH-NPY samples (not shown). MCH is highly expressed in the lateral hypothalamus and the DMH (Bittencourt et al., 1992), indicating there is likely a low level of contamination by non-GFP expressing cells. Double-label immunohistochemistry was used to demonstrate that MCH immunoreactivity was never present in GFP positive neurons or fibers (not shown).
Figure 1. Microdissection of the DMH and ARH from P16-17 NPY-hrGFP mice.
A. Nissl staining of a coronal section. The inserted box shows the location of the DMH and ARH. The right panel illustrates the microdissection technique to obtain isolated DMH and ARH sections from P16-17 NPY-hrGFP mice. B. RT-PCR for Npy, AgRP and Pomc transcript in DMH and ARH-NPY neurons isolated from P16-17 NPY-hrGFP mice. Cyclophilin B was used as an internal control. C. Scatter plot showing DMH-NPY enriched genes (red) and ARH-NPY enriched genes (green) by high stringent analysis. The scale is arbitrary and indicates the level of gene expression in the ARH vs. DMH. The grey dots represent transcripts that are not significantly different between ARH and DMH samples.
3.1. DMH-NPY neuron enriched genes
Using the high stringent analysis (Benjamini and Hockberg corrected p-value over 0.05), 20 DMH-NPY neuron enriched genes and 82 ARH-NPY enriched genes were identified. The scatter plot shows the distribution of these differentially expressed genes and common genes between DMH and ARH-NPY neurons (Figure 1.C). The list of the 20 genes enriched in the DMH is shown in Table 1. Of particular interest were genes involved in transcriptional regulations. BarH-like 2, a mammalian homologue of Drosophila BarH protein is 6.4 fold higher expression in the DMH-NPY than ARH-NPY neurons. During embryonic development, BarH/Barhl is expressed primarily in the central nervous system where it plays an essential role in decisions of cell fate, migration and survival (Reig et al., 2007). Forkhead box A1 (Foxa1, also known as hepatocyte nuclear factor 3 alpha) shows 5 fold higher expression in DMH-NPY neurons. Foxa1 is a member of the Forkhead/winged helix transcription factors and required for the regulation of genes essential for glucose homeostasis in many tissues including pancreas and liver (Kaestner, 2000). Paired-like homeodomain transcription factor 2 (Pitx2) is expressed 4.64 fold higher in the DMH-NPY neurons. Pitx2 is expressed during mouse development in many tissues, including brain and plays a role in modulating the basal and hormonally regulated activity of prolactin (Quentien et al., 2006). Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha (Pgc-1α) expression is higher by 2.14 fold. Pgc1-α is a transcription co-factor involved in adaptive thermogenesis and glucose metabolism (Puigserver and Spiegelman, 2003).
Table 1. DMH-NPY neuron enriched genes: High stringent Analysis.
| Gene Name | Accession number | DMH/ARHRatioa |
|---|---|---|
| Barh like 2 | BB543853 | 6.5 |
| Riken cDNA A430071A18 | BB205989 | 6.0 |
| Forkhead box A1 | NM_008259 | 5.2 |
| Paired-like homeodomain transcription factor Munc 30 | U80011 | 4.6 |
| Acyl-malonyl condensing enzyme 1 | NM_019871 | 2.9 |
| Outer dense fiber of sperm tails 2-like | AK016726 | 2.7 |
| G protein-coupled receptor 177 | BC018381 | 2.6 |
| RIKEN cDNA 5530401A14 | AK017430 | 2.4 |
| Rhabdoid tumor deletion region gene 1 | AK017008 | 2.3 |
| Mdm2, transformed 3T3 cell double minute p53 binding protein | NM_134092 | 2.2 |
| canopy 1 homolog | BB131676 | 2.2 |
| Outer dense fiber of sperm tails 2-like | AK016726 | 2.2 |
| Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | BB745167 | 2.1 |
| Tubulin tyrosine ligase-like family, member 6 | AI429241 | 2.1 |
| Transcribed locus | BB466155 | 2.1 |
| Tubulin tyrosine ligase-like family, member 9 (Ttll9), transcript variant 2 | AK015740 | 2.1 |
| AT-hook transcription factor | BB014626 | 2.0 |
| Homeodomain only protein | BC024546 | 2.0 |
| Transcribed locus | AV118079 | 2.0 |
| Family with sequence similarity 13, member A | BB745929 | 2.0 |
Affymetrix gene chip analysis was performed on DMH-NPY (n=3) and ARH-NPY (n=3) mRNA samples. Each pool contained tissue from 6 animals. Genes that didn't meet the quality cut off for expression level and variability were eliminated from the analysis. Only genes with a greater than 2 fold difference between the DMH and ARH samples were included.
Using a more moderate stringent analysis (raw p-value above 0.01, without the Benjamini and Hockberg correction), an additional 21 DMH-NPY neuron enriched genes were identified (Supplemental Table 1). Of particular interest, signal transducing adaptor family member 2 (Stap2) is the cytosolic protein modulating signal transduction pathways. Stap2 is higher in the DMH-NPY neurons by 3.29 fold and is also known to modulate STAT3 activity (Ikeda et al., 2009). Eph receptor A3, an important player in axon guidance during development, is also higher in the DMH-NPY neurons. CCAAT/enhancer binding protein (C/EBP), alpha (Cebpa) is a transcription factor and is known to bind to the promoter and modulate the expression of leptin (Hwang et al., 1996). The complete list of the genes is found in the supplemental material (Supplemental Table 1).
3.2. ARH-NPY neuron enriched genes
There was a relatively large list of genes that were enriched in the ARH-NPY neurons compared to DMH-NPY neurons (Supplemental Table 2). Most notable was the expression of Activin A receptor, type IC (Acvr1c) which was 32.4 higher in ARH-NPY neurons. Acvr 1c is a Type I Receptor Serine/Threonine Kinase for the TGFβ superfamily and was previously shown to be expressed in the ARH in rat brains (Tsuchida et al., 1996). Acvr 1c is known to be involved in apoptosis and respond to dietary excess, glucose and insulin stimulus (Andersson et al., 2008; Bertolino et al., 2008; Kim et al., 2004). AgRP gene expression, which is exclusively expressed in ARH-NPY neurons (Morton and Schwartz, 2001), was 9.38 fold higher in ARH versus DMH samples.
3.3 RT-PCR confirmation of microarray results
RT-PCR was used to verify the differential expression of Foxa1 and Pgc-1α (Figure 2.A). The DMH-NPY samples clearly showed the expression of Foxa1. However, ARH-NPY samples did not show the expression indicating that the level of the gene in the samples must be below the detection level. Pgc-1α was expressed in both samples, but was qualitatively higher in the DMH versus ARH-NPY neurons.
Figure 2. Verification of microarray analysis.
A. RT-PCR for Foxa1 and Pgc-1α mRNA expression in the DMH and ARH-NPY neurons isolated from P16-17 NPY-hrGFP mice. Cyclophilin B was used an internal control. B. Dark field photomicrograph showing Pgc-1α mRNA localization in the DMH from P16 NPY-hrGFP mouse. C. Fluorescent in situ hybridization for Foxa1 (red) in the DMH (upper) and ARH (lower) of P16 NPY-hrGFP mouse (green). DMHnc: DMH non-compact zone, DMHc: DMH compact zone.
3.4 Localization of PGC-1α and Foxa1 mRNAs and proteins in DMH-NPY neurons
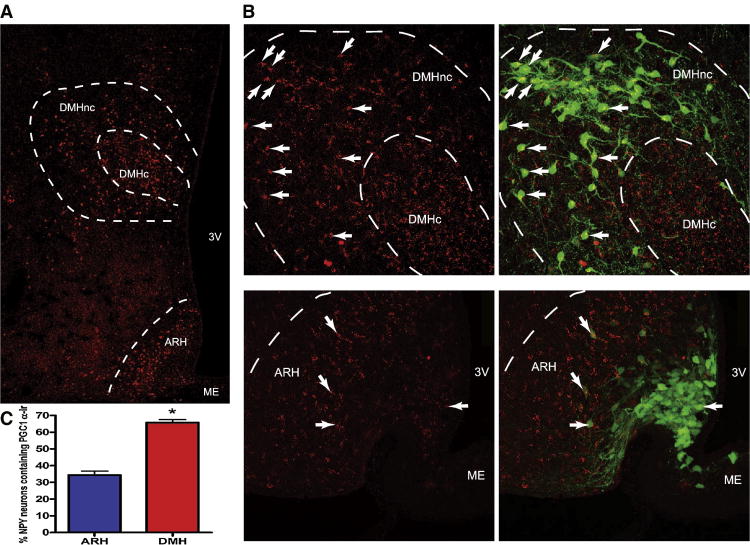
Pgc-1α mRNA expression was localized in the DMH by in situ hybridization using 33P labeled riboprobes in P16 NPY-hrGFP mice (Figure 2.B). Foxa 1 mRNA expression was detected in the ARH and DMH by a fluorescent in situ hybridization (Figure 2.C). In the ARH, Foxa 1 mRNA was highly expressed in the dorsolateral area where NPY expressing neurons are scarce. Foxa 1 mRNA expression was broadly expressed in the DMH, but there was little colocalization between Foxa1 and NPY expressing neurons. PGC-1α and FOXA1 proteins were also localized in the DMH-NPY neurons by immunohistochemistry in P16 NPY-GFP mice. While high levels of PGC-1α immunoreactivity were detected in both the ARH and DMH (Figure 3.A), there was a significantly greater colocalization of PGC-1α in NPY-GFP cells in the DMH (Figure 3. B). Confocal analysis showed that 65% of total DMH-NPY neurons contained PGC-1α immunoreactivity compared to 35% in the ARH (Figure 3. C). Similarly, FOXA1 immunoreactivity was detected in both the ARH and DMH (Figure 4. A). However, only a small population of GFP positive neurons in the DMH were co-localized with FOXA1 (Figure 4.B), with little colocalization in the ARH. FOXA1 immunoreactivity was highly concentrated in the dorsolateral area of ARH, recapitulating the mRNA distribution by in situ hybridization.
Figure 3. Immunohistochemistry for PGC-1α in P16 NPY-hrGFP mouse.
A. 4× objective images of PGC-1α staining in the DMH and ARH. B. Upper panels show 20× confocal images of PGC-1α (red) and overlay image with NPY-GFP (green) in the DMH. Lower panels show 20× confocal images of PGC-1α (red) and overlay image with NPY-GFP (green) in the ARH. The arrows indicate co-localization. C. The statistical analyses showed that 65% of DMH-NPY neurons were co-localized with PGC-1α while only 35% of ARH-NPY neurons were co-localized. (n=3 for each group, P< 0.05 by t-test) DMHnc: DMH non-compact zone, DMHc: DMH compact zone, ME: median eminence.
Figure 4. Immunohistochemistry for FOXA 1 in P16 NPY-hrGFP mouse.
A. 4× objective images of FOXA1 staining in the DMH and ARH. B. Upper panels show 20× confocal images of FOXA1 (red) and overlay image with NPY-GFP (green) in the DMH. Lower panels show 20× confocal images of FOXA1 (red) and overlay with NPY-GFP (green) in the ARH. The arrows indicate co-localization. DMHnc: DMH non-compact zone, DMHc: DMH compact zone, ME: median eminence.
3.5 ObRb expression in the DMH vs. ARH-NPY neurons
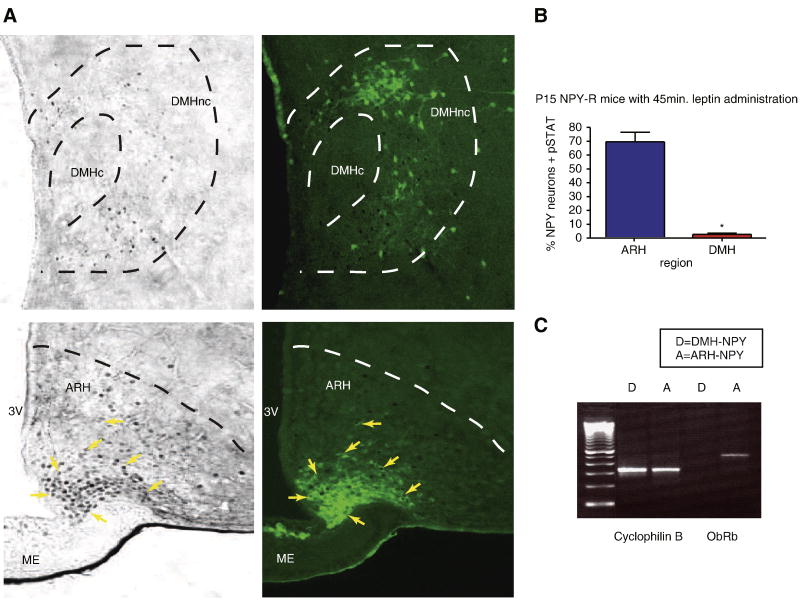
Leptin is known to signal through ObRb to regulate gene transcription. It has been already shown that ObRb mRNA is expressed in the ARH-NPY neurons (Hakansson et al., 1996). While the initial Affymetrix chip analysis did not find a differential expression between the ARH and DMH-NPY neurons, the probe sets on these chips do not distinguish the different isoforms of the leptin receptor. Therefore, we used RT-PCR to compare ObRb expression in DMH and ARH-NPY neurons (Figure 5.C). Using this technique, ObRb was readily detected in ARH-NPY neurons but was not detected in DMH-NPY neurons. To compare leptin responsiveness in the two populations of NPY neurons, we injected P15 NPY-hrGFP mice with 3mg/kg leptin and observed p-STAT3 immuno-reactivity in the ARH and DMH (Figure 5.A). While 70% of NPY positive cells expressed p-STAT3-IR in the ARH, there was only 3-4% of NPY-GFP positive cells in the DMH were p-STAT3 positive (Figure 5.B). These results suggest that leptin may not directly regulate DMH-NPY neurons at P15.
Figure 5. Leptin signaling in DMH and ARH-NPY neurons.
A. p-STAT3 activation in the DMH (upper panels) and ARH (lower panels) 45min after i.p. 3mg/kg leptin administration in P16 NPY-hrGFP mice. NPY neurons are shown as green and p-STAT3-IR are black nuclear staining. The arrows indicate NPY neurons containing p-STAT3 activation. B. The bar graph shows that only 3-4% NPY positive neurons in the DMH contained p-Stat activation compared to near 70% ARH-NPY neurons (n=3 for each group, p=0.0007 by unpaired t-test). C. RT-PCR for ObRb mRNA expression in the DMH and ARH-NPY neurons isolated from P16-17 NPY-hrGFP mice.
3.6 GAD 65 and 67 expression in the DMH vs. ARH-NPY neurons
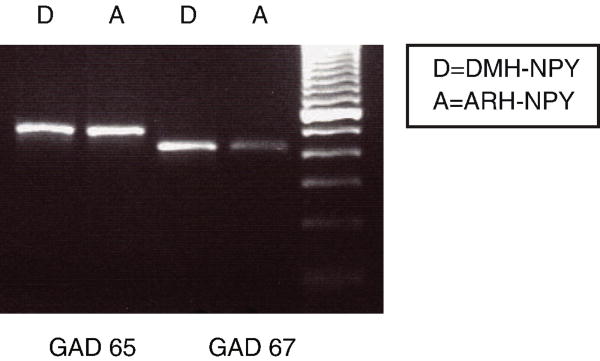
There is an abundance of data showing that ARH-NPY neurons are GABAergic. The microarray data indicated that DMH-NPY neurons express GABA synthesizing enzymes, Glutamic acid decarboxylase (GAD) 65 and 67, at a lower level than ARH-NPY neurons. However, the RT-PCR data showed that DMH-NPY neurons express detectable levels of Gad 65 or 67, suggesting that they may also be GABAergic (Figure 6).
Figure 6. Gad 65 and 67 mRNA expressions in the DMH and ARH-NPY neurons.
DMH and ARH-NPY samples were isolated from P16-17 NPY-hrGFP mice.
Discussion
In this study, the microarray analysis has identified genes that are differentially expressed in DMH-NPY neurons compared to ARH-NPY neurons during the second postnatal week in mice. The role of ARH-NPY neurons in the regulation of energy balance and the phenotype of these neurons has been well characterized. The NPY expression in the ARH is evident from the late gestational period throughout life, with relatively modest changes occurring in response to metabolic changes such as fasting, diet induced obesity, and lactation (Grove et al., 2001; Guan et al., 1998a; Li et al., 1998a; Li et al., 1998b). Unlike the ARH, NPY is transiently expressed in the DMH only during specific physiological conditions. Strong NPY expression in the DMH is observed in hyperphagic animal models such as neonates, lactating dams, and obesity. Recent studies demonstrated that the reduction of DMH-NPY expression caused a significant decrease in food intake and body weight, suggesting a role for DMH-NPY neurons as a major orexigenic signal in chronic hyperphagic conditions (Chen et al., 2004; Yang et al., 2009). Therefore, it is reasonable to speculate that DMH-NPY neurons have a district role and are differentially regulated from ARH-NPY neurons during these conditions. The major goal of this study was to identify genes expressed in DMH-NPY neurons that may explain the role and mechanism of transient NPY expression during the postnatal period.
The analysis of gene expression by microarray and PCR showed that Pgc-1α and Foxa1 mRNA were expressed at higher levels in DMH-NPY neurons in comparison to ARH-NPY neurons. In agreement with the mRNA data, PGC-1α protein expression was observed in the DMH and was colocalized in a large percentage of DMH-NPY neurons. In contrast to the PCR data, Foxa 1 mRNA expression was not localized in the NPY neurons in the DMH using the fluorescent in situ hybridization. This discrepancy might be due to the low sensitivity of FISH assay. However, it is recognized that PCR represents a highly amplified product of the gene. FOXA 1 immuno-reactivity was detected in a small number of DMH-NPY neurons, supporting the microarray and PCR results. Evidence suggests that Foxa 1 is important for food intake since Foxa1 null mutants exhibit progressive starvation, persistent hypoglycemia, hypotriglyceridemia and neonatal mortality between days 2 and 14 (Shih et al., 1999). Moreover, it is possible that Foxa1 might be involved in the regulation of NPY since NPY expression is reduced in the pancreas and gut of Foxa1 -/- mice. However, the expression of NPY in the brain was normal in the hypothalamus in this study. According to our results, it is unlikely that Foxa 1 is a critical differentiated regulator between ARH and DMH-NPY neurons because of the low amount of colocalization.
PGC-1α is a transcription cofactor that interacts with numerous transcription factors that are involved in adaptive thermogenesis and glucose metabolism (Liang and Ward, 2006). Null mutation for PGC-1α resulted in resistance to diet-induced obesity and reduced thermogenic capacity (Lin et al., 2004). These mice had an abnormal morphology in BAT and brain, and became profoundly hyperactive. Another study has also revealed that PGC-1α null mutation eventually leads to abnormal weight control, one of many metabolic disorders caused by the mutation (Leone et al., 2005). Pgc-1α mRNA expression is high during development and is mainly localized in GABAergic neurons throughout the brain (Cowell et al., 2007). This is interesting because DMH-NPY expression also reaches the peak at P14 (Grove and Smith, 2003) and the present study revealed that GABAergic makers are present in DMH-NPY neurons. This high expression of PGC-1α could potentially play an important role in gene transcription for the increased mitochondrial activity and synaptogenesis during this time of substantial metabolic changes. In fact, there are several binding sites for transcription factors that are associated with PGC-1α in the promoter region of the NPY gene. NPY gene transcription in the ARH is regulated by FOXO1 which is known to interact with PGC-1α (Puigserver et al., 2003). However, it is not known whether FOXO1 action on Npy gene transcription is dependent on PGC1-α in the ARH. The present study showed that PGC-1α expression is significantly higher in the DMH-NPY than ARH-NPY neurons. PGC1-α might possibly interact with another transcription factor to regulate Npy gene transcription in the DMH. While both PGC-1α and FOXA 1 are clearly associated with energy homeostasis, it remains to be determined if they are involved in the activation of DMH-NPY neurons in different models of hyperphagia and obesity.
The afferent factors regulating ARH-NPY neurons have been well characterized. It has been shown that the ARH-NPY neurons express the signaling form of leptin receptor, ObRb, and therefore they are under the direct regulation of leptin (Hakansson et al., 1996). ARH-NPY neurons are hyperpolarized by leptin treatment and Npy gene expression is decreased as leptin levels increase (Cowley et al., 2001). Therefore, leptin promotes anorexigenic behavior by inhibiting the orexigenic drive by NPY. The DMH is another site of leptin action in the hypothalamus as evidenced by abundant ObRb mRNA expression and p-STAT3-IR after leptin treatment (Scott et al., 2009).
Our results showed that ObRb mRNA expression was not detected in DMH-NPY neurons and only a small population of DMH-NPY neurons was p-STAT3-IR in response to leptin treatment. Several studies have also reported that ObRb mRNA expression is concentrated in the ventral DMH in both rats and mice (Bi et al., 2003; Caron et al.,; Elmquist et al., 1998b) while NPY is mainly expressed in the compact and dorsal area of the DMH. Chronic food restriction results in NPY upregulation both in the ARH and DMH (the compact zone) in rats (Bi et al., 2003). However, food restricted obese (cp/cp) corpulent rats with a null mutation of the leptin receptor (Williams et al., 1992) showed an altered NPY expression in the ARH, but did not affect DMH-NPY level. This strongly suggests that DMH-NPY neurons may not be under the direct regulation of leptin. This finding was also surprising because DMH-NPY neurons project to the PVH and are believed to play a role in modulating neuroendocrine and autonomic output from the PVH in lactating rats (Li et al., 1998a). In fact, many of the DMH neurons projecting to the PVH are leptin targets. Intravenous leptin treatment activated c-Fos staining in many neurons in the DMH that project to the PVH (Elmquist et al., 1998a). Our results strongly suggest that DMH-NPY neurons may be involved in modulating energy balance via different hormonal or neural inputs. However, it is possible that leptin can influence DMH-NPY neurons indirectly through other leptin responding neurons in the DMH. The role of GABA in regard to energy homeostasis has been overlooked due to the abundant expression of these fast acting neurotransmitters in the brain. However, a recent study showed that mice became resistant to DIO when GABA release is inhibited by disruption of vesicular GABA transporter (VGAT) in NPY/AgRP neurons (Tong et al., 2008). This effect is presumably due to the loss of inhibitory input to the local POMC cells, thus enhancing the neural activity. Another recent study demonstrated that GABA inputs from AgRP neurons to the Parabrachial nucleus (PBN) is also crucial for maintaining feeding (Wu et al., 2009). Ablation of AgRP neurons in adult mice led to starvation, and injection of GABAA receptor agonist into the PBN provided a protection against the starvation phenotype. Therefore it is clear that GABA release from ARH-NPY/AgRP neurons is important for energy balance.
The DMH has been reported to contain GABAergic cell groups, as indicated by widespread Gad 65 and 67 mRNA expression in this area (Okamura et al., 1990). The highest density of Gad 65 and 67 mRNA containing neurons was concentrated in the dorsal and ventral areas of the DMH. Some GABAergic input to the PVH originates from the DMH and may be involved in neuroendocrine and autonomic regulation (Boudaba et al., 1996). Our results showed that DMH-NPY neurons derived from the non-compact zone express GABAergic markers, Gad 65 and 67. Therefore, these cell groups could potentially innervate the PVH and release GABA to participate in the regulation of energy balance. However, further studies are required to characterize the targets of these GABAergic neurons and the role that they play in energy homeostasis.
In conclusion, we have identified DMH-NPY enriched genes during postnatal development in mice, when NPY expression is maximal in the DMH. It should be noted that the expression pattern of these genes may be specific to the postnatal period. It remains to be determined if these differentiated genes are expressed in DMH-NPY neurons in other chronic hyperphagic models such as lactation and DIO. This study also suggests that DMH-NPY neurons may share some properties with ARH-NPY neurons, but could be differentially regulated through other hormonal or neuronal inputs.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson O, Korach-Andre M, Reissmann E, Ibanez CF, Bertolino P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci U S A. 2008;105:7252–6. doi: 10.1073/pnas.0800272105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–42. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bernardis LL. Participation of the dorsomedial hypothalamic nucleus in the “feeding center” and water intake circuitry of the weanling rat. J Neurovisc Relat. 1970;31:387–98. doi: 10.1007/BF02312740. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. Effect of palatable diet on growth, caloric intake and endocrine-metabolic profile in weanling rats with dorsomedial hypothalamic lesions. Appetite. 1986;7:219–30. doi: 10.1016/s0195-6663(86)80027-7. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc Soc Exp Biol Med. 1998;218:284–306. doi: 10.3181/00379727-218-44296. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Holmberg R, Reissmann E, Andersson O, Berggren PO, Ibanez CF. Activin B receptor ALK7 is a negative regulator of pancreatic beta-cell function. Proc Natl Acad Sci U S A. 2008;105:7246–51. doi: 10.1073/pnas.0801285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–6. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–60. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breininger JF, Baskin DG. Fluorescence in situ hybridization of scarce leptin receptor mRNA using the enzyme-labeled fluorescent substrate method and tyramide signal amplification. J Histochem Cytochem. 2000;48:1593–99. doi: 10.1177/002215540004801202. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–40. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 518:459–76. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- Chen P, Williams SM, Grove KL, Smith MS. Melanocortin 4 receptor-mediated hyperphagia and activation of neuropeptide Y expression in the dorsomedial hypothalamus during lactation. J Neurosci. 2004;24:5091–100. doi: 10.1523/JNEUROSCI.0588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 5:S63–7. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Blake KR, Russell JW. Localization of the transcriptional coactivator PGC-1alpha to GABAergic neurons during maturation of the rat brain. J Comp Neurol. 2007;502:1–18. doi: 10.1002/cne.21211. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci U S A. 1998a;95:741–6. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998b;395:535–47. [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–94. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Freeman PH, Wellman PJ. Brown adipose tissue thermogenesis induced by low level electrical stimulation of hypothalamus in rats. Brain Res Bull. 1987;18:7–11. doi: 10.1016/0361-9230(87)90026-8. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582:132–41. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, Brogan RS, Smith MS. Novel expression of neuropeptide Y (NPY) mRNA in hypothalamic regions during development: region-specific effects of maternal deprivation on NPY and Agouti-related protein mRNA. Endocrinology. 2001;142:4771–6. doi: 10.1210/endo.142.11.8498. [DOI] [PubMed] [Google Scholar]
- Grove KL, Allen S, Grayson BE, Smith MS. Postnatal development of the hypothalamic neuropeptide Y system. Neuroscience. 2003;116:393–406. doi: 10.1016/s0306-4522(02)00668-1. [DOI] [PubMed] [Google Scholar]
- Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998a;9:3415–9. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res. 1998b;59:273–9. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Hakansson ML, Hulting AL, Meister B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus--relationship with NPY neurones. Neuroreport. 1996;7:3087–92. doi: 10.1097/00001756-199611250-00059. [DOI] [PubMed] [Google Scholar]
- Hwang CS, Mandrup S, MacDougald OA, Geiman DE, Lane MD. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein alpha. Proc Natl Acad Sci U S A. 1996;93:873–7. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda O, Miyasaka Y, Sekine Y, Mizushima A, Muromoto R, Nanbo A, Yoshimura A, Matsuda T. STAP-2 is phosphorylated at tyrosine-250 by Brk and modulates Brk-mediated STAT3 activation. Biochem Biophys Res Commun. 2009;384:71–5. doi: 10.1016/j.bbrc.2009.04.076. [DOI] [PubMed] [Google Scholar]
- Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab. 2000;11:281–5. doi: 10.1016/s1043-2760(00)00271-x. [DOI] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11:630–7. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- Kim BC, van Gelder H, Kim TA, Lee HJ, Baik KG, Chun HH, Lee DA, Choi KS, Kim SJ. Activin receptor-like kinase-7 induces apoptosis through activation of MAPKs in a Smad3-dependent mechanism in hepatoma cells. J Biol Chem. 2004;279:28458–65. doi: 10.1074/jbc.M313277200. [DOI] [PubMed] [Google Scholar]
- Larsson S. On the hypothalamic organisation of the nervous mechanism regulating food intake. Acta Physiol Scand Suppl. 1954;32:7–63. [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Neuropeptide Y (NPY) neurons in the arcuate nucleus (ARH) and dorsomedial nucleus (DMH), areas activated during lactation, project to the paraventricular nucleus of the hypothalamus (PVH) Regul Pept. 1998a:75–76. 93–100. doi: 10.1016/s0167-0115(98)00057-3. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology. 1998b;139:1645–52. doi: 10.1210/endo.139.4.5905. [DOI] [PubMed] [Google Scholar]
- Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–51. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–35. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW. The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 5:S56–62. doi: 10.1038/sj.ijo.0801915. [DOI] [PubMed] [Google Scholar]
- Okamura H, Abitbol M, Julien JF, Dumas S, Berod A, Geffard M, Kitahama K, Bobillier P, Mallet J, Wiklund L. Neurons containing messenger RNA encoding glutamate decarboxylase in rat hypothalamus demonstrated by in situ hybridization, with special emphasis on cell groups in medial preoptic area, anterior hypothalamic area and dorsomedial hypothalamic nucleus. Neuroscience. 1990;39:675–99. doi: 10.1016/0306-4522(90)90252-y. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–71. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–5. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Quentien MH, Barlier A, Franc JL, Pellegrini I, Brue T, Enjalbert A. Pituitary transcription factors: from congenital deficiencies to gene therapy. J Neuroendocrinol. 2006;18:633–42. doi: 10.1111/j.1365-2826.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- Reig G, Cabrejos ME, Concha ML. Functions of BarH transcription factors during embryonic development. Dev Biol. 2007;302:367–75. doi: 10.1016/j.ydbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DQ, Navas MA, Kuwajima S, Duncan SA, Stoffel M. Impaired glucose homeostasis and neonatal mortality in hepatocyte nuclear factor 3alpha-deficient mice. Proc Natl Acad Sci U S A. 1999;96:10152–7. doi: 10.1073/pnas.96.18.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–73. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008 doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K, Sawchenko PE, Nishikawa S, Vale WW. Molecular cloning of a novel type I receptor serine/threonine kinase for the TGF beta superfamily from rat brain. Mol Cell Neurosci. 1996;7:467–78. doi: 10.1006/mcne.1996.0034. [DOI] [PubMed] [Google Scholar]
- Williams G, Shellard L, Lewis DE, McKibbin PE, McCarthy HD, Koeslag DG, Russell JC. Hypothalamic neuropeptide Y disturbances in the obese (cp/cp) JCR:LA corpulent rat. Peptides. 1992;13:537–40. doi: 10.1016/0196-9781(92)90085-h. [DOI] [PubMed] [Google Scholar]
- Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP. FOXA1: Growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer. 2007;120:1013–22. doi: 10.1002/ijc.22389. [DOI] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–34. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–90. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–25. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.