Abstract
Neurogenesis is the process by which cells divide, migrate, and subsequently differentiate into a neuronal phenotype. Significant rates of neurogenesis persist into adulthood in two brain regions, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles. Cells of the SVZ divide and migrate via the rostral migratory stream (RMS) to the olfactory bulb (OB) where they differentiate into granule and periglomerular cells. With the discovery of large-scale neurogenesis in the adult brain, there have been significant efforts to identify the mechanisms that control this process as well as the role of these cells in neuronal functioning. Neurotrophic factors are a family of molecules that serve critical roles in the survival and differentiation of neurons during development, as well as contribute to continued plasticity throughout life. Several members of the neurotrophin family have been implicated in the control of adult postnatal SVZ neurogenesis. In this review we will address what is currently known regarding neurotrophic factor-dependent control of SVZ neurogenesis and place these findings in the context of what is known regarding other growth factors.
Keywords: neurotrophins, SVZ, neurogenesis, BDNF, TrkB, olfactory bulb
Introduction
Until recently, neurogenesis was largely understood to be restricted to prenatal and early postnatal development, with no neuronal regeneration in the adult brain. This notion was initially challenged by Altman and Das, who, using tritiated-thymidine labeling, identified newly dividing cells in the hippocampus and lateral ventricles of the adult rat (Altman, 1962, 1969; Altman and Das, 1965). Since that time, the advent of new and more accessible techniques to label and track newly born neurons has led to an explosion in the study of adult neurogenesis. Many recent studies have been focused on understanding the basic mechanisms controlling this process, the role of these cells for adult neuronal functioning, and the potential therapeutic benefits that these cells may provide. Despite intensive study, still many basic questions remain.
The two brain regions that contain the majority of neural stem cells are the subgranular zone (SGZ) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles. These stem cell populations divide to form neuroblasts, which eventually differentiate into neuronal or glial phenotypes and are integrated into the existing network. Neuroblasts of the SGZ primarily replace granule cells of the dentate gyrus (DG). Neuroblasts that originate in the SVZ migrate long distances along a rostral migratory stream (RMS) (Luskin, 1993; Lois and Alvarez-Buylla, 1994) to the OB where the majority differentiate into granule cells (∼95%) and a small population become periglomerular cells (∼5%). Within the SVZ, as many as 50,000 cells are born each day (Winner et al., 2002). The majority of neuroblasts that reach the OB die, with about 40% of newly born cells surviving throughout the life of the animal (Petreanu and Alvarez-Buylla, 2002; Winner et al., 2002). Approximately 95% of the newly born cells differentiate into a neuronal phenotype and functionally integrate into the existing circuitry. Nearly all of these cells become GABA or Calretinin positive inhibitory interneurons (Winner et al., 2002; Batista-Brito et al., 2008). However, a small percentage of these cells become tyrosin-hydroxylase (TH) positive dopaminergic neurons (Baker et al., 2001; Saino-Saito et al., 2004). This “turnover” of cells, represents an ∼6–10% replacement of the existing cellular population within the OB granule cell layer.
SVZ Neurogenesis and Olfactory Functioning
The incorporation of new cells into the OB occurs in an odor-specific, learning-dependent manner (Alonso et al., 2006; Mandairon et al., 2006a,b), suggesting that the influence of granule cell activity on odor representations is in turn regulated by the selective survival and differentiation of these migrating neuroblasts. The signaling molecules that modulate cell migration and eventual survival are not yet well understood. However, studies of OB neurogenesis, though correlational, have provided some of the clearest evidence for the functional importance of granule cells in olfactory performance. Computational models of the activity-dependent survival of newborn granule cells suggest that these interneurons modulate the tuning of bulbar activity to enhance olfactory discrimination performance and potentially regulate the categorization of novel odorants (Cecchi et al., 2001). Indeed, low rates of neurogenesis have been linked to impairments in olfactory discrimination, and the increased survival of newly born neurons has been associated with enhanced olfactory short-term memory (Gheusi et al., 2000; Mandairon et al., 2003, 2006a,b; Saghatelyan et al., 2005). In this context, the olfactory system provides a potential model system for examining the functional importance of adult neurogenesis.
Role for Neurotrophins in SVZ Neurogenesis
BDNF and SVZ Neurogenesis
A number of mechanisms involving diffusible signaling molecules, such as neurotransmitters, neuropeptides, and growth factors, have been proposed to regulate SVZ neurogenesis and its effects on olfactory tuning and plasticity. One growth factor implicated in the control of adult SVZ neurogenesis is brain-derived neurotrophic factor (BDNF). BDNF exists in two forms (proBDNF and mature BDNF) that may play divergent roles during neuronal development and throughout the life of the animal. BDNF mediates its diverse actions by binding to two structurally distinct receptors; a member of the Trk family of receptor tyrosine kinases (TrkB) and the p75 NTR receptor, a member of the tumor necrosis factor (TNF) receptor superfamily. BDNF signaling via these two pathways has typically been dichotomized, with positive effects, such as neuronal differentiation, dendritic branching, cell survival, and synaptic long-term potentiation (LTP) being attributed to mature BDNF signaling via TrkB, and processes such as cell death and synaptic long-term depression (LTD) being attributed to proBDNF signaling through p75NTR (Chao, 2003; Gentry et al., 2004; Lu et al., 2005). Thus far, the majority of studies that have focused on the role of BDNF in regulating postnatal SVZ neurogenesis have attributed their effects to the mature form of BDNF. However, future studies will be needed to disentangle effects attributable to pro relative to mature BDNF in regulating such processes.
As mature BDNF has been shown to promote the survival and differentiation of a variety of neuronal populations, it was an excellent candidate molecule to regulate the survival and/or differentiation of neural stem cells and neuroblasts in the adult brain. Goldman and coworkers demonstrated that BDNF administered to rat SVZ derived neuroblasts in vitro, promoted the long-term survival of these cells (Kirschenbaum and Goldman, 1995). In related studies, Luskin and coworkers demonstrated that following infusion of BDNF into the lateral ventricles of adult rats there was a near doubling of newly born neurons in the OB (Zigova et al., 1998). Furthermore, following intraventricular infusion of BDNF other groups also observed increases in the number of newly born neurons in adjacent structures, such as the striatum and septum (Pencea et al., 2001). These initial findings have been replicated by a number of labs using either BDNF infusion or viral overexpression of BDNF to attempt to augment SVZ neurogenesis (Benraiss et al., 2001; Chmielnicki et al., 2004; Henry et al., 2007). In addition, the viral overexpression of BDNF in typically nonneurogenic regions, such as the striatum, can support the survival of grafted progenitor cells, further supporting a role for BDNF as a trophic factor capable of promoting neurogenesis (Chen et al., 2007). It should also be noted here that some recent studies in mouse have failed to replicate the neurogenic properties of infusion of BDNF into the lateral ventricles (Galvao et al., 2008). This same group has also found in rats that the administration of BDNF into the lateral ventricles led to a decrease in SVZ neurogenesis (Galvao et al., 2008). Further studies may be required to clarify how these results fit with previous studies. Furthermore, alternative forms of BDNF exist (pro and mature) which may regulate opposing phenotypic outcomes. In the context of these studies demonstrating that exogenous BDNF can alter (either positively or negatively) SVZ neurogenesis, the role of endogenously produced BDNF and proBDNF remain to be determined.
Advances in transgenic mouse technologies have allowed for the development of a series of genetically altered mice in which the levels of BDNF expression in vivo can be altered and then alterations in basal rates of neurogenesis can be assessed. In mice in which a single copy of BDNF has been deleted, we have found a significant reduction in the number of newborn neurons in the OB (Bath et al., 2008). Interestingly, in these BDNF haploinsufficient mice, we found no effect of reduced BDNF levels on cell proliferation in the SVZ. From these data, we hypothesized that endogenous BDNF plays a significant role in the migration or survival of neuroblasts following cell division. It should be noted, that in these mice 50% of BDNF has been missing throughout the development of the animal, and these effects on postnatal neurogenesis may be the result of an earlier disruption in the organization of the neurogenic niche. Indeed, previous reports from Miller and coworkers have shown that disruptions in BDNF signaling lead to impairments in cortical development and subsequently a thinning of the subventricular zone (Bartkowska et al., 2007). However, in relation to OB development, others have shown that the complete loss of BDNF or any of its receptors, leads to no impairments in the embryonic development of the OB (Nef et al., 2001). Furthermore, in BDNF null mice, defects in SVZ neurogenesis are not detectable until at least 2 weeks after birth (Linnarsson et al., 2000). Using realtime quantitative PCR, we tested for changes in BDNF expression within the OB across development and found BDNF levels to significantly increase with age [Fig. 1(a)]. This increase in BDNF expression was mirrored by a decrease in p75NTR expression and no significant changes in total TrkB expression [Fig. 1(b,c)]. These findings taken together could indicate that SVZ derived stem cells destined for the OB cells may not depend upon BDNF signaling during embryonic and early postnatal development. Instead, during the first several weeks of life there may be a shift in the sensitivity of these cells to extrinsic factors such as BDNF. Such findings may explain why grafted cells derived from postnatal day 1 mice that lack the BDNF receptor TrkB, might not show impairment in migration or survival (Galvao et al., 2008). However, similar studies to those described in Galvao et al. would need to be performed using TrkB null neural stem cells derived from adults to support such a hypothesis.
Figure 1.
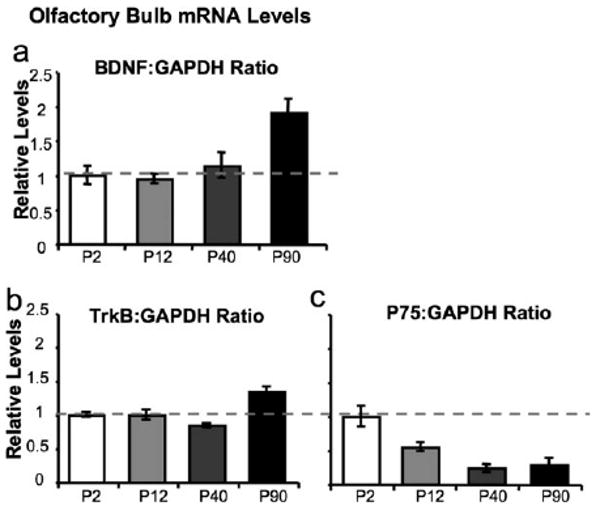
Developmental expression of BDNF, TrkB, and p75NTR in mouse OB. Histograms depict relative levels of (a) BDNF, (b) TrkB, and (c) p75NTR mRNA in the olfactory bulb of mice at different developmental time points (n = 3 per group). All samples were run in triplicate and normalize to GAPDH.
A major question that remains to be addressed is the localization of the source of BDNF regulating SVZ neurogenesis. Reports by Goldman and coworkers have suggested that endothelial cells are a primary source of BDNF (Leventhal et al., 1999). Much of this data comes from studies of SVZ explants cocultured with endothelial cells. They demonstrated that endothelial cells produce significant amounts of BDNF, and when cocultured, lead to increased survival of SVZ-derived cells that can be blocked by TrkB-Fc, a fusion complex that can sequester free BDNF. A role for endothelial cells as a significant source of BDNF has been supported by recent studies from Saghatelyan and coworkers (Snapyan et al., 2009). They demonstrated through the use of targeted knockout mice and biochemical means, that sequestering BDNF from the vasculature leads to significant impairments in neuroblast migration in the RMS. Recently, we generated a targeted knock-in mouse in which we replaced the wildtype BDNF with BDNF that contains a uniquely human single nucleotide polymorphism (SNP), a valine to methionine substitution in the prodomain (BDNF Val66Met) (Chen et al., 2006). This SNP leads to a significant reduction in regulated but not constitutive secretion of BDNF. In those same mice, we found a significant reduction in the survival of newly born OB granule cells (Bath et al., 2008). These findings are important as endothelial cells lack a regulated release mechanism, and thus do not explain the observed decrease in survival of newly born neurons in BDNF Val66Met mice. On the basis of these findings, we have hypothesized that a second source of BDNF exists, which is released in an activity-dependent manner, and can regulate the survival of newly born neurons in the OB. BDNF is released from neurons in an activity-dependent manner, a process known to support synaptic formation and neuronal excitability. BDNF Val66Met mutant mice, in which regulated BDNF secretion is impaired, may have defects in synaptic formation in the OB. Thus, newly born neurons, which may depend upon synaptic formation and activity to promote their integration into circuits and eventual survival, may fail to survive in Val66Met mice.
TrkB and SVZ Neurogenesis
Evidence for a role of BDNF in mediating neurogenesis has been quite compelling. However, BDNF can signal through either of two receptors (TrkB or p75NTR). As such, significant questions remain regarding which of these receptors is responsible for mediating the observed effects of BDNF on neurogenesis in vitro and in vivo. We will first focus on reviewing what is known regarding the role of TrkB in SVZ neurogenesis.
TrkB exists in four distinct isoforms, two full-length form (TrkB.FL) which differ in a short amino acid insert on the extracellular domain, both of which contain an active kinase domain with multiple phosphorylation sites. In addition, two truncated forms of TrkB have been identified, TrkB.T1 and TrkB.T2 which all lack the intracellular kinase domain (Reichardt, 2006). Activation of TrkB.FL can lead to downstream signaling via PLC-gamma, ERK, and/or PI3Kinase. Given the absence of the kinase domain, the truncated forms of TrkB have been largely believed to function as endogenous dominant negatives that bind excess BDNF but do not signal. However, in recent reports, Mattson and coworkers have identified a potential novel signaling pathway for TrkB.T1, through the activation of a G-protein coupled receptor and protein kinase C (Rose et al., 2003; Cheng et al., 2007). They have shown that activation of this pathway can significantly impact the cell fate decision of neural stem cells during early postnatal development and possibly guide them toward a glial fate.
Given the presence of an active kinase domain, TrkB.FL has received the most attention as a potential regulator of SVZ neurogenesis. Using antibodies specific to the intracellular domain, TrkB protein has been found to be highly enriched within subpopulations of cells of the SVZ across species (Yan et al., 1997; Zigova et al., 1998; Tonchev et al., 2007). However, the specific subpopulation of cells within this region that express TrkB has been somewhat controversial, with some groups identifying TrkB on migrating neuroblasts (Chiaramello et al., 2007; Bath et al., 2008) while others have shown TrkB to be restricted to astocytes (Galvao et al., 2008; Snapyan et al., 2009). Using an antibody specific to the phosphorylated form of TrkB (pTrkB), we found pTrkB to be selectively localized to migrating neuroblasts within the SVZ, RMS, and subependymal layer of the OB of mice (Bath et al., 2008). These findings are consistent with in vitro and in vivo studies where TrkB has been shown to be localized to PSA-NCAM positive migrating neuroblasts (Chiaramello et al., 2007). Consistent with these results, others have demonstrated a similar selective expression of TrkB on postmitotic immature neuroblasts within the SGZ of the hippocampus (Donovan et al., 2008; Li et al., 2008). This localization of TrkB to migrating neuroblasts is consistent with results from BDNF infusion and BDNF knockout animal studies, demonstrating a role for BDNF in the migration or survival but not proliferation of neuroblasts (Kirschenbaum and Goldman, 1995; Benraiss et al., 2001; Chiaramello et al., 2007; Bath et al., 2008; Snapyan et al., 2009). Through careful in vitro studies, TrkB signaling via PI3-K and ERK has shown to be critical for the appropriate migration of neuroblasts (Chiaramello et al., 2007). In further studies, BDNF stimulation of TrkB has been shown to be capable of rescuing inactivation or loss of the polysialated head (PSA) of PSA-NCAM, a molecule critical for cell adhesion during the migratory process (Vutskits et al., 2001). Consistent with these data, we found a significant reduction in the survival but not proliferation of newly born cells in the OB of TrkB heterozygous mice (Bath et al., 2008) an effect that is also observed in the hippocampus of these same mice (von Bohlen und Halbach et al., 2003; Bath et al., 2009). In recent reports, Alvarez-Buylla and coworkers show a similar trend toward a decrement in survival of newly born cells (14% fewer) in TrkB heterozygous mice compared to wildtype controls, through with a small n (three wild-type and four heterozygous mice), this difference approached but did not reach significance (Galvao et al., 2008).
Little data exists with regard to the role of the truncated forms of TrkB in the regulation of SVZ neurogenesis. Recent reports have shown that TrkB.T1 protein and message are enriched within the SVZ (Galvao et al., 2008) and in SVZ-derived cells in culture (Tervonen et al., 2006). Using cultures of SVZ-derived progenitor cells from mice in which the truncated TrkB.T1 was overexpressed, Tervonen and coworkers found a significant reduction in the number of neurosphere-forming progenitors compared with cells derived from wild-type mice. Interestingly, these same cells showed an increased rate of cell proliferation and differentiated into a neuronal phenotype at higher rates than wild-type-derived cells (Tervonen et al., 2006). These data suggest multiple roles for TrkB.T1 in regulating SVZ neurogenesis, proliferation, and differentiation. In addition, during early postnatal cortical development, TrkB.T1 has also been identified as playing a significant role in the regulation of gliogenesis (Cheng et al., 2007). Recently, a mouse has been generated in which the TrkB.T1 form of the TrkB receptor has been selectively knocked out (Carim-Todd et al., 2009). Future studies from this mouse will be important for understanding potential unique contributions of the truncated form of TrkB to the process of adult SVZ neurogenesis.
p75NTR and SVZ Neurogenesis
p75NTR is a member of the tumor necrosis receptor superfamily and is composed of an extracellular domain that includes four cysteine-rich motifs, a single transmembrane domain, and a cytoplasmic domain that includes a “death” domain (Liepinsh et al., 1997; He and Garcia, 2004). p75NTR exerts its potent effects on nervous system development through a variety of mechanisms. p75NTR is capable of inhibiting the activation of some forms of tropomysin-related kinase (Trk) receptors, the preferred receptor for neurotrophins (Benedetti et al., 1993; Bibel et al., 1999; Brennan et al., 1999). p75NTR can also interact with multiple novel signaling partners such as Nogo, Lingo, and Par-3 (Mi et al., 2004; Chan et al., 2006) to enhance responsiveness to ligands other than neurotrophins (e.g., oligodendrocyte myelin glycoprotein (OMgP), Nogo-66, and myelin-associated glycoprotein (MAG)) (reviewed in Bandtlow and Dechant, 2004; Barker, 2004; Gentry et al., 2004). p75NTR interaction in such signaling pathways occurs primarily during embryonic and early postnatal development to facilitate axonal outgrowth, axon targeting, synaptic stabilization, and myelination (Barker, 2004; Gentry et al., 2004). During postnatal brain development, p75NTR is significantly down-regulated in most neuronal populations including the OB [Fig. 1(c)]. This may in part be due to p75NTR's ability to induce cell death in response to high concentrations of neurotrophin or proneurotrophins which are upregulated postnatally. Such effects of p75NTR are most apparent following injury when p75NTR and proneurotrophins can be upregulated and induce death of cells in the injured area (Volosin et al., 2008).
In recent years p75NTR has also been implicated as a controller of adult SVZ neurogenesis. Using Western Blot analysis, Barde and coworkers have shown that stem cells that have been induced to differentiate into a neuronal phenotype, express high levels of p75NTR early postplating. p75NTR expression then decreases with a concomitant increase in TrkB expression (Bibel et al., 2004). These findings have been replicated in SVZ-derived neuroblasts in vitro (Gascon et al., 2005). Consistent with these findings, in initial immunohistochemical studies of the SVZ, a high degree of colocalization was found between p75NTR and nestin, a marker that labels proliferating cells within the SVZ and RMS (Giuliani et al., 2004). Others, including our lab, have found similar localization of p75NTR to nestin-positive cells using antibodies from different companies (Giuliani et al., 2004; Young et al., 2007; Bath et al., 2008), but the percent colocalization between p75NTR-nestin positive cells was extremely low (5–10%). It should also be noted that in recent reports, others have identified p75NTR colocalization with PSA-NCAM (Galvao et al., 2008) or doublecortin (Snapyan et al., 2009), markers found on migrating neuroblasts. The disparities in the subpopulations of cells identified may be due to the use of different antibodies by different groups. However, this is difficult to disentangle as all groups report the use of different antibodies and some groups report the use of multiple antibodies (Galvao et al. lists five antibodies for p75NTR). In an attempt to rectify the disparity between our and Young et al.'s findings, and those of Giuliani et al. regarding the percent of p75NTR-positive cells that are also nestin-positive, we conducted a comparison of p75NTR labeling in the rostral migratory stream (RMS) using several of the commercially available p75NTR antibodies in wildtype and p75NTR-null mice. As a control we included labeling in the basal forebrain, a p75NTR-rich region. We found that in the basal forebrain, the c-terminal [Fig. 2(a)] but not n-terminal [Fig. 2(e)] antibodies from Santa Cruz detect p75NTR-positive neurons and that staining is largely abolished in p75NTR null mice [Fig. 2(b,f)]. Both Santa Cruz antibodies showed a high degree of staining through the RMS of sections from a wild-type mouse [Fig. 2(c,g)]. However, much of the staining in the RMS remained in knockout tissue for both antibodies [Fig. 2(d,h)]. Antibodies from R&D showed greater specificity, detecting p75NTR in wild-type sections on basal forebrain neurons [Fig. 2(i)], staining that was abolished in tissue from p75NTR-null sections [Fig. 2(j)]. In addition, using this antibody we detected a small population of p75NTR-positive cells in the RMS [Fig. 2(k), arrows]. Labeling in the RMS was completely abolished in p75NTR null tissue [Fig. 2(l)].
Figure 2.
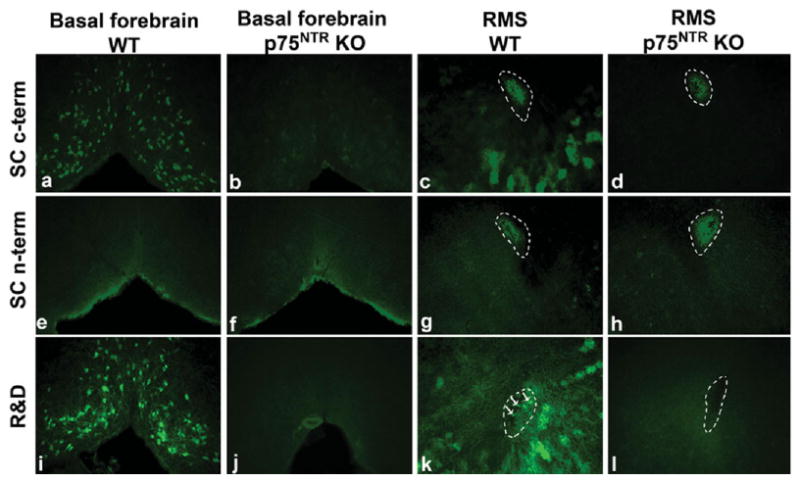
Comparison of commercially available p75NTR antibodies. Photomicrographs of p75NTR immunoreactivity in brains of wild-type (WT) and p75NTR-null (p75NTR KO) mice. Comparisons were made between the c-terminal (c-term) and n-terminal (n-term) antibodies from Santa Cruz Biotechnology, (SC- Cat#'s sc6188 and sc6189 respectively) and a c-terminal antibody from R&D Systems (R&D- Cat# AF1157). Immunolabeling was carried out in adjacent sections from a single wild-type and a single p75NTR null mouse. Immunoreactivity was compared in the basal forebrain (a, b, e, f, i, and j) and rostral migratory stream (RMS) (c, d, h, k, and l).
To assess the in vivo and in vitro consequence of disruptions in p75NTR signaling on adult SVZ neurogenesis, we and others have taken advantage of p75NTR null mice. Using FACS sorting, Bartlett and coworkers have shown that only a small population of cells (∼0.3%) within the SVZ are p75NTR-positive (Young et al., 2007). Using in vitro assays they argue that this population of cells is responsible for the production of all neurospheres, and that p75NTR-positive cells alone are neurogenic. These data are in part supported by their findings that p75NTR-null mice show a 70% reduction in their neurogenic potential in vitro. For in vivo studies they conducted whole mount staining of PSA-NCAM from wild-type and p75NTR-null mice and found a significant reduction in pixel density in p75NTR-null animals. However, no quantification of total cell density was conducted. Furthermore, they weighed OB's of p75NTR-null and wild-type mice and found a significant reduction in OB weight in p75NTR-null animals (Young et al., 2007). From these data they argue that p75NTR significantly contributes to neurogenesis in vivo. We carried out similar studies in wild-type and p75NTR-null mice and found no effect of the loss of p75NTR on cell proliferation in the SVZ or neuron survival in the OB using BrdU labeling and stereological sampling (Bath et al., 2008). Similarly, we found no reduction in volume of the olfactory bulbs of p75NTR-null mice compared to age matched wild-type controls using Cavaleri estimation or magnetic resonance microscopy. It should be noted, that Snapyan et al., also implicate p75NTR as a potential regulating of neurogenesis through its control of neuroblast migration. Based upon these three disparate findings, the question of p75NTR's role as a regulator of neurogenesis remains to be clarified.
Other Neurotrophin Family Members
Thus far, few studies exist characterizing the role of other members of the neurotrophin family of genes on adult SVZ neurogenesis. One group has shown that the intraventricular administration of NGF can increase SVZ proliferation in vivo (Fiore et al., 2002). However, they also demonstrate that this same protocol leads to an increased BDNF expression in the SVZ (Tirassa et al., 2003), making the individual contribution of NGF to SVZ neurogenesis less clear. This same group has also shown that in EAE rats, that message for NGF and its cognate receptor TrkA can be detected within the SVZ (Triaca et al., 2005). In addition, in vitro application of NGF can augment the survival and dendritic outgrowth of cells derived from the SVZ of rats (Gascon et al., 2005), but likely through p75NTR and not TrkA as this same group, as well as others, was unable to detect TrkA protein or mRNA in the SVZ (Giuliani et al., 2004; Gascon et al., 2005; Galvao et al., 2008).
Another neurotrophin family member, NT-3 has also been implicated in the proliferation of cells within the perinatal SVZ. However, instead of impacting neurogenesis, loss of NT-3 seems to selectively impact the survival and proliferation of SVZ-derived oligodendrocytes (Kahn et al., 1999). In subsequent studies using NT-3 null mice or mice lacking TrkC, the primary receptor to which NT-3 binds, no alterations were found in the development, organization, or size of the OB (Nef et al., 2001). To date, we are not aware of any studies that have assessed adult SVZ neurogenesis in NT-3 mutant mice or of studies assessing the expression of NT-3 or TrkC protein within the SVZ.
Other Growth Factors
Fibroblast Growth Factor (FGF-2)
In addition to neurotrophic factors, several other growth factors have been implicated in the control of SVZ neurogenesis. The first, fibroblast growth factor (FGF)-2, has also been shown to be capable of augmenting neurogenesis following intraventricular administration (Kuhn et al., 1997; Tropepe et al., 1999). In studies of rat brain, FGF-2 is expressed by GFAP-positive cells (Mudo et al., 2007), and the FGF receptors (FGFR-1 and FGFR-2) are present on the proliferating precursor and ependymal cells (Gonzalez et al., 1995). Use of FGF-2 in cultures promote both cell proliferation as well as increased survival and neurite outgrowth (Pincus et al., 1998). The primary action of FGF-2 seems to be in regulating cell cycle as mice lacking FGF-2 show significant impairments in SVZ cell proliferation (Raballo et al., 2000; Zheng et al., 2004). However, more work is required to specifically identify if FGF-2 regulates cell cycle length, cell cycle progression, or cell cycle reentry.
Epidermal Growth Factor (EGF)
A second growth factor shown to significantly influence adult SVZ neurogenesis is epidermal growth factor (EGF). Like FGF-2, EFG administration into lateral ventricles leads to significant upregulation of cell proliferation within the SVZ (Seroogy et al., 1995; Craig et al., 1996; Okano et al., 1996; Morshead et al., 2003). However, despite serving as a significant modulator of cell proliferation within the SVZ and in culture, the role of EGF in regulating neurogenesis per se, is somewhat less clear due to questions regarding the levels of its endogenous expression in neural tissue. In recent studies, Alvarez-Buylla and coworkers have shown that intraventricular infusion of EGF leads to augmentation in cell proliferation and migration of newly born cells into structures adjacent to the lateral ventricles. However, instead of becoming neurons, the vast majority of these cells upon differentiation express an oligodendrocyte or glial-like phenotype (Gonzalez-Perez et al., 2009). These findings are supported by data showing that suppression of EGFR signaling following injury leads to a decrease in the migration of SVZ-derived cells to the site of the lesion and subsequent oligodendrogenesis (Aguirre and Gallo, 2007).
Transforming Growth Factor (TGF)
Like other growth factors studied, transforming growth factor (TGF) also plays a significant role in regulating proliferation of cells of the SVZ and act on the same receptors as EGF (the EGF receptor; EGFR). TGF has been shown to be highly expressed in the striatum, a region adjacent to the SVZ (Wilcox and Derynck, 1988; Seroogy et al., 1993). Intraventricular infusion of TGF-alpha leads to a dramatic increase in cell proliferation in the SVZ (Craig et al., 1996). Furthermore, mice that lack TGF-alpha have significantly decreased rates of cell proliferation within the SVZ and fewer newly born cells within the OB (Tropepe et al., 1997). Interestingly, the intranasal administration of TGF-beta1 to mice after stroke leads to an increased SVZ neurogenesis and can abrogate some of the deleterious effects of stroke (Ma et al., 2008).
Vascular Endothelial Growth Factor (VEGF)
One of the most well studied growth factors capable of regulating adult SVZ neurogenesis is vascular endothelial growth factor (VEGF). Again, like other growth factors, intraventricular infusion of VEGF leads to increased cell proliferation in the SVZ (Jin et al., 2002; Sun et al., 2006). Mice lacking VEGF-B also show significant impairments in SVZ neurogenesis, with fewer cells reaching the OB (Sun et al., 2006). Using targeted KO mice, release of VEGF-A from GFAP positive cells stimulates VEGFR1 and VEGFR2 to regulate proliferation and migration of SVZ neuroblasts, and ultimately alters rates of surviving cells in vivo (Wittko et al., 2009). Finally, the overexpression of VEGF can increase rates of SVZ cell proliferation and also leads to increased migration of newly dividing cells to sites of ischemic injury in mouse models (Wang et al., 2007a,b).
Ciliary Neurotrophic Factor (CNTF)
Cilliary neurotrophic factor (CNTF) is a growth factor that is exclusively expressed within the CNS (Ip et al., 1993; Ip and Yancopoulos, 1996) and predominantly localized to astrocytes (Sendtner et al., 1994; Dallner et al., 2002). The CNTF receptor, CNTFR-alpha, is expressed in the adult SVZ (Ip et al., 1993) and may reflect a potential role in regulating adult SVZ neurogenesis. In recent reports, Emsley and Heff localized CNTFR-alpha to a subset of GFAP positive cells within the SVZ (Emsley and Hagg, 2003). They found that forebrain administration of CNTF increased the incorporation of BrdU into cells of the adult SVZ and increased the percentage of CNTFR-alpha BrdU double-positive cells. Furthermore, anti-CNTF antibody administration abrogated these effects, and led to a reduction in BrdU labeling in the SVZ (Emsley and Hagg, 2003). Mice in which CNTF has been genetically ablated have an ∼20% reduction in cell proliferation within the SVZ (Yang et al., 2008). This same group identified dopaminergic activation of CNTF-positive astrocytes as one potential mechanism by which CNTF may regulate adult neurogenesis (Yang et al., 2008). CNTF is also a potent regulator of adult neurogenesis in brain regions other than the SVZ, including the dentate gyrus (Emsley and Hagg, 2003; Muller et al., 2009), the hypothalamus (Kokoeva et al., 2005, 2007).
Discussion
Uncovering the molecular mechanisms that guide adult neurogenesis will pave the way to answering questions that have to this point remained elusive. Specifically, what is the functional relevance of adult neurogenesis and can these cells be recruited to aid in the treatment of neurodegenerative diseases or injury? Recently, several groups have begun to apply what has been learned regarding neurotrophic control of neurogenesis to such ends. Using adenoviral over-expression of BDNF following quinolinic acid lesioning of the striatum, one group was able to recruit SVZ-derived cells to the site of injury (Henry et al., 2007). Similarly, another group has shown that following the induction of stroke in rats, BDNF infusions leads to an augmentation in SVZ neurogenesis and newly born cells migrating to the striatum of the affected hemisphere (Schabitz et al., 2007). In yet another series of studies, infusions of BDNF in combination with VEGF increased SVZ neurogenesis and resulted in significant functional improvements in mice following stroke (Chen et al., 2005). Despite these early successes, much more work will be required to truly discover the potential and harness the power of these cells for such purposes.
References
- Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3:209–220. doi: 10.1017/S1740925X08000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Baker H, Liu N, Chun HS, Saino S, Berlin R, Volpe B, Son JH. Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci. 2001;21:8505–8513. doi: 10.1523/JNEUROSCI.21-21-08505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci STKE. 2004;2004:24. doi: 10.1126/stke.2352004pe24. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: Novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134:4369–4380. doi: 10.1242/dev.008227. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Voss HU, Jing D, Anderson S, Hempstead B, Lee FS, Dyke JP, et al. Quantitative intact specimen magnetic resonance microscopy at 3.0 T. Magn Reson Imaging. 2009;27:672–680. doi: 10.1016/j.mri.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, et al. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci. 2004;7:1003–1009. doi: 10.1038/nn1301. [DOI] [PubMed] [Google Scholar]
- Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- Carim-Todd L, Bath KG, Fulgenzi G, Yanpallewar S, Jing D, Barrick CA, Becker J, et al. Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29:678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi GA, Petreanu LT, Alvarez-Buylla A, Magnasco MO. Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci. 2001;11:175–182. doi: 10.1023/a:1012849801892. [DOI] [PubMed] [Google Scholar]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Henry RA, Hughes SM, Connor B. Creating a neurogenic environment: The role of BDNF and FGF2. Mol Cell Neurosci. 2007;36:108–120. doi: 10.1016/j.mcn.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Coksaygan T, Tang H, Khatri R, Balice-Gordon RJ, Rao MS, Mattson MP. Truncated tyrosine kinase B brain-derived neurotrophic factor receptor directs cortical neural stem cells to a glial cell fate by a novel signaling mechanism. J Neurochem. 2007;100:1515–1530. doi: 10.1111/j.1471-4159.2006.04337.x. [DOI] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, et al. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner C, Woods AG, Deller T, Kirsch M, Hofmann HD. CNTF and CNTF receptor alpha are constitutively expressed by astrocytes in the mouse brain. Glia. 2002;37:374–378. [PubMed] [Google Scholar]
- Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18:435–439. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- Fiore M, Triaca V, Amendola T, Tirassa P, Aloe L. Brain NGF and EGF administration improves passive avoidance response and stimulates brain precursor cells in aged male mice. Physiol Behav. 2002;77:437–443. doi: 10.1016/s0031-9384(02)00875-2. [DOI] [PubMed] [Google Scholar]
- Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon E, Vutskits L, Zhang H, Barral-Moran MJ, Kiss PJ, Mas C, Kiss JZ. Sequential activation of p75 and TrkB is involved in dendritic development of subventricular zone-derived neuronal progenitors in vitro. Eur J Neurosci. 2005;21:69–80. doi: 10.1111/j.1460-9568.2004.03849.x. [DOI] [PubMed] [Google Scholar]
- Gentry JJ, Barker PA, Carter BD. The p75 neurotrophin receptor: Multiple interactors and numerous functions. Prog Brain Res. 2004;146:25–39. doi: 10.1016/S0079-6123(03)46002-0. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A, D'Intino G, Paradisi M, Giardino L, Calza L. p75(NTR)-immunoreactivity in the subventricular zone of adult male rats: Expression by cycling cells. J Mol Histol. 2004;35:749–758. doi: 10.1007/s10735-004-9609-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal growth factor induces the progeny of subventricular zone Type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XL, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304:870–875. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]
- Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- Ip NY, McClain J, Barrezueta NX, Aldrich TH, Pan L, Li Y, Wiegand SJ, et al. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Ip NY, Yancopoulos GD. The neurotrophins and CNTF: Two families of collaborative neurotrophic factors. Annu Rev Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn MA, Kumar S, Liebl D, Chang R, Parada LF, De Vellis J. Mice lacking NT-3, and its receptor TrkC, exhibit profound deficiencies in CNS glial cells. Glia. 1999;26:153–165. [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Ilag LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnarsson S, Willson CA, Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Brain Res Mol Brain Res. 2000;75:61–69. doi: 10.1016/s0169-328x(99)00295-8. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Ma M, Ma Y, Yi X, Guo R, Zhu W, Fan X, Xu G, et al. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;9:117. doi: 10.1186/1471-2202-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006a;24:3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Jourdan F, Didier A. Deprivation of sensory inputs to the olfactory bulb up-regulates cell death and proliferation in the subventricular zone of adult mice. Neuroscience. 2003;119:507–516. doi: 10.1016/s0306-4522(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Linster C. Olfactory enrichment improves the recognition of individual components in mixtures. Physiol Behav. 2006b;89:379–384. doi: 10.1016/j.physbeh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Garcia AD, Sofroniew MV, van Der Kooy D. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience. 2007;145:470–483. doi: 10.1016/j.neuroscience.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Muller S, Chakrapani BP, Schwegler H, Hofmann HD, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009;27:431–441. doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- Nef S, Lush ME, Shipman TE, Parada LF. Neurotrophins are not required for normal embryonic development of olfactory neurons. Dev Biol. 2001;234:80–92. doi: 10.1006/dbio.2001.0240. [DOI] [PubMed] [Google Scholar]
- Okano HJ, Pfaff DW, Gibbs RB. Expression of EGFR-, p75NGFR-, and PSTAIR (cdc2)-like immunoreactivity by proliferating cells in the adult rat hippocampal formation and forebrain. Dev Neurosci. 1996;18:199–209. doi: 10.1159/000111408. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: Role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, et al. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, et al. Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron. 2005;46:103–116. doi: 10.1016/j.neuron.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Saino-Saito S, Sasaki H, Volpe BT, Kobayashi K, Berlin R, Baker H. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J Comp Neurol. 2004;479:389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Dittrich F, Hughes RA, Thoenen H. Actions of CNTF and neurotrophins on degenerating motoneurons: Preclinical studies and clinical implications. J Neurol Sci. 1994;124(Suppl):77–83. doi: 10.1016/0022-510x(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Gall CM, Lee DC, Kornblum HI. Proliferative zones of postnatal rat brain express epidermal growth factor receptor mRNA. Brain Res. 1995;670:157–164. doi: 10.1016/0006-8993(94)01300-7. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Lee DC, Guthrie KM, Gall CM. Cellular localization of transforming growth factor-alpha mRNA in rat forebrain. J Neurochem. 1993;60:1777–1782. doi: 10.1111/j.1471-4159.1993.tb13403.x. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: Evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329–335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Tervonen TA, Ajamian F, De Wit J, Verhaagen J, Castren E, Castren M. Overexpression of a truncated TrkB isoform increases the proliferation of neural progenitors. Eur J Neurosci. 2006;24:1277–1285. doi: 10.1111/j.1460-9568.2006.05010.x. [DOI] [PubMed] [Google Scholar]
- Tirassa P, Triaca V, Amendola T, Fiore M, Aloe L. EGF and NGF injected into the brain of old mice enhance BDNF and ChAT in proliferating subventricular zone. J Neurosci Res. 2003;72:557–564. doi: 10.1002/jnr.10614. [DOI] [PubMed] [Google Scholar]
- Tonchev AB, Yamashima T, Guo J, Chaldakov GN, Takakura N. Expression of angiogenic and neurotrophic factors in the progenitor cell niche of adult monkey subventricular zone. Neuroscience. 2007;144:1425–1435. doi: 10.1016/j.neuroscience.2006.10.052. [DOI] [PubMed] [Google Scholar]
- Triaca V, Tirassa P, Aloe L. Presence of nerve growth factor and TrkA expression in the SVZ of EAE rats: Evidence for a possible functional significance. Exp Neurol. 2005;191:53–64. doi: 10.1016/j.expneurol.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL, Carter BD, et al. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency in trkB and/or trkC neurotrophin receptors causes structural alterations in the aged hippocampus and amygdala. Eur J Neurosci. 2003;18:2319–2325. doi: 10.1046/j.1460-9568.2003.02953.x. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Djebbara-Hannas Z, Zhang H, Paccaud JP, Durbec P, Rougon G, Muller D, et al. PSA-NCAM modulates BDNF-dependent survival and differentiation of cortical neurons. Eur J Neurosci. 2001;13:1391–1402. doi: 10.1046/j.0953-816x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Guo X, Qiu MH, Feng XY, Sun FY. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J Neurosci Res. 2007a;85:73–82. doi: 10.1002/jnr.21091. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007b;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Derynck R. Developmental expression of transforming growth factors alpha and beta in mouse fetus. Mol Cell Biol. 1988;8:3415–3422. doi: 10.1128/mcb.8.8.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]
- Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28:2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]


