Abstract
N9741 is a clinical trial in patients with metastatic colorectal cancer that was originally written in 1997 and completed patient accrual in 2004. One thousand seven hundred thirty-one patients were enrolled in the study. During the conduct of the trial, N9741 was repeatedly modified to adapt to toxicity findings, to add evaluation of oxaliplatin to what was originally a trial examining various schedules of irinotecan-based therapy, and to ask evolving questions. The trial led to a new U.S. Food and Drug Administration indication for 5-fluorouracil, leucovorin, and oxaliplatin as indicated for the treatment of previously untreated patients with metastatic colorectal cancer and helped to change the standard of care for the disease in the U.S. and worldwide. The data from the trial have been used to study multiple regimens, pharmacogenetics, and quality of life issues, to correlate plasma protein levels with outcomes, to inform trial methodology, and to perform economic analyses. To date nearly 30 papers and an even larger number of abstracts have been based upon data arising from the study. The history of the trial and the major findings are summarized in this review.
Keywords: Colorectal cancer meta-analysis, Clinical trial phase III, Pharmacogenetics
INTRODUCTION
The N9741 trial led by the North Central Cancer Treatment Group (NCCTG) enrolled patients with metastatic colorectal cancer (MCRC) on combination chemotherapy regimens in the late 1990s through 2003. The trial opened at an opportune time in the management of MCRC, when, after 50 years using the single active agent 5-fluorouracil (5-FU), two new cytotoxic agents, irinotecan and oxaliplatin, proved to be active in MCRC. Data derived from N9741 led to published observations into treatment, trial methodology, pooled analyses, and pharmacogenetics. Additional analyses are planned. This review catalogues the trial’s evolution and summarizes observations published by a variety of investigators to date. We will also attempt to glean some lessons from the study’s evolution in the hope that other investigator teams can use this as a case study to drive the productivity of their current and future studies.
This phase III study was a partnership among the enrolled patients; the National Cancer Institute (NCI) of the U.S. and the National Cancer Institute of Canada (NCIC); NCI-sponsored cooperative groups, industry, and investigators at academic centers, community clinical oncology programs, and private practices. It was approved and monitored through the local institutional review board of each institution where the study was open for enrollment according to institutional policy. The patients completed periodic quality of life (QOL) assessments using validated tools during the course of their therapy. Because of the willingness of investigators from around the globe to share data, various clinical scientists have pooled N9741 data with those from other studies for meta-analyses and to provide insights into and refine clinical trial methodology. Over 500 of the >1,700 study patients permitted the banking of germline DNA and plasma samples. Consequently, this is one of the largest populations with cancer available to date for pharmacogenetic studies and for the study of other biomarkers. Tumor tissue was not collected because of the paucity of tissue available in the metastatic disease setting.
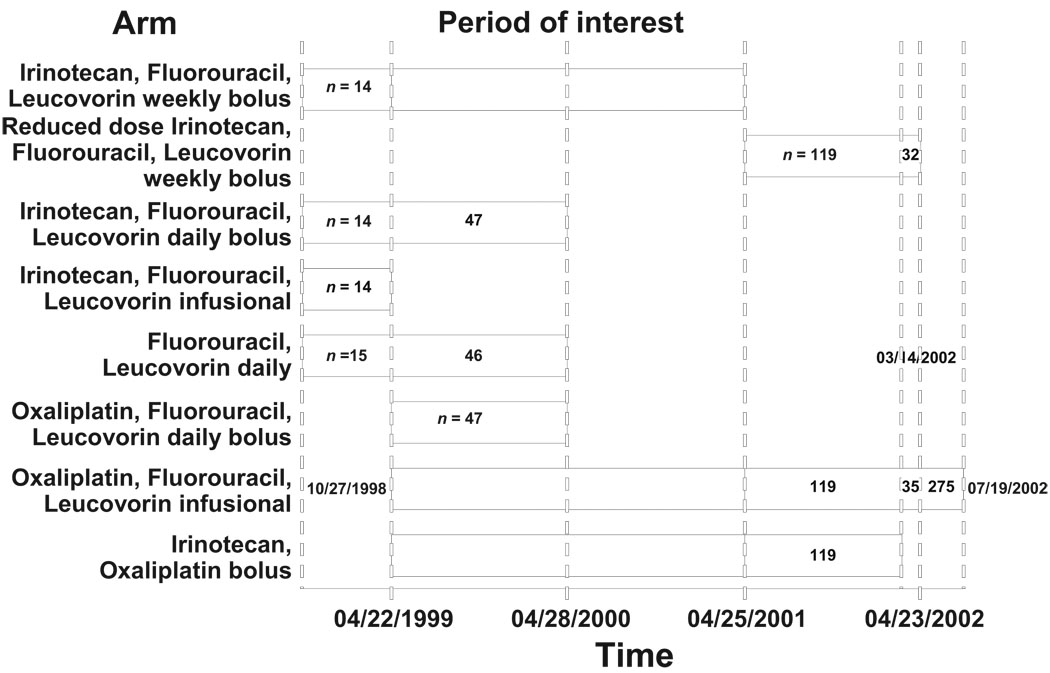
Initiated in 1997, the trial so far has spawned 26 published or in press papers and 39 abstracts (some of the abstracts presented similar data at different meetings). For nine consecutive years, 1999–2007, a variety of investigators, ranging from fellows to senior investigators, clinicians to health services researchers, statisticians to basic scientists, stood at podiums at the annual meeting of the American Society of Clinical Oncology (ASCO) to present on one or more aspects of the trial. Additional poster presentations occurred at ASCO, and poster and podium presentations based on the study continue to be heard at meetings across the world. In some ways, the study has taken on its own life and this paper is its biography. Figure 1 presents a schematic of the trial through its accrual period, as described in the next section.
Figure 1.
Timeline for N9741 trial.
HISTORY
In the fall of 1997, representatives from the NCI, NCI-sponsored cooperative groups, and the pharmaceutical industry met in Crystal City, Virginia, to brainstorm about the design of a phase III trial in MCRC incorporating the new agent irinotecan in the first-line setting. The prospect of developing a trial focused at optimizing the incorporation of the first new agent to show activity in colorectal cancer beyond 5-FU generated considerable excitement and some controversy. After discussion of a variety of approaches, there was a consensus reached to launch a four-arm trial comparing the then standard of care Mayo Clinic 5-FU and leucovorin (LV) control arm with three approaches for delivering irinotecan and 5-FU. These three programs administered irinotecan with bolus weekly 5-FU (IFL), with bolus 5-FU given for five consecutive days based on the Mayo Clinic regimen [1], and with a 24-hour 5-FU infusion [Arbeitsgemeinschaft Internische Oncologie (AIO) regimen]. After discussions with the U.S. Food and Drug Administration (FDA) about its potential to serve as a licensing study, this intergroup study opened to accrual in October 1998, led by the NCCTG and cosponsored by other major cooperative groups including the Cancer and Leukemia Group B (CALGB), Eastern Cooperative Oncology Group (ECOG), and Southwest Oncology Group. The NCIC joined the study later. Upon enrollment, and periodically while they received treatment on the study, patients completed QOL assessments, including the validated Symptom Distress Scale (SDS) and the Uniscale. Initially, there was no provision for blood collection, but that was added while the study was in progress.
In 1998 de Gramont et al. [2] and Giacchetti et al. [3] reported preliminary results with response rates of ~50% when oxaliplatin was combined with infused 5-FU and LV (FOLFOX4) in two separate trials. The N9741 study team and representatives from the NCI discussed these findings and decided that amending the trial to incorporate arms testing oxaliplatin was the most practical and expedient way to rapidly get oxaliplatin into a phase III trial in the U.S. The study reopened in 1999 with six arms. The IFL, Mayo Clinic study combination of irinotecan and 5-FU, and 5-FU–LV control arm remained. Three arms were added, including FOLFOX4 and irinotecan plus oxaliplatin (IROX). An additional arm used the five consecutive day bolus 5-FU–LV regimen combined with oxaliplatin based on a phase I study [4].
REAL-TIME TOXICITY MONITORING
A real success of this trial was the implementation of a novel, real-time toxicity monitoring scheme. This scheme was designed because of the relatively modest experience with the bolus combination regimens and a desire on the part of the study team to monitor toxicity as it was occurring in patients undergoing treatment at widely dispersed study sites. This simple plan required investigators to fax a one-page form to the NCCTG operations office within 24 hours of any event resulting in grade 4 or 5 toxicity or a patient hospitalization [5, 6]. These data were entered into the study’s computerized database immediately upon receipt and generated nightly e-mails to the study chair and lead statistician with cumulative summaries of the toxicity observed to date by arm.
In addition to ongoing monitoring of grade ≥4 toxicity, we also included an assessment of 60-day all-cause mortality as part of the real-time toxicity monitoring scheme. We chose the metric of 60-day all-cause mortality as a surveillance tool to monitor early deaths potentially resulting from toxicity because the eligibility criteria mandated that patients have an ECOG performance status (PS) score ≤2 and most patients had a PS score of 0 or 1. This metric eliminated the subjective issue of investigator attribution for defining the relationship of these events to the chemotherapy treatment and allowed us to monitor early deaths potentially resulting from toxicity.
The real-time toxicity monitoring system provided the study team with some initial signals requiring intervention in the study under the direction of the NCCTG Data Safety Monitoring Board (DSMB). We noted that the regimens coupling oxaliplatin or irinotecan to the five consecutive day bolus 5-FU regimens had higher 60-day all-cause death rates— 8.5% and 8.2%, respectively—as well as augmented severe but nonlethal toxicity. This early death rate compared with much lower rates in the other regimens, of around 2%, during the early stages of the six-arm study [7, 8]. As a result, those two regimens were quickly dropped from the trial.
IFL TOXICITY IN A THREE-ARM TRIAL
At the same time as these toxicity data came to light, IFL had emerged as the U.S. standard of care based on publication of a study sponsored by Pharmacia comparing it with the Mayo Clinic 5-FU–LV regimen [9]. Consequently, the IFL regimen replaced the Mayo regimen as the control arm of the study and the Mayo Clinic 5-FU–LV regimen was dropped from the trial. The study reopened on April 28, 2000, as a three-arm trial comparing IFL, FOLFOX, and IROX. That segment of the study was intended to accrue a total of 1,125 patients.
As accrual to the three-arm trial was continuing, through continuous monitoring, the study team noted that the then current standard of care regimen, the IFL arm, was associated with a higher severe (grade ≥3) toxicity rate and a 60-day all-cause mortality rate of 4.5%, compared with a 1.8% early death rate for the other two study arms. Because of this finding and its implications for patients receiving standard care outside the realm of clinical trials with IFL, the NCI convened a panel of experts who were uninvolved with the conduct of the trial to review the early findings. This team, led by Mace Rothenberg, discerned that, in the first cycle of therapy, a small percentage of patients developed both neutropenia and gastrointestinal tract toxicity manifested by diarrhea leading to grade 4 or 5 toxicity events [8]. They warned the oncology community of the need for vigilant monitoring, aggressive supportive care, and dose reductions in subsequent cycles of therapy.
These data were subsequently brought to the attention of the CALGB Data Safety Monitoring Committee, which was overseeing a phase III trial of IFL versus the Mayo Clinic 5-FU–LV regimen in the adjuvant setting (C89803) [10]. The IFL regimen in that study was also confirmed to have a higher early death rate than was observed with the 5-FU–LV control regimen.
At that juncture, the study team decided to explore the possibility that a reduced dose of irinotecan and 5-FU might lead to better outcomes because most of the severe toxicity in the IFL arm seemed to occur early in the first course of treatment. Pharmacogenetic studies from N9741 would later provide some insight into this phenomenon. We observed that the dose modification strategy written in to the original study worked. That is, once the standardized doses specified in the protocol were individualized based upon the patient’s first-course toxicity, those individuals tolerated their subsequent treatment much better. The study was therefore modified by reducing the doses of irinotecan and 5-FU in the IFL arm—the dose of the irinotecan was decreased from 125 mg/m2 to 100 mg/m2 and the bolus 5-FU dose was reduced from 500 mg/m2 to 400 mg/m2 when therapy was initiated, resulting in the reduced-dose IFL (rIFL) regimen [11].
The trial continued at this point as a three-arm trial testing rIFL, FOLFOX, and IROX. After an additional accrual of approximately 100 patients per arm, further accrual to the IROX arm was terminated per protocol design. Subsequently, on April 23, 2002, the NCCTG DSMB met to review a planned interim analysis. At that time, the DSMB released the data to the study team because the progression-free survival (PFS) advantage for FOLFOX surpassed the early stopping rules that were specified at the time of the protocol’s design. The results indicated median PFS times of 6.9 months, 8.7 months, and 6.5 months for the IFL, FOLFOX (p = .0014 versus IFL), and IROX arms, respectively. The results for the comparative response rates were also consistent—IFL, 31%; FOLFOX, 45%; and IROX, 34%. When median overall survival (OS) data were reported later, the results were confirmatory: IFL, 14.8 months; FOLFOX, 19.5 months (p = .0001 versus IFL); and IROX, 17.4 months [12].
ADDRESSING CRITICISMS
As the N9741 primary manuscript underwent peer review, this phase of the trial faced two criticisms. First, the FOLFOX arm contained 5-FU infusion whereas IFL employed bolus 5-FU. This raised the question of whether the outcome of the trial would have been different had we employed an optimized irinotecan-containing regimen based around a 5-FU infusion, such as that used in the 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) regimen. That almost certainly would have been the case, because Christophe Tournigand and colleagues published a trial in the Journal of Clinical Oncology in the same issue as the original N9741 report showing that FOLFOX and FOLFIRI have comparable activity [13]. Although the Tournigand trial was underpowered for an equivalence comparison, the OS time in a number of subsequent studies with the FOLFIRI regimen has been very consistent with that in the Tournigand trial. Also, the OS time in our study and the Saltz et al. [9] IFL study were very consistent, suggesting the value of the infusion of 5-FU when coupling it with irinotecan. In the design of N9741, we used, by necessity, the then standard of care in the U.S., IFL, as our control arm.
The second criticism was that oxaliplatin was experimental during the early phase of the study and was only available either as a part of one of a few clinical trials for second-line treatment or to patients who went to Europe to obtain the drug under the guidance of a European oncologist. For that reason, only a small percentage of patients found a way to get FOLFOX in the second line. In contrast, in patients initially randomized to FOLFOX, irinotecan was available upon disease progression. This issue was subsequently addressed as the study evolved.
Regarding the reduction in the IFL doses employed midway through the trial, this strategy succeeded, and we noted less severe early toxicity among the 151 patients who received rIFL. Activity parameters still favored FOLFOX over rIFL for all endpoints—response rate, 47% versus 32% (p = .008); median time to progression (TTP), 10.1 months versus 6.5 months (p < .001); and median OS time, 20.5 months versus 16.3 months (p = .023). Of particular importance to the overall interpretation of the study was the experience of the patients after they completed protocol-specified therapy. In this later portion of the study, 75% of the patients in both arms went on to receive second-line, off-study therapy. By coincidence, 56% of the rIFL patients were treated with FOLFOX and 56% of the FOLFOX patients received a second-line irinotecan-based regimen. Although crossover was not mandated, and these results are therefore suggestive, we believe that this has largely resolved the question of whether the outcome of the original three-arm study was skewed because of the inability of patients to get access to oxaliplatin in the second-line setting. The benefits of first-line FOLFOX were confirmed.
Based on the N9741 results with the IROX regimen, in some cases for which 5-FU is contraindicated, such as in patients with dihydropyrimidine dehydrogenase deficiency or 5-FU hypersensitivity, the combination of IROX could be an attractive chemotherapy choice. We documented that toxicity, while tolerable, was substantial with IROX. The grade ≥3 toxicity rates were 32% for neutropenia, 28% for diarrhea, and 21% for vomiting [14]. Patients aged ≥70 years exhibited poor tolerance for the regimen. In all, 52% of patients required dosage reductions. Activity parameters were intermediate between FOLFOX and IFL, as previously noted.
A SHIFT TO FOLFOX
In its final iteration, the trial continued with a single arm—FOLFOX. It thus served as an access portal to permit patients to receive FOLFOX prior to its FDA approval as indicated for the treatment of patients with MCRC in the first-line setting. Submission of the appropriate data to the FDA in support of the new indication and their analysis required some months. On January 8, 2004, FOLFOX was approved for that indication.
This approval had significant implications for patients, for practice patterns, and for industry (principally for Pharmacia, who owned the patent on irinotecan in the U.S., and for Sanofi, who owned the patent for oxaliplatin). The N9741 results and subsequent oxaliplatin approval gave patients an additional therapeutic option. It enticed physicians in the U.S. to transition from using bolus 5-FU to using 5-FU infusion and, although subsequent studies showed that FOLFOX and FOLFIRI had similar activity profiles with distinctive toxicity profiles, the preference in the U.S. quickly transitioned from favoring first-line use of irinotecan to favoring oxaliplatin. Marketing data suggest that that preference persists. These shifts in practice patterns had substantial economic impacts on pharmaceutical companies and their personnel.
SUBSEQUENT ANALYSES
As previously noted, the study team and individuals who have requested data from the study have leveraged the results of this trial to provide additional insights into the management of MCRC beyond the primary study goals as well as using the data for other purposes. These secondary analyses can be divided into five categories: pooled analyses, subset analyses, economic analyses, investigations into clinical trial methodology, and pharmacogenetic/biomarker studies. The next part of this review focuses on selected outcomes from those analyses.
Subset Analyses
As this trial was recruiting patients, both European and American surgical groups reported that resection of liver or/and lung limited MCRC could be curative in a subset of patients. The evolution of this approach later led to randomized studies supporting resection of oligometastatic disease as the standard of care. Most advanced disease trials now exclude patients judged to be resectable and permit resection in those patients whose disease responds sufficiently to make that plausible. When this study was written, however, those distinctions were not made. There was no specific policy applied to enrollees with resectable disease and resection was permitted in N9741 when clinically indicated. We reported a subset analysis of the 24 of 795 patients who were taken to the operating room for potentially curative surgery [15]. These individuals had a markedly longer median OS time (42 months) than the median survival time for all enrolled patients. In addition, the majority of patients (22 of 24) who underwent resection received oxaliplatin-containing regimens.
We also focused on the 62 of 1,508 patients (4%) enrolled in all stages of the trial who achieved a complete remission (CR) as a consequence of their therapy [16]. The CR rates by arm were 6.2% for FOLFOX, 1.9% for IFL, and 2.9% for IROX. Some patients had their residual disease resected or ablated, but 35 received only chemotherapy. The median TTP for the CR patients was 15.3 months, compared with 7.3 months for those not achieving a CR. This retrospectively identified a subgroup of patients proven to have a very favorable prognosis, with a median survival time of 44.3 months.
Recently, we updated the results of this trial after a median of 5 years of follow-up on the entire population of 1,691 patients who had follow-up data available [17]. The 5-year OS rates were 9.8% for FOLFOX-treated patients, 3.7% for IFL-treated patients, and 5.1% for IROX-treated patients. We also examined the role of risk-adjusted stratification using multiple clinical and laboratory parameters to develop high-, medium-, and low-risk groups. The median OS time for the low-risk group was 20.7 months; for the medium-risk group it was 17.4 months and for the high-risk group it was 6.7 months. FOLFOX treatment proved to be superior in all three risk groups and was the most important predictor of survival on multivariate analysis. The risk group blend enrolled in any individual clinical trial can clearly influence the outcomes, perhaps accounting for optimistic findings in phase II studies that are not corroborated in randomized, phase III trials. This classification may prove valuable in comparing populations enrolled when making cross-study comparisons.
Two papers explored the monetary costs associated with oxaliplatin use in the U.S. The first used an incremental cost-effectiveness (ICE) projection based on data derived from the trial to compare IFL with FOLFOX [18]. The ICE for FOLFOX was projected to add approximately $117,000 per life-year to the costs of caring for MCRC patients. In a separate analysis, we evaluated the ICE of combinations of cytotoxic drugs as well as the cost of adding targeted therapies in the management of MCRC, examining data from a number of studies, including N9741, and used these data to run simulations [19]. Incremental gains in outcome measures came at considerable cost. For multiagent chemotherapy without biologic agents, the ICE ratio was approximately $100,000 per discounted life-year. This compared favorably with the $170,000 cost when biologic therapy was added to multiagent chemotherapy in this Markov model.
Pooled Analyses
Patient data from N9741 have been combined with data from other trials in five efforts. In the first of these, data from seven trials were pooled to examine the contribution of patient exposure to all three classes of cytotoxic agents to OS [20]. In that study, it was clear that the use of combination therapy in the first-line setting led to a statistically significant 3.5-month survival advantage over single-agent treatment. There was a very strong correlation of receipt of second-line therapy and exposure to all three cytotoxic classes with OS.
We used the experience of the patients in the FOLFOX arm of the study pooled with another first-line study, a second-line study, and the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) adjuvant trial to examine whether patients aged >70 had different outcomes with respect to either activity or toxicity than younger patients enrolled in these trials. None of the individual studies had the power to discern this [21]. We found that the benefits from FOLFOX were no different for older or younger patients. Older patients did have modestly, but significantly, higher rates of neutropenia, thrombocytopenia, and fatigue but did not experience worse neuropathy or gastrointestinal toxicity, nor did they have a higher rate of early deaths in the setting of first-line treatment of MCRC.
A similar meta-analysis was performed to examine the relative value of standard versus experimental therapies comparing patients with a PS score of 0 or 1 with those with a PS score of 2 enrolled in nine phase III trials using a variety of regimens [22]. There were >6,000 individual patient records pooled for this analysis, of which 509 were classified by their treating physicians as having a PS score of 2. Nausea, vomiting, and 60-day all-cause mortality were significantly higher in the patients with a PS score of 2. Patients with a PS score of 2 had a worse prognosis than did patients with PS scores of 0 or 1, with median OS times of 8.5 months versus 17.3 months, respectively. Nonetheless, patients with a PS score of 2 derived a proportional benefit from superior therapy similar to less symptomatic patients across the pooled trials.
Clinical Trial Methodology
Data from N9741 pooled with that from 32 other NCCTG studies, leading to a population of 2,374 patients, were used to examine clinical trial endpoints and tumor measurements [23]. We examined the value of measuring a single, a few, and up to 10 lesions as markers for response [16]. With measurement of the two largest lesions, there was excellent concordance with the results obtained from measuring a larger number of lesions in assessing response and TTP. In addition, requiring that a response be confirmed by a second scan at an interval of 4–8 weeks after the scan that established response did not materially influence the determination of the response rate. These data were instrumental in modifying the Response Evaluation Criteria in Solid Tumors to simplify measurement requirements from a maximum of 10 lesions to a maximum of five lesions in phase II trials and three lesions in phase III trials.
A similar pooled analysis was done of 26 trials, including N9741, to evaluate the utility of cataloging adverse events (AEs) regardless of grade [24]. In those studies >75,000 AEs were reported, of which >8,000 required clarification after review at the statistical center. In all, 83% of required reports were for data prespecified in the protocol for which the toxicity grade was 0 (not present) or mild. Overall, 3% of AEs were grade ≥3. We concluded that the majority of reported events were of no real utility in terms of monitoring and establishing patient safety, even in cases in which investigational agents were part of the treatment regimen. The massive efforts required to collect, verify, refine, and report these data required significant resource expenditure for questionable benefit.
In the Genentech-sponsored AVF2107 trial that established the benefit of bevacizumab with IFL in managing MCRC, it had been noted that patients with stable as well as responding disease had better PFS and OS when treated with bevacizumab [25]. It was not clear if this finding was related to receiving the superior regimen or to the inclusion of a biologic agent in that regimen. To evaluate this, we repeated the analysis in N9741, showing that the benefit was related to the receipt of a better regimen rather than a specific agent [26]. These data also suggested that measurable tumor shrinkage is not necessary in an individual patient for that person to benefit from therapy in terms of longer PFS and OS times.
Translational Studies
While N9741 was under way, an amendment led to the collection of blood from willing patients to obtain both germline DNA and plasma. The primary motivation for this was to perform pharmacogenetic studies of key drug activating, transporting, and metabolizing genes in order to characterize polymorphisms relevant to response and toxicity. To date, the plasma has also been used to explore the relevance of the insulin-like growth factor (IGF) pathway to both QOL and outcomes.
IGF-I and IGF-II stimulate neoplastic cell growth in mouse models, and IGF-binding protein 3 (IGFB-3) inhibits the bioavailability of IGF. In 527 patients who contributed blood samples, we correlated the pretreatment levels of these three factors as well as C-peptide with outcomes [27]. IGFB-3 levels were positively and significantly associated with higher response rates (p = .03). Higher levels of all three IGFs were associated with longer OS. Higher IGFB-3 was also significantly associated with longer TTP. These findings suggest that new drugs that target this pathway may have therapeutic potential in MCRC.
We correlated the same four protein levels with QOL parameters as assessed by the validated QOL instruments the Uniscale and the SDS [28]. We found that lower levels of IGF-I were associated with greater concern about appearance and worse appetite, cough, and nausea. Lower IGF-II levels correlated with worse appetite, fatigue, nausea, and pain and greater concern about appearance. These data suggest that baseline molecular biomarkers may be predictive of cancer patient QOL.
At the ASCO 2004 plenary session, QOL and pharmacogenetic data from N9741 were used to generate a presentation entitled “Preliminary evidence of a relationship between genetic markers and oncology patient QOL before treatment begins” [29]. We examined polymorphisms in 22 candidate genes that relate to folate metabolism, cellular homeostasis, and the transport, activation, and metabolism of the three chemotherapy agents employed in the treatment regimens. Although these genes were chosen to evaluate the effect of polymorphisms on drug activation and metabolism, we performed an exploratory analysis to discern if they had relevance to QOL. The DPYD*5 gene was most correlated with worse initial QOL (p = .008). In all, 13 of 80 relationships proved to be significant, including polymorphisms in ACBC1, ERCC2, several genes in the TYMS family, and others. Although this work is clearly not definitive, it suggests that individual genetic makeup assessed using polymorphisms relevant to drug metabolism may also have relevance to the QOL of patients with relatively uniform disease characteristics before therapy begins.
We examined 34 variants in 15 candidate genes to discern if these polymorphisms could predict activity and toxicity upon exposure of patients with MCRC to 5-FU, irinotecan, and oxaliplatin (McLeod et al., submitted manuscript). The homozygous UGT1A1*28 allele observed in 9% of patients was associated with a risk for grade 4 neutropenia in patients on IROX (55% versus 12%; p = .001). Deletion in GSTM1 was associated with grade 4 neutropenia after FOLFOX (28% versus 16%; p = .02). Patients with a homozygous variant genotype for GSTP1 were more likely to discontinue FOLFOX because of neurotoxicity (24% versus 10%; p = .01). The presence of a CYP3A5 variant was significantly associated with response rate to IFL (29% versus 60%; p = .0074). Most previously published genotype–toxicity or efficacy relationships were not validated in this study. Because of the multiple comparisons done in this analysis, these results are considered to be hypothesis generating. Nonetheless, this 520-patient analysis is one of the largest, well-characterized, relatively homogeneous cancer bearing populations available for such analyses.
The data from this analysis were instrumental in discerning the predictive value of the UGT1A1*28 allele for neutropenia in patients exposed to irinotecan. In retrospect, this neutropenia when linked to mucosal damage in the gastrointestinal tract may explain the early severe toxicity noted in the irinotecan arms in the initial phases of the study. Unfortunately, DNA samples were not available from those early patients whose severe side effects occurred before the study was amended to collect blood. However, in a pooled analysis of a number of studies using varying doses of irinotecan, it appears that higher absolute doses of the agents are more commonly associated with high-grade toxicity [30].
We also examined the potential for an association between pharmacogenetics and differences in outcomes observed between whites and blacks who enrolled in the study and provided blood samples [31]. The response rate was significantly higher in whites (41%) than in blacks (28%) (p = .009). Grade ≥3 toxicity was also higher in whites (48%) than in blacks (34%) (p = .004) mainly because of lower rates of severe diarrhea in black patients. These relationships were maintained in multivariate models adjusting for arm, age, sex, and PS score. There was no racial difference in dose intensity of delivered therapy. Significant racial differences in the distribution of polymorphisms in key candidate genes were observed between races, although the sample size was too small to investigate the relationship among treatment, race, and genotype. This observation does, however, suggest the possibility of individualizing therapy based on an additional patient characteristic.
CONCLUSION
The N9741 trial proved to be an important study in many ways. Its large size and the facts that prospective QOL data collection was built into the trial and blood sample collection for studies of germline DNA and plasma banking was incorporated allowed for many secondary analyses that have informed current practice and ongoing scientific exploration on the biology of colorectal cancer and colorectal cancer treatment. The study team that managed the trial included the protocol principal investigator (PI) Richard Goldberg, the lead statistician Daniel Sargent, the protocol development coordinator Jeanine Hadley, the lead data coordinator Carol Leonard, and the NCCTG PI Roscoe Morton as well as numerous others. The NCCTG DSMB was also heavily involved in its management. The study team adopted a generous policy of data sharing that permitted a number of outside investigators to bring forward concepts for use of the wealth of data collected. Authors of studies included individuals of diverse backgrounds, some of whom were only peripherally associated with the NCCTG. Coauthors have included fellows and doctoral students, junior and senior faculty, and community oncologists. In addition, statisticians, laboratory investigators, QOL experts, economists, and pharmaceutical company personnel were involved in various analyses. We believe that this model of liberal access policies to data is an advantageous way to maximize the value of a large public/private partnership such as this study and other cooperative group trials.
ACKNOWLEDGMENTS
Supported by CA25224 (NCCTG) and CA114740 (NCCTG Biospecimen Resource), Sanofi-Aventis, and Pharmacia.
Footnotes
Disclosures: Richard M. Goldberg: Consultant/advisory role: American Society of Clinical Oncology (ASCO), Cancer and Leukemia Group B (CALGB), National Surgical Adjuvant Breast and Bowel Project (NSABP); Honoraria: Myriad, Amgen, Genentech, ImClone, Sanofi-Aventis, Poniard, Bristol-Myers Squibb, Genomic Health; Research funding/contracted research: Pfizer, Sanofi-Aventis, Amgen, Abbott, Enzon; Daniel J. Sargent: None; Roscoe F. Morton: None; Erin Green: None; Hanna K. Sanoff: None; Howard McLeod: None; Jan Buckner: None.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
AUTHOR CONTRIBUTIONS
Conception/Design: Richard Goldberg, Erin Green, Howard McLeod, Roscoe F. Morton, Daniel Sargent
Administrative support: Richard Goldberg, Jan Buckner, Erin Green, Howard McLeod, Daniel Sargent
Provision of study material or patients: Richard Goldberg, Erin Green, Howard McLeod, Roscoe F. Morton, Daniel Sargent
Collection and/or assembly of data: Richard Goldberg, Erin Green, Howard McLeod, Roscoe F. Morton, Hanna K. Sanoff, Daniel Sargent
Data analysis and interpretation: Richard Goldberg, Erin Green, Howard McLeod, Roscoe F. Morton, Hanna K. Sanoff, Daniel Sargent
Manuscript writing: Richard Goldberg
Final approval of manuscript: Richard Goldberg, Jan Buckner, Erin Green, Howard McLeod, Roscoe F. Morton, Hanna K. Sanoff, Daniel Sargent
REFERENCES
- 1.Goldberg RM, Kaufmann SH, Atherton P, et al. A phase I study of sequential irinotecan and 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:1674–1680. doi: 10.1093/annonc/mdf260. [DOI] [PubMed] [Google Scholar]
- 2.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 3.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Szanto J, Roth A, et al. Evaluation of the addition of oxaliplatin to the same Mayo or German 5FU regimen in advanced refractory colorectal cancer [abstract] Proc Am Soc Clin Oncol. 1999;18:234a. [Google Scholar]
- 5.Goldberg RM, Sargent DJ, Morton RF, et al. Early detection of toxicity and adjustment of ongoing clinical trials: The history and performance of the North Central Cancer Treatment Group’s real-time toxicity monitoring program. J Clin Oncol. 2002;20:4591–4596. doi: 10.1200/JCO.2002.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Sargent DJ, Goldberg RM, Mahoney MR, et al. Rapid reporting and review of an increased incidence of a known adverse event. J Natl Cancer Inst. 2000;92:1011–1013. doi: 10.1093/jnci/92.12.1011. [DOI] [PubMed] [Google Scholar]
- 7.Delaunoit T, Goldberg RM, Sargent DJ, et al. Mortality associated with daily bolus 5-fluorouracil/leucovorin administered in combination with either irinotecan or oxaliplatin: Results from Intergroup Trial N9741. Cancer. 2004;101:2170–2176. doi: 10.1002/cncr.20594. [DOI] [PubMed] [Google Scholar]
- 8.Rothenberg ML, Meropol NJ, Poplin EA, et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: Summary findings of an independent panel. J Clin Oncol. 2001;19:3801–3807. doi: 10.1200/JCO.2001.19.18.3801. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, Cox JV, Blanke C, et al. Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 10.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American Intergroup trial. J Clin Oncol. 2006;24:3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 14.Ashley AC, Sargent DJ, Alberts SR, et al. Updated efficacy and toxicity analysis of irinotecan and oxaliplatin (IROX): Intergroup Trial N9741 in first-line treatment of metastatic colorectal cancer. Cancer. 2007;110:671–677. doi: 10.1002/cncr.22831. [DOI] [PubMed] [Google Scholar]
- 15.Delaunoit T, Alberts SR, Sargent DJ, et al. Chemotherapy permits resection of metastatic colorectal cancer: Experience from Intergroup N9741. Ann Oncol. 2005;16:425–429. doi: 10.1093/annonc/mdi092. [DOI] [PubMed] [Google Scholar]
- 16.Dy GK, Krook JE, Green EM, et al. Impact of complete response to chemotherapy on overall survival in advanced colorectal cancer: Results from Intergroup N9741. J Clin Oncol. 2007;25:3469–3474. doi: 10.1200/JCO.2007.10.7128. [DOI] [PubMed] [Google Scholar]
- 17.Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillner BE, Schrag D, Sargent DJ, et al. Cost-effectiveness projections of oxaliplatin and infusional fluorouracil versus irinotecan and bolus fluorouracil in first-line therapy for metastatic colorectal carcinoma. Cancer. 2005;104:1871–1884. doi: 10.1002/cncr.21411. [DOI] [PubMed] [Google Scholar]
- 19.Wong YN, Meropol NJ, Speier W, et al. Cost implications of new treatments for advanced colorectal cancer. Cancer. 2009;115:2081–2091. doi: 10.1002/cncr.24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 22.Sargent DJ, Köhne CH, Sanoff HK, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:1948–1955. doi: 10.1200/JCO.2008.20.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillman S, An M, O’Connell MJ, et al. Evaluation of the optimal number of lesions needed for tumor evaluation using the response evaluation criteria in solid tumors: a North Central Cancer Treatment Group investigation. J Clin Oncol. 2009;27:3205–3210. doi: 10.1200/JCO.2008.18.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahoney MR, Sargent DJ, O’Connell MJ, et al. Dealing with a deluge of data: An assessment of adverse event data on North Central Cancer Treatment Group (NCCTG) trials. J Clin Oncol. 2005;23:9275–9281. doi: 10.1200/JCO.2004.00.0588. [DOI] [PubMed] [Google Scholar]
- 25.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 26.Grothey A, Hedrick EE, Mass RD, et al. Response-independent survival benefit in metastatic colorectal cancer: A comparative analysis of N9741 and AVF2107. J Clin Oncol. 2008;26:183–189. doi: 10.1200/JCO.2007.13.8099. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs CS, Goldberg RM, Sargent DJ, et al. Plasma insulin-like growth factors, insulin-like binding protein-3, and outcome in metastatic colorectal cancer: Results from intergroup trial N9741. Clin Cancer Res. 2008;14:8263–8269. doi: 10.1158/1078-0432.CCR-08-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerhardt JA, Sloan JA, Sargent DJ, et al. Associations between plasma insulin-like growth factor proteins and C-peptide and quality of life in patients with metastatic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1402–1410. doi: 10.1158/1055-9965.EPI-04-0862. [DOI] [PubMed] [Google Scholar]
- 29.Sloan JA, Mcleod H, Sargent D, et al. Preliminary evidence of relationship between genetic markers and oncology patient quality of life (QOL) Proc Am Soc Clin Oncol. 2004;23:2. [Google Scholar]
- 30.Hoskins JM, Goldberg RM, Qu P, et al. UGT1A1*28 genotype and irinotecan-induced neutropenia: Dose matters. J Natl Cancer Inst. 2007;99:1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 31.Sanoff H, Sargent DJ, Green EM, et al. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: A subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27:4109–4115. doi: 10.1200/JCO.2009.21.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]