Abstract
A long wavelength boronic acid-modified TTP (NB-TTP) has been synthesized and enzymatically incorporated into DNA. Such DNA shows intrinsic fluorescent changes upon carbohydrate addition.
In addition to their critical roles in storing genomic information, DNA molecules are also very important in the development of aptamers,1 nanoelectronic assemblies, nanosensors, nanoclusters, nanocomputing,2 and DNA rotary machine, to name a few.3 DNA functionalization brings in additional properties for broadened applications. For example, gold-nanoparticle functionalized DNA assemblies have been used in sensor development with extraordinary sensitivity;3 and side-chain functionalized DNA has been used in aptamer selection with improved affinity and properties.4–5
Our lab has had a long-standing interest in using the boronic acid moiety, which is known to interact with diols/carbohydrates, fluoride, cyanide, and other nucleophiles/Lewis bases, as a recognition group for sensing and other applications.6–7 Recently, we have reported the synthesis of a quinolineboronic acid-modified thymidine triphosphate (QB-TTP, Fig. 1),8 its enzymatic incorporation into DNA, and discussed the feasibility of using it in DNA aptamer selection. Along this line, we desire to incorporate a fluorescent boronic acid into DNA that has the following properties: (1) the excitation and emission wavelengths are longer than the λmax of nucleobases; (2) shows fluorescent property changes upon carbohydrate binding; (3) is stable under PCR conditions; (4) is stable under copper-mediated “click” conditions;9–10 and (5) is relatively easy to synthesize. Such boronic acid-modified DNA may have applications in carbohydrate and glycoprotein sensing as well as the construction of DNA assemblies with unique properties.7 For example, nucleic acid and peptide-templated multi-boronic acid compounds are artificial lectin mimics; boronic acid-containing compounds have been used in the preparation of polymers with reversible properties; boronic acid-modified proteins can be used as lectin mimics with improved affinity and for protein purification; and boronic acid-containing polymers have been used for the development of glucose-sensitive insulin release systems. Beyond simple sensing, incorporation of fluorescent boronic acid-modified thymidine into genomic DNA may allow for the probe of changes in the nuclear environment, nuclear imaging, and cell division genomic DNA tracing. Herein, we report the very first side chain functionalized naphthalimide-based boronic acid-modified TTP (NB-TTP, Fig. 1) that by itself shows significant intrinsic fluorescent property changes upon carbohydrate binding and retains its ability to show fluorescent changes upon binding after DNA incorporation.
Fig. 1.
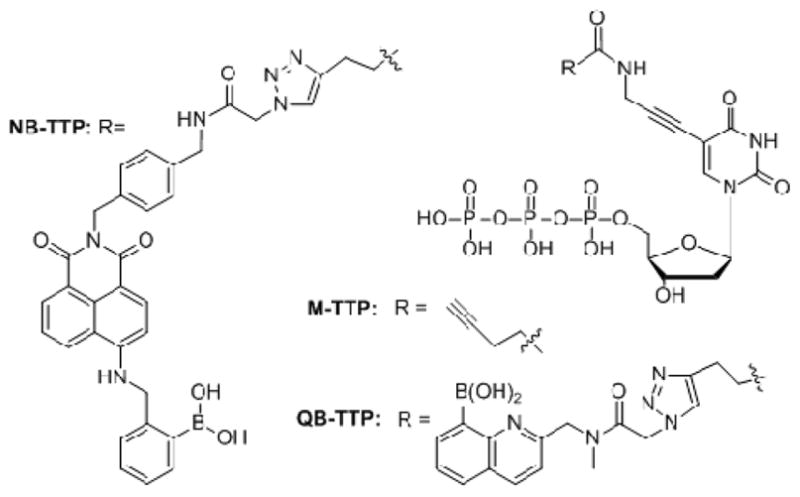
M-TTP, QB-TTP and NB-TTP
4-Amino-1,4-naphthalimide was chosen as the fluorophore after careful consideration and evaluation.11–14 Many boronic acids that change fluorescent properties have been reported. However, not all fluorescent boronic acids retain their fluorescent properties after incoporation into DNA. Such is the case with quinolineboronic acid (Fig. 1. QB-TTP), which loses its ability to show significant fluorescent intensity changes (over 40 fold) in response to sugar binding after DNA incorproation.8 Furthermore, when we applied the criteria set out above, the naphthalimide-based boronic acid moiety in Fig. 1 seemed to represent the best choice because of its relatively long excitation and emission wavelengths, stability, and ease of synthesis. For example, under constant irradiation at 490 nm for one hour, the fluorescence of boronic acid 7 (Scheme 1) didn’t show noticeable changes (Fig. S1, SI). Thus, the problem of photo bleaching faced by many fluorophores15–16 should not be a major issue in this case. In this design, Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC),17 which has been studied in previous boronic acid synthesis,8, 18–19 was used to conjugate the thymidine triphosphate moiety with the boronic acid moiety. This was done because of the difficulty in performing triphosphorylation in the presence of a boronic acid moiety (hygroscopic) and the difficulty in installing a boronic acid group in the presence of a triphosphate. The synthesis of the boronic acid moiety is described in Scheme 1 and the synthesis of M-TTP (Fig. 1) has been described previously.8
Scheme 1.
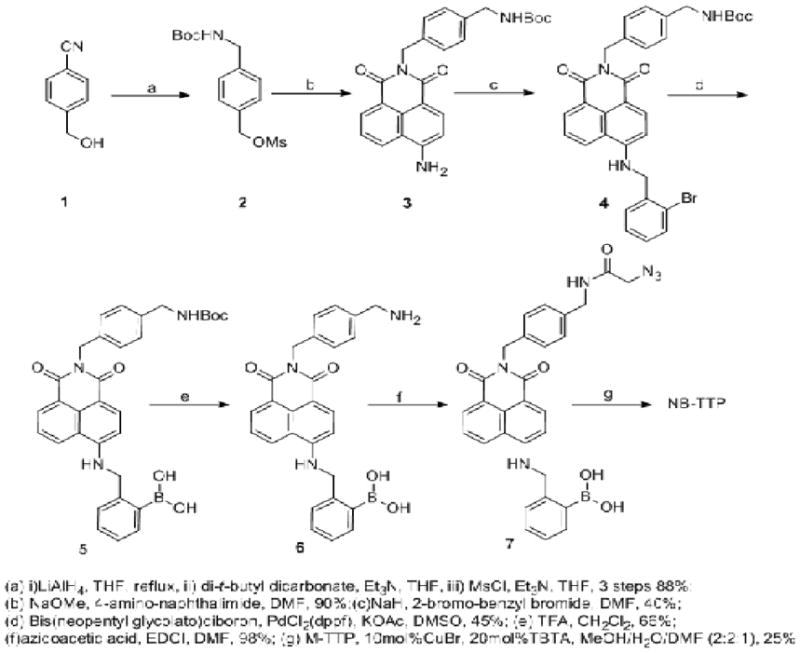
Synthesis route of NB-TTP
During the experiment, it was observed in Steps (e) and (f) (Scheme 1) that the solubility of boronic acids 6 and 7 in methanol was very low if the solution is acidic and treating with K2CO3 brought the boronic acids into solution. The synthesized NB-TTP has a solubility of about 170 μM in water as determined using UV. This solubility is high enough for most enzymatic reaction requirements.
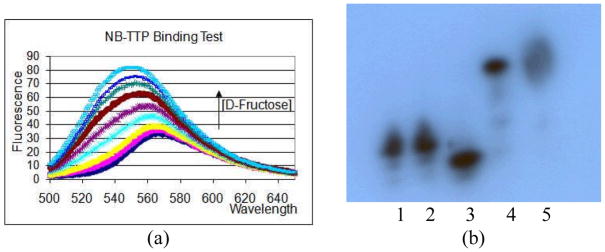
Since the NB-TTP compound was designed for its ability to change fluorescent properties, we studied its binding with a model sugar, fructose. As shown in Fig. 2a, the fluorescence intensity of NB-TTP increased upon D-fructose addition in a concentration dependent fashion (λex: 490 nm). The Ka was determined to be 73 ± 5 M−1 (see SI for details). Such results demonstrate that even after conjugation with a nucleobase (thymidine), the naphthalimide-based boronic acid maintains its ability to change fluorescent properties upon sugar binding.
Fig. 2.
(a) Fluorescent binding test of NB-TTP with D-fructose (b) Primer extension analyzed on 15% PAGE: 1) primer only; 2) reaction without enzyme; 3) reaction without dNTPs; 4) reaction with natural dNTPs; 5) reaction with NB-TTP and other dNTPs
Next we studied whether this NB-TTP can be incorporated into DNA in an enzyme-catalyzed reaction. In this study, NB-TTP was incorporated into a DNA sequence of a 14-nt primer (Table 1) using a 21-nt template (Template 0, Table 1) through Klenow fragment catalyzed primer extension.20 The primer and template sequences were designed in such a way that the first base to be incorporated is a T, which led to a “none-or-all” case in the extension. In the control reaction, full extension of the primer using natural dNTPs yielded a DNA with m/z 6520.4 (calculated [M+H]+: 6519.3) as the major product peak in MALDI. The minor peak of m/z 6850.6 could have resulted from a non-specific extension with an extra nucleotide. In the NB-TTP (replacing TTP) reaction, primer extension yielded a DNA with m/z 7142.8 as the major product peak and a new peak of m/z 7160.6 after treatment with H2O2. Each was assigned as the deborylated (calc. [M+H-HBO2]+: 7142.5) and oxidative deborylated NB-TTP-DNA sequence (calc. [M+H-HBO]+: 7158.5), respectively. Thus, it was concluded that the boronic acid of NB-TTP was intact after incorporation into the DNA sequence and deborylation happened during the ionization process, which is common with arylboronic acids.21
Table 1.
Designed sequences for NB-TTP-DNA construction
| Primer | 3′-GGTCGTTGGGCGAT-5′ |
| Template 0 | 5′-GGTTCCACCAGCAACCCGCTA-3′ |
| Template 1 | 5′-GGTTCCAACCAGCAACCCGCTA-3′ |
| Template 2 | 5′-GGTTCACACCAGCAACCCGCTA-3′ |
| Template 3 | 5′-GGTTACCACCAGCAACCCGCTA-3′ |
| Template 4 | 5′-GGTATCCACCAGCAACCCGCTA-3′ |
| Template 5 | 5′-GGATTCCACCAGCAACCCGCTA-3′ |
| Template 6 | 5′-GAGTTCCACCAGCAACCCGCTA-3′ |
| Template 7 | 5′-AGGTTCCACCAGCAACCCGCTA-3′ |
DNA from primer extension using NB-TTP was studied using gel electrophoresis. The 14-nt primer was labeled with 32P at the 5′-end using γ-32P-ATP and T4 kinase (Figure 2b, Lane 1). Negative control reactions without the Klenow fragment (Fig. 2b Lane 2) and without dNTPs (Fig. 2b, Lane 3) showed no full length DNA sequence. On the other hand, positive control reactions in the presence of both the enzyme and natural dNTPs (Fig. 2b, Lane 4) showed full length DNA. The shorter length of DNA in the Lane 3 without dNTPs could result from the 3′→5′ exonuclease activity of Klenow fragment.20 Primer extension using NB-TTP and three other natural dNTPs gave a full length of DNA sequence (Fig. 2b, Lane 5). Its smear band could be due to the interaction of the boronic acid moiety with the gel matrix or DNA conformation(s) disrupted by the large naphthalimide moiety. Thus, both mass spectrometry and gel electrophoresis results indicated that NB-TTP was recognized by DNA polymerase as a substrate and incorporated into DNA.
After incorporating NB-TTP into the DNA sequence, we were interested in studying whether sugar addition to the DNA solution would still cause fluorescence property changes. Since dNTPs and oligonucleotides are known to quench fluorescence,22–24 the retention of NB-TTP’s fluorescent property in DNA was uncertain. Fructose in the concentration range of 1 to 100 mM was used as a model sugar. Fig. 3 shows that the fluorescence intensity of NB-TTP-DNA increased by about 1.5-fold upon fructose addition at its highest concentration used (100 mM) (λex: 490 nm), which demonstrated that the fluorescent properties of NB-TTP were retained. Again, this is the very first example of side-chain functionalized DNA showing intrinsic fluorescent property changes upon sugar binding and such changes do not rely on FRET.
Fig. 3.
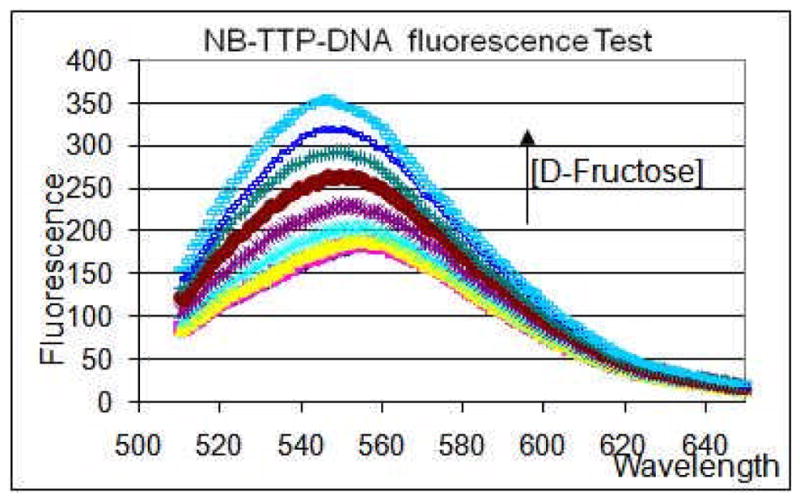
Fluorescent binding tests of NB-TTP-DNA with D-fructose
Since fluorophores can self-quench through stacking and DNA can also modify the fluorescent properties of a fluorophore if intercalation occurs, we were also interested in examining whether spacing between two boronic acid-modified T would affect the intrinsic fluorescent properties of the fluorophore. Therefore, templates 1–7 were designed in such a way that two A bases at the 5′-end were separated by 0–6 bases in between while keeping the rest of bases the same (Table 1), which would lead to DNA sequences with two boronic acid-modified T separated from 0 to 6 bases.
The same model sugar, fructose, was chosen for the fluorescent test. Double stranded NB-TTP-DNA sequences were generated through primer extension reactions with primer/template ratio of 1:1 and no heat denaturing before the binding test. The 21-nt template denoted as Template 0 was used as the control (Table 1). From the testing results (Fig S3.1, SI), it can be seen that all boronic acid-modified DNA showed fluorescent intensity changes upon sugar addition. Such results indicate that having more than one fluorophore in the DNA sequence did not affect its ability to fluoresce. The same studies were conducted using the ssDNA. Again, the boronic acid fluorophore retained its ability to change fluorescence (Fig S3.2, SI). It should be noted that the fructose studies were meant to test the intrinsic ability for the fluorophore in question to change fluorescence. This is not a “sensor” for fructose or any other sugar.
Conclusions
A novel fluorescent boronic acid conjugated TTP analogue (NB-TTP) was successfully synthesized. NB-TTP was readily recognized by the Klenow fragment and incorporated into DNA. NB-TTP modified DNA showed fluorescence intensity increases upon binding with fructose. Spacing two boronic acids in various positions does not fundamentally affect its ability to change fluorescence upon sugar binding. The boronic acid-modified thymidine will be very useful for preparation of fluorescent DNA-based probes/materials, genomic DNA incorporation to study specific properties in the nucleus, and in cell lineage studies.
Supplementary Material
Acknowledgments
We appreciate the financial support by the Georgia Cancer Coalition, Georgia Research Alliance, the National Institutes of Health (Grants CA123329, CA113917, GM084933, and GM086925), and the GSU MBD program. We also thank Dr. Siming Wang, Director of GSU MS Facilities, for her extensive help with the mass spectrometry work.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
References
- 1.Wilson DS, Szostak JW. Annu Rev Biochem. 1999;68:611–647. doi: 10.1146/annurev.biochem.68.1.611. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 2.Adleman LM. Science. 1994;266:1021–1024. doi: 10.1126/science.7973651. [DOI] [PubMed] [Google Scholar]
- 3.Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 4.Sakthivel K, Barbas CF. Angew Chem, Int Ed. 1998;37:2872–2875. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2872::AID-ANIE2872>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Eaton BE, Pieken WA. Annu Rev Biochem. 1995;64:837–863. doi: 10.1146/annurev.bi.64.070195.004201. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 6.Hall DG. Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine. Wiley-VCH; Weinheim: 2005. and references cited therein. [Google Scholar]
- 7.Jin S, Cheng Y, Reid S, Li M, Wang B. Med Res Rev. 2009 doi: 10.1002/med.20155. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin N, Yan J, Huang Z, Altier C, Li M, Carrasco N, Suyemoto M, Johnston L, Wang S, Wang Q, Fang H, Caton-Williams J, Wang B. Nucleic Acids Res. 2007;35:1222–1229. doi: 10.1093/nar/gkl1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gierlich J, Burley GA, Gramlich PME, Hammond DM, Carell T. Org Lett. 2006;8:3639–3642. doi: 10.1021/ol0610946. [DOI] [PubMed] [Google Scholar]
- 10.Bouillon C, Meyer A, Vidal S, Jochum A, Chevolot Y, Cloarec JP, Praly JP, Vasseur JJ, Morvan F. J Org Chem. 2006;71:4700–4702. doi: 10.1021/jo060572n. [DOI] [PubMed] [Google Scholar]
- 11.DiCesare N, Adhikari DP, Heynekamp JJ, Heagy MD, Lakowicz JR. J Fluoresc. 2002;12:147–154. [PMC free article] [PubMed] [Google Scholar]
- 12.Jin S, Wang JF, Li MY, Wang BH. Chem Eur J. 2008;14:2795–2804. doi: 10.1002/chem.200701785. [DOI] [PubMed] [Google Scholar]
- 13.Trupp S, Schweitzer A, Mohr GJ. Org Biomol Chem. 2006;4:2965–2968. doi: 10.1039/b604716e. [DOI] [PubMed] [Google Scholar]
- 14.Wang JF, Jin S, Akay S, Wang BH. Eur J Org Chem. 2007:2091–2099. [Google Scholar]
- 15.Soper SA, Nutter HL, Keller RA, Davis LM, Shera EB. Photochem Photobiol. 1993;57:972–977. [Google Scholar]
- 16.Ambrose WP, Goodwin PM, Jett JH, Van Orden A, Werner JH, Keller RA. Chem Rev. 1999;99:2929–2956. doi: 10.1021/cr980132z. [DOI] [PubMed] [Google Scholar]
- 17.Kolb HC, Finn MG, Sharpless KB. Angew Chem, Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Scrafton DK, Taylor JE, Mahon MF, Fossey JS, James TD. J Org Chem. 2008;73:2871–2874. doi: 10.1021/jo702584u. [DOI] [PubMed] [Google Scholar]
- 19.Jin S, Choudhary G, Cheng YF, Dai CF, Li MY, Wang BH. J Chem Soc-Chem Commun. 2009:5251–5253. doi: 10.1039/b909575f. [DOI] [PubMed] [Google Scholar]
- 20.Klenow H, Henningsen I. Proc Natl Acad Sci U S A. 1970;65:168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cragg RH, Todd JFJ, Weston AF. Org Mass Spectrom. 1972;6:1077–1081. [Google Scholar]
- 22.Rachofsky EL, Osman R, Ross JBA. Biochemistry. 2001;40:946–956. doi: 10.1021/bi001664o. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins ME, Pfleiderer W, Balis FM, Porter D, Knutson JR. Anal Biochem. 1997;244:86–95. doi: 10.1006/abio.1996.9879. [DOI] [PubMed] [Google Scholar]
- 24.Arkin MR, Stemp EDA, Turro C, Turro NJ, Barton JK. J Am Chem Soc. 1996;118:2267–2274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.